Back to Journals » Drug Design, Development and Therapy » Volume 17
Tripterygium wilfordii Hook. F. and Its Extracts for Psoriasis: Efficacy and Mechanism
Authors Wang Y, Tian Z, Huang S, Dang N
Received 9 September 2023
Accepted for publication 21 November 2023
Published 20 December 2023 Volume 2023:17 Pages 3767—3781
DOI https://doi.org/10.2147/DDDT.S439534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yingchao Wang,1,2 Zhaochun Tian,3 Shuhong Huang,4 Ningning Dang1,2
1Department of Dermatology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Jinan, Shandong, People’s Republic of China; 2Department of Dermatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 3Science and Technology Innovation Center, Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 4School of Clinical and Basic Medical Sciences, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China
Correspondence: Ningning Dang, Department of Dermatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, 324 Jingwuweiqi Road, Jinan, Shandong, 250021, People’s Republic of China, Email [email protected]
Abstract: Psoriasis is an inflammatory autoimmune skin condition that is clinically marked by chronic erythema and scaling. The traditional Chinese herb Tripterygium wilfordii Hook. F. (TwHF) is commonly used in the treatment of immune-related skin illnesses, such as psoriasis. In clinical studies, PASI (Psoriasis Area and Severity Index) were dramatically decreased by TwHF and its extracts. Their benefits for psoriasis also include relief from psoriasis symptoms such as itching, dryness, overall lesion scores and quality of life. And the pathological mechanisms include anti-inflammation, immunomodulation and potentially signaling pathway modulations, which are achieved by modulating type-3 inflammatory cytokines including IL-22, IL-23, and IL-17 as well as immune cells like Th17 lymphocytes, γδT cells, and interfering with IFN-SOCS1, NF-κB and IL- 36α signaling pathways. TwHF and its extracts may cause various adverse drug reactions, such as gastrointestinal responses, aberrant hepatocytes, reproductive issues, and liver function impairment, but at adequate doses, they are regarded as an alternative therapy for the treatment of psoriasis. In this review, the effectiveness and mechanisms of TwHF and its extracts in psoriasis treatment are elucidated.
Keywords: efficacy, mode of action, Tripterygium wilfordii Hook. F., psoriasis, type-3 inflammation
Introduction
Introduction of Psoriasis
Psoriasis is an inflammatory immune-mediated skin condition characterized by chronic widespread papules, scales, and erythema. It is more prevalent in high-income countries, with frequency ranging from 0.1% in East Asia to 1.5% in Western Europe.1 Approximately 120 million individuals are affected globally.2 The severity of the illness is assessed using PASI (Psoriasis Area and Severity Index), which takes into consideration the presence of erythema, scaling, thickness, infiltration, and the extent of the lesions.3 Different clinical phenotypes of psoriasis exist, however, persistent plaque or psoriasis vulgaris is the most common and most easily recognized.1 Psoriasis is an autoimmune disease that affects the innate and adaptive immune systems, with KCs (keratinocytes), T lymphocytes, and DCs (dendritic cells) playing important roles.4 Both environmental and genetic factors, such as smoking, can influence the clinical progression of psoriasis.5,6 (Figure 1) Originally, psoriasis was thought to be caused by a disorder of epidermal keratinocytes.7 Subsequent research has shown that Th1 cytokines such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-12 (IL-12) play an important role in the development of psoriasis.8 Recent studies have also revealed that psoriasis is characterized by immunological abnormalities in Th17 cells and Th17 cytokines such as IL-17A and IL-17F, so as known as type-3 inflammation, as well as abnormal keratinocyte differentiation and proliferation.9,10 Furthermore, the IL-23/Th17 pathway/axis takes a critical role in the etiology of psoriasis.11–13 Patients with psoriasis have a much higher risk of depression and suicide, according to studies.14,15 Therefore, finding an effective treatment for psoriasis is essential to lessening the severe effects of the condition on one’s physical, social, and psychological well-being. Currently, traditional psoriasis therapies include the topical application of drugs including corticosteroids, vitamin D3 analogues, calcineurin inhibitors, keratolytics, and combination topical medication, as well as systemic medications like methotrexate (MTX), apremilast, ciclosporin, and acitretin.1 Over the last 20 years, monoclonal biologics have been successfully approved for psoriasis treatment targeting highly specific cytokines like TNF-α, IL-12, IL-17, and IL-23, which have higher efficacy than chemical drugs. Physical therapy and topical medicines are recommended for those with mild to severe psoriasis. Systemic medicine and monoclonal biologics are suitable for moderate to severe psoriasis.16 There are still limitations in the systemic treatment of psoriasis. For example, Acitretin, a retinoid used to treat psoriasis, is associated with dose-dependent pneumonia as the most common side effect, along with other adverse effects such as eye problems, liver inflammation, and teratogenicity, particularly contraindicated in women of childbearing age, and may also cause minor side effects like dose-dependent hair loss and dry skin.2,17 At the same time, MTX, a frequently employed primary systemic therapy for psoriasis, exhibits teratogenicity and potential adverse effects, including the risk of hepatotoxicity, myelosuppression, and other detrimental reactions, potentially resulting in liver cirrhosis. Therefore, long-term, vigilant monitoring of hepatic function and hematological parameters is imperative.1,18 Moreover, the limitations of monoclonal biologics include a much greater expense than some other psoriasis therapies and there is still a not insignificant percentage of patients who do not respond to the drug at all.19 Therefore, the current selection of pharmacological treatments for psoriasis is not satisfactory for medical needs due to its recurrent lengthy course, high recurrence rate, and low efficiency of psoriasis treatment.20 And there may be potential benefits in developing traditional medicines, particularly novel traditional medicines as a form of therapy. To mitigate the profound impact of psoriasis on an individual’s physical, social, and psychological well-being, there is a compelling need for further research into novel medications that can efficiently address the condition, all the while offering enhanced affordability and safety compared to current treatment options.
