Back to Journals » Journal of Inflammation Research » Volume 17
Triggering Receptor Expressed on Myeloid Cells 2 Mediates the Involvement of M2-Type Macrophages in Pulmonary Tuberculosis Infection
Authors Shang X, Maimaiti N, Fan J, Wang L, Wang Y, Sun H, Lv J, Zhang X, Wang J, Ma X
Received 15 September 2023
Accepted for publication 14 March 2024
Published 27 March 2024 Volume 2024:17 Pages 1919—1928
DOI https://doi.org/10.2147/JIR.S435216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Xiaoqian Shang,1,* Naifeisha Maimaiti,1,* Jiahui Fan,1 Liang Wang,2 