 |
Figure 1 The pathogenesis of psoriasis. |
Introduction of Compounds in the Treatment of Psoriasis
Chinese Herbal Medicine, owing to its rich repository of potentially bioactive compounds, has found extensive application in the management of autoimmune disorders such as psoriasis, rheumatoid arthritis, and ulcerative colitis. This widespread usage can be attributed to the relatively extensive safety profile and multifaceted therapeutic benefits it offers.21 It globally recognized as promising drug candidates for the treatment of a broad spectrum of chronic diseases due to their sustained safety profile, even during extended usage, and the high level of patient compliance they engender.22
A considerable amount of evidence on the efficacy and safety of Chinese Herbal Medicine has already been established through previous studies.23 Notably, resveratrol, prevalent in red grapes and wine, exhibits antioxidative and anti-inflammatory attributes.24 Curcumin, derived from turmeric, is renowned for its potent anti-inflammatory and antioxidant effects, often necessitating strategies to enhance its bioavailability.25 Boswellic acids, obtained from Boswellia serrata, hold promise for their anti-inflammatory potential.25 Epigallocatechin gallate (EGCG), extracted from green tea, offers antioxidant and anti-inflammatory properties.26 Triptolide, derived from the Thunder God Vine, presents potent anti-inflammatory and immunosuppressive actions, which accompanied by complex ADME characteristics, including concerns regarding toxicity.27 Indirubin, a traditional Chinese medicine component, has been recognized for its anti-inflammatory benefits.28 Olibanum, sourced from Boswellia trees, has demonstrated anti-inflammatory and immune-modulating effects.29 Phytocannabinoids, found in cannabis, exhibit anti-inflammatory and immune-modulating properties and its ADME properties are contingent on specific compounds and consumption methods.21 Licorice, containing glycyrrhizin, showcases anti-inflammatory and immune-modulating effects.30 Quercetin, a flavonoid distributed across diverse plants, demonstrates antioxidant and anti-inflammatory attributes.31 Aloe-emodin, present in plants such as aloe vera, exhibits laxative and anti-inflammatory characteristics which ADME profiles are influenced by source and preparation.32 These compounds present a promising avenue for further research into effective psoriasis treatments and these ADME variations underscore the necessity for tailored approaches and therapeutic strategies, while their unique properties underscore the need for tailored approaches and therapeutic strategies in clinical practice. (Table 1) In particular, Tripterygium wilfordii Hook. F. has been used for centuries to relieve symptoms of immune-mediated inflammatory disease such as RA (rheumatoid arthritis), SLE (systemic lupus erythematosus), psoriasis, ankylosing spondylitis, and idiopathic IgA nephropathy, as well as cancer treatment.33–35
 |
Table 1 Material Basis of Compounds in the Treatment of Psoriasis |
Introduction of Tripterygium wilfordii Hook. F. and Its Application
Tripterygium wilfordii Hook. F. (TwHF) is a vine plant belonging to the genus Tripterygium of the Celastraceae family that is also known as “Lei Gong Teng”, “Thunder God Vine” and “Huang Teng”, and it has a long history of cultivation mainly in southern China.38 It was initially featured as a cure for several types of arthritis in Mao Lan’s book “Dian Nan Ben Cao” in 1476.39 TwHF contains over 400 natural products that have been isolated and characterized, including sesquiterpenes, diterpenes, triterpenes, lignans, glycosides, and alkaloids.38 Among these compounds, triptolide (diterpene) and celastrol (triterpene) are the most biologically active and promising.40 (Figure 2) TwHF tablets and TwHF multiglycoside tablets have been used as a traditional Chinese medicine to treat rheumatoid arthritis symptoms since the 1970s.41–44 The woody root of TwHF contains the majority of the traditionally and legally used medicinal ingredients and is recommended by Chinese dermatologists for the treatment of psoriasis.45 Subsequent studies have revealed that TwHF and its extract possess significant beneficial pharmacological activity, including anti-inflammatory,46,47 immunosuppressive,48,49 antineoplastic,50,51 and antiangiogenic52 properties, so that they have been approved in China for treating autoimmune and inflammatory diseases such as RA and SLE.35,53 Their pharmacological mechanisms and effects have been extensively studied in the treatment of immune-related disorders including RA, SLE, ankylosing spondylitis, and various skin diseases including psoriasis.38,40,43 The negative effects of TwHF and its extracts, on the other hand, have severely limited their potential clinical applicability in the treatment of disorders, and identifying the right treatment plan is critical.54 This suggests that TwHF may be applicable to all immune-mediated inflammatory diseases, and the shift in therapeutic strategy in recent years from broad-spectrum immunomodulators to the use of highly specific targeted agents suggests that tight control of inflammation is critical to disease outcome.55 Even though the exact causes of psoriasis are still unknown, it is known that a number of pathways connected to inflammation have a role in the pathogenesis. Given this, it becomes sense to assume that TwHF’s underlying mechanism involves a multicomponent, multi-targeted action that modifies the immune system and the systemic inflammatory state linked to the symptoms of psoriasis.23
 |
Figure 2 Structural formula of chemical components isolated from Tripterygium wilfordii Hook. F. |
TwHF and its extracts have been commonly used in psoriasis treatment in recent years, with the use of TwHF and its extracts for the treatment of psoriasis has gained popularity in recent years, with Triptolide and celastrol serving as the primary active ingredients. While there have been reports on the effectiveness of TwHF and its extracts in treating psoriasis, this review aims to provide a concise overview of the clinical efficacy, mechanism, and safety of TwHF and its extracts in treating psoriasis, in order to emphasize its potential for clinical use. The review will provide a useful reference for relevant research and contribute to the ongoing development and clinical use of TwHF by summarizing the effectiveness and mechanisms of TwHF and its extracts. Therefore, this review is essential for serving as a reference point for pertinent research, advancing the progress and clinical application of TwHF and its extracts.
Efficacy of Tripterygium in Psoriasis Treatment
Clinical Trial and Case Report
Several clinical studies have demonstrated the efficacy of TwHF and its extracts in treating psoriasis. A clinical comparative study including 91 subjects evaluated the tolerability of a new emollient balm containing celastrol whether topically used alone or in combination with topical or systemic medication therapies or phototherapy. The researchers discovered that using a celastrol-containing emollient balm for topical treatment on the entire body once a day can help with psoriasis symptoms including itching, dryness, and global lesion scores and the quality of patients’ life is improved, according to the patient-reported outcome questionnaire. In conclusion, it is crucial to assure long-term compliance that patients with psoriasis vulgaris tolerate this novel emollient cream containing celastrol effectively, whether used alone or in conjunction with medication or phototherapy. Pruritus, symptoms, and quality of life are improved after one month of daily usage.56 For the systemic treatment of TwHF, the results of a small simple prospective randomized clinical trial (RCT), enrolling 115 moderate to severe psoriasis vulgaris patients (PASI score ≥10 and body surface area afflicted by psoriasis ≥ 10%), show no significant difference in treatment effectiveness between the TwHF group (20 mg, 3 times a day) and acitretin group (20 mg, 3 times a day) within 8 weeks, however, the TwHF group had fewer treatment-related side events. This suggests that Tripterygium wilfordii Hook F. may be an efficient and secure therapy for people with moderate to severe psoriasis vulgaris, while its usage may be constrained by side effects such menstruation abnormalities.57 What’s more, in another randomized controlled trial conducted by Fu et al, it was found that the combination of tripterygium wilfordii glycoside (TWGs) tablets and acitretin was more effective and safer in treating moderate to severe plaque psoriasis (MSPP). 36 MSPP patients were collected and separated into three groups: group A (12 patients received TWG tablets plus acitretin capsules), group B (12 patients received compound glycyrrhizin capsules plus acitretin capsules), and group C (12 patients received acitretin capsules). In terms of serum parameters, clinical effectiveness, PASI score, and incidence of adverse events, group A’s therapeutic impact were clearly superior to that of the other two groups.58 As such, these studies show that TwHF and its extracts positively affect treating psoriasis (Table 2).
 |
Table 2 Clinical Trial of TwHF and Its Extracts on Psoriasis |
In addition, not only clinical trials but also many case reports have shown significant efficacy of TwHF psoriasis treatment. In a retrospective analysis including 26 patients with generalized pustular psoriasis in southwest China, 11 among them were effective with TwHF.59 An open clinical and one-year-follow study enrolling 103 patients with psoriasis vulgaris evaluated the effect and safety of triptolide tablets with a PASI score, and the results proved an overall efficacy rate of 75% in 103 patients. In addition, only 5 patients experienced adverse effects.60
Systematic Review
Recently, researchers have conducted multiple clinical trials to evaluate the efficacy of TwHF and its extracts in treating psoriasis. They can be used alone or in combination with systemic medications like acitretin. Han et al evaluated ten randomized or quasi-randomized clinical controlled studies of TwHF extracts with psoriasis patients, in which the efficacy of existing authorized psoriasis medicines was compared by using TwHF extracts alone or in combination with them. The results of the meta-analysis of these ten clinical studies showed a statistically significant improvement in psoriasis following therapy with TwHF extract, demonstrating that TwHF has a beneficial effect on plaque, pustular, and erythrodermic psoriasis types.61 Another meta-analysis and systematic review including 20 randomized controlled studies with 1872 psoriasis vulgaris evaluated the clinical effectiveness and safety of TwHF in psoriasis treatment. The results revealed that despite the mild adverse effects of TwHF, it reduces the Psoriasis Area Severity Index (PASI) scores of patients and remains effective in the treatment of psoriasis. It is evident that TwHF can clearly improve overall efficacy in the treatment of psoriasis vulgaris when used in conjunction with other medication therapies, whether herbal tonics or western pharmaceuticals.62
Outcome and Prospect
In establishing diagnostic criteria for psoriasis in clinical trials, the choice is made in accordance with the specific clinical indications of the drug under consideration. For instance, in the case of topically applied creams containing celastrol, Thouvenin et al opted for patients exhibiting stable mild-to-moderate body plaque psoriasis lasting for over 6 months, with a PASI score of less than 10 and a pruritus intensity of at least 3 on a Numerical Rating Scale. On the other hand, in randomized controlled trials comparing Tripterygium wilfordii Hook F to Acitretin, researchers, such as Wu et al, focused on patients with moderate to severe psoriasis vulgaris, with inclusion criteria based on a PASI score greater than or equal to 10 or a psoriasis-affected body surface area of 10% or more. Meanwhile, Fu et al included adults with a PASI score ranging from 7 to 20 points. It is noteworthy that nearly all studies excluded patients who had received drug treatment and topical medication for skin lesions within the preceding 6 months to ensure control over relevant variables. Above all, the positive effects of TwHF and its extracts in the treatment of psoriasis, including a significant decrease in PASI and a relief from psoriasis symptoms such as itching, dryness, overall lesion scores and quality of life, suggest that they could be an alternative as a therapeutic drug for psoriasis.
The Mechanism of Action
The primary pharmacological effects of TwHF are anti-inflammatory and immune regulation. Notably, the compounds celastrol and triptolide derived from TwHF exhibit efficacy against conditions characterized by inflammation, such as RA.63 These compounds target various signaling pathways, including NF‑κB, endoplasmic reticulum Ca2+‑ATPase, myeloid differentiation factor 2, toll‑like receptor 4, pro‑inflammatory chemokines, DNA damage, cell cycle arrest, apoptosis, receptor activator of NF‑κB (RANK)/RANK ligand/osteoprotegerin, cyclooxygenase‑2, matrix metalloproteases, and cytokines. These actions contribute to immune response modulation, which is frequently overactive in psoriasis. Additionally, TwHF can regulate signal pathways involved in both the inflammatory response and immune system function, helping to restore balance to the overactive immune response observed in psoriasis. In summary, aside from its well-known anti-inflammatory and immune regulatory functions, TwHF also possesses mechanisms for repairing damage. This summarizes the targets and signaling pathways associated with TwHF and its formulations in the treatment of psoriasis (Table 3 and Figure 3).
 |
Table 3 Pharmacological Mechanisms of TwHF and Its Extracts on Psoriasis |
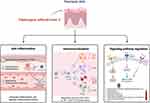 |
Figure 3 Pharmacological mechanisms of TwHF and its extracts on psoriasis. |
Effect of Tripterygium wilfordii Hook. F. on Inflammation
Psoriasis development is closely associated with inflammation, where abnormally activated skin dendritic cells secreting IL-23 and TNF-α.77 High levels of pro-inflammatory cytokines such as IL-1, IFN, IL-12, IL-17, IL-22, and IL-23 will interact with keratinocytes, leading to possible hyperproliferation and activation.78 In addition, activated keratinocytes release proinflammatory cytokines, chemokines, and antimicrobial peptides in inflamed skin, recruiting and triggering immune cells.79 TwHF and its extracts can eliminate inflammation and consequently regulate inflammatory factors.
HaCaT cells, immortalized human KCs, exhibiting similar features to KCs in psoriasis, such as excessive proliferation and aberrant differentiation. Triptolide, for example, reduces the proliferation of HaCaT cells (immortalized human keratinocytes) triggered by IL-22 and promotes KC differentiation by upregulating miR-181b-5p, implying that triptolide might be a possible psoriasis treatment.65 Several studies have investigated the use of carriers for celastrol, such as encapsulation of celastrol by niosomes, to enhance its therapeutic efficacy in treating psoriasis. These studies have consistently demonstrated the significant effectiveness of celastrol in reducing erythema and scaling in imiquimod-induced psoriasis mouse models and in decreasing the levels of inflammatory cytokines like TNF-α, IL-6, IL-22, IL-23, and IL-17 in HaCaT cells.67–69 Additionally, Tripterygium wilfordii poly-glycosides (TWP), extracted from Tripterygium wilfordii Hook. f., have been shown to possess anti-inflammatory and immunosuppressive effects. TWP suppressed HaCaT cell proliferation and production of inflammatory cytokines by lowering the ratio of neutrophil elastase to Trappin-2 levels, offering a novel insight into TWP’s anti-psoriasis mechanism.73 In conclusion, TwHF and its extracts can reduce the degree of inflammation in psoriasis by modulating inflammatory cytokines such as TNF-α, IL-6, IL-22, IL-23, and IL-17.
Effect of Tripterygium wilfordii Hook. F. on Immunomodulation
Psoriasis is an immune-related skin disease, in which the abnormal immune function of Th17 and Treg as well as their cytokines, play an essential part in the pathogenetic mechanism.11 The importance of the IL-23/Th17 axis in psoriasis pathophysiology has been proven in recent clinical trials and investigations. The paradigm of cytokine research has evolved from Th1 to Th17, with IL-17 and IL-22 serving as Th17 cytokines in psoriasis patients to activate and stimulate the proliferation of KCs.80–83 TwHF and its extracts have the capacity for immune modulation through interacting with relevant immune cells, cytokines, and chemokines.
TwHF and its extracts have been shown to have a beneficial modulatory impact on immunomodulation in psoriasis in several investigations. According to a study, in keratinocytes, celastrol enriched extract (CEE) inhibits both Th17 and Th22 differentiation and factors stimulated by IL-17, IL-22, and IFN-α, thereby lowering the levels of Th17/Th22 cytokines such as IL-19, IL-23, IL-36c, CCL5, CCL20, CXCL1, and IL-8, which are major inflammatory parameters, and key biomarkers associated with psoriasis, implying that CEE could be used as adjuvant therapy for psoriasis.71 In mice with imiquimod-induced psoriasis-like dermatitis, celastrol gel was shown to decrease the release of IL-23 from Langerhans cells, downregulate the connection between Langerhans cells and γδ T cells, and decrease the number of T cells that are stimulated and the concomitant IL-17 production. It ameliorates psoriasis-like dermatitis and also has a glucocorticoid-like effect, which successfully prevents psoriasis recurrence.70 Another study demonstrated that GTW (multi-glycoside of Tripterygium wilfordii Hook. f.) reduced the level of inflammation in lesions developing psoriasis in mice after topical IMQ administration, which was linked to substantially lower mRNA levels of Th17 cytokines like IL-17A, IL-17F, and IL-22 as well as a reduction of IL-17-secreting CD4+ immune cells in the spleen of IMQ-exposed mice.74 Ru et al found that TwHF root decoction (TwHF-RD) could attenuate psoriatic lesions induced by IMQ through modulating KC proliferation and apoptosis, inhibiting the differentiation of T cells and Treg, and reducing the expression of pro-inflammatory cytokines.76 Overall, immune function plays a key role in the occurrence of psoriasis, whereas TwHF and its extracts have the function of immunomodulation by regulating immune cells such as Th17 and Th22 lymphocytes and γδ T cells.
Effect of Tripterygium wilfordii Hook. F. on Potential Signaling Pathway Regulation
The pathology of psoriasis is complex and dynamic, and the mechanisms through which TwHF exerts its therapeutic effects remain unclear. Recent studies have focused on investigating the specific pathways, cytokines, chemokines, and targets that TwHF acts on in psoriasis. It has been suggested that TWP could be able to treat psoriasis by inhibiting the IL-17 signaling pathway and the differentiation of Th17 cells, which may be common therapeutic methods. TWP effectively improves skin lesions, lessens inflammation, and prevents the differentiation of Th1/Th17 cells, according to animal research. The effects of TWP on MAPK14, IL-2, and IL-6 were discovered using molecular docking and qPCR confirmation. TWP also inhibits the growth of Th17 cells and the IL-17 signaling pathway. However, it has also been found that TWP has potential hepatotoxic effects, revealing 145 hepatotoxic targets, including ALB, CASP3, and HSP90AA1, which are associated with the development of Th17 cells and the IL-17 signaling cascade.75 Triptolide, a component of TwHF, has been shown to disrupt the IFN-γ signaling pathway by suppressing the expression of IFN-γ receptor α (IFN‐γRα), the activation of Jak2 (Janus kinase 2), and STAT1 (signal transducer and activator of transcription 1), and up-regulating the expression of SOCS1 (suppressor of cytokine signaling 1). This suggests that triptolide acts directly on skin cells (KCs) and thus plays an anti-inflammatory role, which further supports the therapeutic value of TwHF in treating IFN-γ-dependent skin inflammatory illnesses like psoriasis.66 Celastrol, another component of TwHF, has been linked to inducing apoptosis in HaCaT cells, through death receptor and mitochondrial pathways, as well as the suppression of the NF-κB pathway.72 Another study found that a safer triptolide derivative LLDT-8 ((5R)-5-hydroxytriptolide) could reduce the expression of IL-36α and block IL-36α signaling, notably alleviating psoriasis-like skin inflammation in IMQ-induced mice through inhibiting the IL-36α signaling pathway.64 Above importantly, these findings provide new insights into the mechanisms underlying the therapeutic effects of TwHF in psoriasis and suggest potential targets for psoriasis treatment.
The studies cited above provide evidence that the therapeutic effects of TwHF and its extracts in treating psoriasis can be attributed to their anti-inflammation, immunomodulation, and potentially signaling pathway modulatory functions, which are achieved by modulating inflammatory cytokines such as IL-22, IL-23, IL-17 and immune cells such as Th17 lymphocytes, γδT cells and interfering with IFN-SOCS1, NF-κB and IL- 36α signaling pathways.
Adverse Effects
Even though TwHF and its extracts have been proven effective in treating psoriasis, their potential side effects should be carefully considered. Han et al reported that gastrointestinal complaints, aberrant hepatocytes, and reproductive dysfunction are the most common side effects of TwHF extracts.61 A meta-analysis of 14 studies revealed TwHF-related toxicity in systemic application, including menstrual disorders in women, dry mouth, gastrointestinal complaints, swelling of the lower limbs, abnormal hepatocytes, and abnormal routine blood results.62 Another meta-analysis reveals that TwHF may also cause higher reproductive toxicity, severe skin responses, hematologic problems, and cardiovascular events.84 TwHF-related adverse effects are systemic, and organ-specific, and are associated with factors such as dosing schedule, co-interventions, and medication dosage.54,85 In addition, large dosages of TwHF may cause significant side effects such as cardiac shock and renal failure86 (Table 4).
 |
Table 4 Adverse Effects of Celastrol, Triptolide and TwHF in Psoriasis Treatment |
In comprehensive examination of TwHF and its extracts, we have meticulously elucidated the complex landscape of its toxicity profile, specifically focusing on organ systems. Hepatotoxicity is a well-documented adverse effect of TwHF. It can cause liver injury, often marked by elevated liver enzymes and oxidative stress. Metabolomic analysis revealed that the hydroxyl group at C-14 in the molecular structure of triptolide is associated with hepatotoxicity. Triptolide-induced liver toxicity occurs in a dose- and time-dependent manner and is characterized by apoptosis. Triptolide and celastrol induced hepatotoxicity by reducing the substrate affinity, activity and expression of cytochrome P450 (CYP450) at the transcriptional and protein levels which related to PI3K/AKT, MAPK, TNFα and p53 signal pathways.87–89 Moreover, TwHF can induce renal damage characterized by injured renal tubules and oxidative stress, which damage proximal tubules and affect the tight junction complex and paracellular permeability. It is mediated by the GSK-3β/Fyn pathway and leads to degradation of Nrf2 and renal tubular cell apoptosis.90,91 Additionally, TwHF can induce gastrointestinal symptoms, including nausea, vomiting and diarrhea, hematological abnormalities, and potential bone density issues, necessitating in-depth exploration of mechanisms and clinical implications.61 Cardiotoxicity as well as adverse reproductive effects, are also notable findings.92–94 Importantly, potential drug interactions, which may impact treatment outcomes, should be a point of clinical consideration (Table 5).
 |
Table 5 Summary of Toxicity Findings of TwHF and Its Extracts |
In conclusion, despite the efficacy of TwHF and its extracts in treating psoriasis, there exist potential adverse effects, mainly including gastrointestinal reactions, hepatic impairment, reproductive problems, hepatic impairment, skin adverse reactions, cardiovascular events, and renal failure. Because the toxicity of TwHF shows a correlation with the dose, it is critical to prescribe a safe dose to minimize adverse effects.
Conclusion and Future Perspective
This article presents a review of current scientific research on the composition, clinical efficacy, and mechanism of action of TwHF in the treatment of psoriasis. Although the therapeutic potential of TwHF in the treatment of psoriasis has been previously investigated, this review specifically focuses on the latest developments in TwHF research, with the aim of providing a comprehensive understanding of the mechanisms underlying TwHF’s effectiveness in treating psoriasis and informing future clinical investigations.
The pathogenesis of psoriasis has not been fully studied so far, and the present treatment of psoriasis is primarily with western drugs, such as methotrexate and acitretin, and biologics, including Adalimumab and Etanercept, but they must be closely monitored for adverse reactions and increase the financial burden of patients due to the long-term and repeated recurrence of psoriasis. A large number of studies have shown that TwHF and its extracts have positive efficacy on psoriasis with fewer side effects, and their pharmacological effects such as anti-inflammation, immunomodulation, and potential signaling pathway modulation have been widely and deeply understood and studied.
TwHF and its extracts have demonstrated some efficacy in the treatment of psoriasis, although they are not without adverse effects. Many clinical investigations have identified gastrointestinal responses, aberrant hepatocytes, reproductive issues, hepatic impairment, cutaneous adverse reactions, cardiovascular events, and renal failure as side effects of TwHF and its extracts. Hepatic impairment is the most prevalent of these problems. As a result, unfavorable responses to TwHF and its extracts during clinical application should be taken seriously, which limits TwHF’s widespread usage. However, there is some evidence of clinical efficacy of TwHF from the clinical trials, but high quality randomized double-blind placebo-controlled studies are missing. From the data presented it may be concluded that TwHF extracts might be preferably used topically to avoid systemic side effects.
Although TwHF and its extracts have been utilized for treating psoriasis, there remain several issues that need to be resolved. First, comprises several active components, necessitating systematic inheritance, development, and innovative studies on their dose-related toxicity, efficacy, and mechanisms of action to achieve safe and effective clinical application and improve social and economic benefits. Second, TwHF and its extracts are typically administered orally or topically in clinical psoriasis treatment. To better exploit TwHF’s clinical benefits and lay the groundwork for its further theoretical clinical implementation, a novel drug delivery mechanism that overcomes TwHF’s physical and chemical limitations should be investigated. Lastly, the existing pharmacodynamic studies of TwHF and its extracts lack a thorough explanation of the structure, function, and guiding principles of its active components, focusing mainly on skin damage treatment effectiveness. It should be investigated at the cellular and molecular levels, and future research should focus on its pharmacokinetics and metabolites.
Acknowledgments
Figures of this review were created with Biorender.com (https://www.biorender.com).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work. N-N.D. was responsible for its financial supports and the corresponding works.
Funding
This study was supported by grants from the Shandong Provincial Natural Science Foundation of China (numbers ZR2022MH242), the National Natural Science Foundation of China (number 82273527).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301–1315. doi:10.1016/S0140-6736(20)32549-6
2. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945. doi:10.1001/jama.2020.4006
3. Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210:194–199. doi:10.1159/000083509
4. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082. doi:10.1038/nrdp.2016.82
5. Amani H, Shahbazi M-A, D’Amico C, et al. Microneedles for painless transdermal immunotherapeutic applications. J Control Release. 2021;330:185–217. doi:10.1016/j.jconrel.2020.12.019
6. Wang J, Li X, Zhang P, et al. Chrna5 is overexpressed in psoriasis patients and promotes psoriasis-like inflammation in mouse models. J Invest Dermatol. 2022. doi:10.1016/j.jid.2022.04.014
7. Griffiths CEM, Barker JN. Psoriasis 1 Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9):263–271. doi:10.1016/S0140-6736(07)61128-3
8. Ogawa E, Sato Y, Minagawa A, Okuyama R. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2017;9:264–272.
9. Boyman O, Hefti HP, Conrad C, et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J Exp Med. 2004;6:731. doi:10.1084/jem.20031482
10. Sanchez AP, da Costa A, Del Rey C, Silva B, Romiti R. The overview of the immunobiology of interleukin-23 associated with immune-mediated inflammatory disorders: a narrative review. J Drugs Dermatol. 2023;22:375–385. doi:10.36849/JDD.7017
11. Lowes MA, Suárez-Fariñas M, Krueger JG. Immunology of Psoriasis. Annu Rev Immunol. 2014;32:227–255. doi:10.1146/annurev-immunol-032713-120225
12. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11:763–776. doi:10.1038/nrd3794
13. Navarro-Compán V, Puig L, Vidal S, et al. The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases. Front Immunol. 2023;14:1191782. doi:10.3389/fimmu.2023.1191782
14. Liang SE, Cohen JM, Ho RS. Screening for depression and suicidality in psoriasis patients: a survey of US dermatologists. J Am Acad Dermatol. 2019;80:1460–1462. doi:10.1016/j.jaad.2019.01.025
15. Yang E, Beck K, Sanchez I, Koo J, Liao W. The impact of genital psoriasis on quality of life: a systematic review. PTT. 2018;8:41–47. doi:10.2147/PTT.S169389
16. Wu JJ, Lynde CW, Kleyn CE, et al. Identification of key research needs for topical therapy treatment of psoriasis - a consensus paper by the international psoriasis council. J Eur Acad Dermatol Venereol. 2016;30:1115–1119. doi:10.1111/jdv.13614
17. Ortiz NEG, Nijhawan RI, Weinberg JM. Acitretin. Dermatol Ther. 2013;26:390–399. doi:10.1111/dth.12086
18. Lluch-Galcerá JJ, Carrascosa JM, González-Quesada A, et al. Safety of biologic therapy in combination with methotrexate in moderate to severe psoriasis: a cohort study from the BIOBADADERM registry. Br J Dermatol. 2023;382. doi:10.1093/bjd/ljad382
19. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140:645–653. doi:10.1016/j.jaci.2017.07.004
20. Kupetsky EA, Mathers AR, Ferris LK. Anti-cytokine therapy in the treatment of psoriasis. Cytokine. 2013;61:704–712. doi:10.1016/j.cyto.2012.12.027
21. Chen H, Su Z, Pan X, et al. Phytochemicals: targeting autophagy to treat psoriasis. Phytomedicine. 2023;120:155041. doi:10.1016/j.phymed.2023.155041
22. Ren J-L, Yang L, Qiu S, Zhang A-H, Wang X-J. Efficacy evaluation, active ingredients, and multitarget exploration of herbal medicine. Trends Endocrinol Metab. 2023;34:146–157. doi:10.1016/j.tem.2023.01.005
23. Jo H-G, Kim H, Lee D. Oral administration of East Asian herbal medicine for inflammatory skin lesions in plaque psoriasis: a systematic review, meta-analysis, and exploration of core herbal materials. Nutrients. 2022;14:2434. doi:10.3390/nu14122434
24. Elgewelly MA, Elmasry SM, Sayed NSE, Abbas H. Resveratrol-loaded vesicular elastic nanocarriers gel in imiquimod-induced psoriasis treatment: in vitro and in vivo evaluation. J Pharm Sci. 2022;111:417–431. doi:10.1016/j.xphs.2021.08.023
25. Zhang S, Wang J, Liu L, et al. Efficacy and safety of curcumin in psoriasis: preclinical and clinical evidence and possible mechanisms. Front Pharmacol. 2022;13:1.
26. Chamcheu JC, Siddiqui IA, Adhami VM, et al. Chitosan-based nanoformulated (–)-epigallocatechin-3-gallate (EGCG) modulates human keratinocyte-induced responses and alleviates imiquimod-induced murine psoriasiform dermatitis. Int J Nanomed. 2018;13:4189–4206. doi:10.2147/IJN.S165966
27. Ma J, Dey M, Yang H, et al. Anti-inflammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry. 2007;68:1172–1178. doi:10.1016/j.phytochem.2007.02.021
28. Lin Y-K, See L-C, Huang Y-H, et al. Efficacy and safety of Indigo naturalis extract in oil (Lindioil) in treating nail psoriasis: a randomized, observer-blind, vehicle-controlled trial. Phytomedicine. 2014;21:1015–1020. doi:10.1016/j.phymed.2014.02.013
29. Halim SA, Khan A, Csuk R, Al-Rawahi A, Al-Harrasi A. Diterpenoids and triterpenoids from frankincense are excellent anti-psoriatic agents: an in silico approach. Front Chem. 2020;8:486. doi:10.3389/fchem.2020.00486
30. Hoffmann J, Gendrisch F, Schempp CM, Wölfle U. New herbal biomedicines for the topical treatment of dermatological disorders. Biomedicines. 2020;8:27. doi:10.3390/biomedicines8020027
31. Zhang Y, Gong S, Liu L, et al. Cyclodextrin-coordinated liposome-in-gel for transcutaneous quercetin delivery for psoriasis treatment. ACS Appl Mater Interfaces. 2023;15:40228–40240. doi:10.1021/acsami.3c07582
32. Dong X, Zeng Y, Liu Y, et al. Aloe-emodin: a review of its pharmacology, toxicity, and pharmacokinetics. Phytother Res. 2020;34:270–281. doi:10.1002/ptr.6532
33. Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry. 2007;68(6):732–766. doi:10.1016/j.phytochem.2006.11.029
34. Bao J, Dai S-M. A Chinese herb tripterygium wilfordii hook F in the treatment of rheumatoid arthritis: mechanism, efficacy, and safety. Rheumatol Int. 2011;31:1123–1129. doi:10.1007/s00296-011-1841-y
35. Law SK-Y, Simmons MP, Techen N, et al. Molecular analyses of the Chinese herb Leigongteng (Tripterygium wilfordii Hook.f.). Phytochemistry. 2011;72:21–26. doi:10.1016/j.phytochem.2010.10.015
36. Zhang Q, Xie J, Li G, et al. Psoriasis treatment using Indigo Naturalis: progress and strategy. J Ethnopharmacol. 2022;297:115522. doi:10.1016/j.jep.2022.115522
37. Efferth T, Oesch F. Anti-inflammatory and anti-cancer activities of frankincense: targets, treatments and toxicities. Semin Cancer Biol. 2022;80:39–57. doi:10.1016/j.semcancer.2020.01.015
38. Tong L, Zhao Q, Datan E, et al. Triptolide: reflections on two decades of research and prospects for the future. Nat Prod Rep. 2021;38:843–860. doi:10.1039/D0NP00054J
39. Huang W, Liu W-J, Xiao Y-H, et al. Tripterygium and its extracts for diabetic nephropathy: efficacy and pharmacological mechanisms. Biomed Pharmacother. 2020;121:109599. doi:10.1016/j.biopha.2019.109599
40. Chen S-R, Dai Y, Zhao J, et al. A mechanistic overview of triptolide and celastrol, natural products from tripterygium wilfordii Hook F. Front Pharmacol. 2018;9:104. doi:10.3389/fphar.2018.00104
41. Marks WH. Tripterygium wilfordii Hook F. versus Sulfasalazine in the treatment of rheumatoid arthritis: a well-designed clinical trial of a botanical demonstrating effectiveness. Fitoterapia. 2011;82:85–87. doi:10.1016/j.fitote.2010.11.024
42. Astry B, Venkatesha SH, Laurence A, et al. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin Immunol. 2016;20:228–238.
43. Venkatesha SH, Dudics S, Astry B, Moudgil KD. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog Dis. 2016;74:ftw059. doi:10.1093/femspd/ftw059
44. Tao X, Cush JJ, Garret M, Lipsky PE. A Phase I study of ethyl acetate extract of the Chinese antirheumatic herb Tripterygium wilfordii hook F in rheumatoid arthritis. J Rheumatol. 2001;28:2160–2167.
45. China Association of Chinese Medicine. Dermatology branch psoriasis Chinese medicine treatment expert consensus (2017 edition). Chin J Dermatovenereol Integr Tradit West Med. 2018;17:273–277.
46. Xue M, Jiang -Z-Z, Wu T, et al. Anti-inflammatory effects and hepatotoxicity of Tripterygium-loaded solid lipid nanoparticles on adjuvant-induced arthritis in rats. Phytomedicine. 2012;19:998–1006. doi:10.1016/j.phymed.2012.06.006
47. Zhang Y, Ma X. Triptolide Inhibits IL-12/IL-23 Expression in APCs via CCAAT/Enhancer-Binding Protein alpha. J Immunol. 2010;184:3866–3877. doi:10.4049/jimmunol.0903417
48. Chen Y-Z, Gao Q, Zhao X-Z, et al. Meta-analysis of tripterygium Wilfordii Hook F in the Immunosuppressive Treatment of IgA Nephropathy. Intern Med. 2010;49:2049–2055. doi:10.2169/internalmedicine.49.3704
49. Wang Y, Jia L, Wu C-Y. Triptolide inhibits the differentiation of Th17 cells and suppresses collagen-induced arthritis. Scand J Immunol. 2008;68:383–390. doi:10.1111/j.1365-3083.2008.02147.x
50. Jiang X, Huang X-C, Ao L, et al. Total alkaloids of Tripterygium hypoglaucum (levl.) Hutch inhibits tumor growth both in vitro and in vivo. J Ethnopharmacol. 2014;151:292–298. doi:10.1016/j.jep.2013.10.045
51. Zhou Z-L, Yang Y-X, Ding J, Li Y-C, Miao Z-H. Triptolide: structural modifications, structure-activity relationships, bioactivities, clinical development and mechanisms. Nat Prod Rep. 2012;29:457–475. doi:10.1039/c2np00088a
52. He M-F, Liu L, Ge W, et al. Antiangiogenic activity of Tripterygium wilfordii and its terpenoids. J Ethnopharmacol. 2009;121:61–68. doi:10.1016/j.jep.2008.09.033
53. Goldbach-Mansky R, Wilson M, Fleischmann R, et al. Comparison of tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis a randomized trial. Ann Intern Med. 2009;151:229–W51. doi:10.7326/0003-4819-151-4-200908180-00005
54. Ru Y, Luo Y, Zhou Y, et al. adverse events associated with treatment of tripterygium wilfordii Hook F: a quantitative evidence synthesis. Front Pharmacol. 2019;10:1250. doi:10.3389/fphar.2019.01250
55. McInnes IB, Gravallese EM. Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol. 2021;21:680–686. doi:10.1038/s41577-021-00603-1
56. Thouvenin MD, Dalmon S, Theunis J, et al. Tolerance and efficacy of a new celastrol-containing balm as adjunct care in psoriasis. J Eur Acad Dermatol Venereol. 2020;34:10–16. doi:10.1111/jdv.16691
57. Wu C, Jin H-Z, Shu D, et al. Efficacy and Safety of Tripterygium wilfordii Hook F Versus Acitretin in Moderate to Severe Psoriasis Vulgaris: a Randomized Clinical Trial. Chinese Med J. 2015;128:443–449. doi:10.4103/0366-6999.151069
58. Fu Q, Zhu Y, Fang Y, Dai C. Efficacy and safety of tripterygium wilfordii glycoside tablets combined with acitretin capsules in the treatment of moderate to severe plaque psoriasis: a randomized controlled trial. Appl Bionics Biomech. 2022;2022:2252500. doi:10.1155/2022/2252500
59. Wang Q, Liu W, Zhang L. Clinical features of von Zumbusch type of generalized pustular psoriasis in children: a retrospective study of 26 patients in southwestern China. An Bras Dermatol. 2017;92:319–322. doi:10.1590/abd1806-4841.20175536
60. Shao-xi W, Ning-ru G. Clinical observation on effect of triptolide tablet in treating patients with psoriasis vulgaris. Chin J Integr Med. 2005;11:147–148. doi:10.1007/BF02836473
61. Han R, Rostami-Yazdi M, Gerdes S, Mrowietz U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases: triptolide for inflammatory diseases. Br. J. Clin. Pharmacol. 2012;74:424–436. doi:10.1111/j.1365-2125.2012.04221.x
62. Lv M, Deng J, Tang N, Zeng Y, Lu C. Efficacy and safety of tripterygium wilfordii Hook F on Psoriasis vulgaris: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2018;2018:1–10.
63. Song X, Zhang Y, Dai E. Therapeutic targets of thunder god vine (Tripterygium wilfordii hook) in rheumatoid arthritis (Review). Molecul Med Rep. 2020;21:2303–2310. doi:10.3892/mmr.2020.11052
64. Qi Q, Li Q, Zhu H, et al. Triptolide analog LLDT-8 ameliorates psoriasis-like dermatitis in BALB/c mice via suppressing the IL-36α signaling pathway. Pharmacol Res. 2021;169:105678. doi:10.1016/j.phrs.2021.105678
65. He Q, Zhang B, Hu F, et al. Triptolide Inhibits the Proliferation of HaCaT Cells Induced by IL22 via Upregulating miR-181b-5p. DDDT. 2020;14:2927–2935. doi:10.2147/DDDT.S254466
66. Hongqin T, Xinyu L, Heng G, et al. Triptolide Inhibits IFN-γ Signaling via the Jak/STAT Pathway in HaCaT Keratinocytes. Phytother Res. 2011;25:1678–1685. doi:10.1002/ptr.3471
67. Xi L, Lin Z, Qiu F, et al. Enhanced uptake and anti-maturation effect of celastrol-loaded mannosylated liposomes on dendritic cells for psoriasis treatment. Acta Pharm Sin B. 2022;12:339–352. doi:10.1016/j.apsb.2021.07.019
68. Qiu F, Xi L, Chen S, et al. Celastrol niosome hydrogel has anti-inflammatory effect on skin keratinocytes and circulation without systemic drug exposure in psoriasis mice. Int J Nanomed. 2021;16:6171–6182. doi:10.2147/IJN.S323208
69. Meng S, Sun L, Wang L, et al. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf B. 2019;182:110352. doi:10.1016/j.colsurfb.2019.110352
70. Liu L, Chen X, Lu Y, et al. Celastrol gel ameliorates imiquimod-induced psoriasis-like dermatitis in mice by targeting Langerhans cells. Biomed Pharmacother. 2022;147:112644. doi:10.1016/j.biopha.2022.112644
71. Nguyen T, Lestienne F, Cousy A, Mengeaud V, Castex‐Rizzi N. Effective inhibition of Th17/Th22 pathway in 2D and 3D human models of psoriasis by Celastrol enriched plant cell culture extract. J Eur Acad Dermatol Venereol. 2020;34:3–9. doi:10.1111/jdv.16475
72. Zhou -L-L, Lin Z-X, Fung K-P, et al. Celastrol-induced apoptosis in human HaCaT keratinocytes involves the inhibition of NF-kappa B activity. Eur J Pharmacol. 2011;670:399–408. doi:10.1016/j.ejphar.2011.09.014
73. Chen N, Sun J, Song Y, et al. Tripterygium wilfordii polyglycoside reduces the proliferation and inflammatory cytokines secretion of Hacat cells by regulating the balance of neutrophil elastase and trappin-2. Int J Clin Exp Pathol. 2016;9:1.
74. Zhao J, Di T, Wang Y, et al. Multi-glycoside of Tripterygium wilfordii Hook. f. ameliorates imiquimod-induced skin lesions through a STAT3-dependent mechanism involving the inhibition of Th17-mediated inflammatory responses. IntJ Mol Med. 2016;38:747–757. doi:10.3892/ijmm.2016.2670
75. Chen Y, Wang Y-F, Song -S-S, et al. Potential shared therapeutic and hepatotoxic mechanisms of Tripterygium wilfordii polyglycosides treating three kinds of autoimmune skin diseases by regulating IL-17 signaling pathway and Th17 cell differentiation. J Ethnopharmacol. 2022;296:115496. doi:10.1016/j.jep.2022.115496
76. Ru Y, Li H, Zhang R, et al. Role of keratinocytes and immune cells in the anti-inflammatory effects of Tripterygium wilfordii Hook. f. in a murine model of psoriasis. Phytomedicine. 2020;77:153299. doi:10.1016/j.phymed.2020.153299
77. Mabuchi T, Takekoshi T, Hwang S. Epidermal CCR6+ γδ T Cells Are Major Producers of IL-22 and IL-17 in a Murine Model of Psoriasiform Dermatitis. J Immunol. 2011;187:5026–5031. doi:10.4049/jimmunol.1101817
78. Lee M, Kim SH, Kim T-G, et al. Resident and monocyte-derived Langerhans cells are required for imiquimod-induced psoriasis-like dermatitis model. J Dermatological Sci. 2018;91:52–59. doi:10.1016/j.jdermsci.2018.04.003
79. Nograles KE, Davidovici B, Krueger JG. New Insights in the Immunologic Basis of Psoriasis. Semin Cutan Med Surg. 2010;29:3–9. doi:10.1016/j.sder.2010.03.001
80. Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th17 axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol. 2006;18:670–675. doi:10.1016/j.coi.2006.09.008
81. Mitra A, Fallen RS, Lima HC. Cytokine-Based Therapy in Psoriasis. Clin Rev Allerg Immunol. 2013;44:173–182. doi:10.1007/s12016-012-8306-2
82. Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T Cells. J Invest Dermatol. 2008;128:1207–1211. doi:10.1038/sj.jid.5701213
83. Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, et al. Low Expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7427. doi:10.4049/jimmunol.181.10.7420
84. Zhang C, Sun -P-P, Guo H-T, et al. Safety profiles of tripterygium wilfordii Hook F: a systematic review and meta-analysis. Front Pharmacol. 2016;7. doi:10.3389/fphar.2016.00402
85. Wang T, Shen F, Su S, et al. Comparative analysis of four terpenoids in root and cortex of Tripterygium wilfordii Radix by different drying methods. BMC Complement Altern Med. 2016;16:476. doi:10.1186/s12906-016-1453-x
86. Brown AC. Kidney toxicity related to herbs and dietary supplements: online table of case reports. Part 3 of 5 series. Food and Chemical Toxicology. 2017;107:502–519. doi:10.1016/j.fct.2016.07.024
87. Zhao J, Xie C, Wang K, et al. Comprehensive analysis of transcriptomics and metabolomics to understand triptolide-induced liver injury in mice. Toxicol Lett. 2020;333:290–302. doi:10.1016/j.toxlet.2020.08.007
88. Lu Y, Xie T, Zhang Y, et al. Triptolide Induces hepatotoxicity via inhibition of CYP450s in Rat liver microsomes. BMC Complement Altern Med. 2017;17:15. doi:10.1186/s12906-016-1504-3
89. Jin C, Wu Z, Wang L, Kanai Y, He X. CYP450s-activity relations of celastrol to interact with triptolide reveal the reasons of hepatotoxicity of tripterygium wilfordii. Molecules. 2019;24:2162. doi:10.3390/molecules24112162
90. Wu M, Chen W, Yu X, et al. Celastrol aggravates LPS-induced inflammation and injuries of liver and kidney in mice. Am J Transl Res. 2018;10:2078–2086.
91. Sun L, Li H, Huang X, et al. Triptolide alters barrier function in renal proximal tubular cells in rats. Toxicol Lett. 2013;223:96–102. doi:10.1016/j.toxlet.2013.08.014
92. Tang X, Wang C, Hsieh Y, et al. Triptolide induces toxicity in inner ear stem cells via promoting DNA damage. Toxicol in vitro. 2019;61:104597. doi:10.1016/j.tiv.2019.104597
93. Singla N, Challana S. Reproductive toxicity of triptolide in male house rat, Rattus rattus. ScientificWorldJournal. 2014;2014:879405. doi:10.1155/2014/879405
94. Zeng Y, Sun H, Li Y, et al. Exposure to triptolide affects follicle development in NIH mice: role of endoplasmic reticulum stress in granulosa cell apoptosis. Hum Exp Toxicol. 2017;36:82–92. doi:10.1177/0960327116638725
95. Zhao W, Xiao L, Pan L, et al. Cardiac toxicity of Triptergium wilfordii Hook F. may correlate with its inhibition to hERG channel. Heliyon. 2019;5:e02527. doi:10.1016/j.heliyon.2019.e02527
96. Xi Y, Wang W, Wang L, et al. Triptolide induces p53-dependent cardiotoxicity through mitochondrial membrane permeabilization in cardiomyocytes. Toxicol Appl Pharmacol. 2018;355:269–285. doi:10.1016/j.taap.2018.07.011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
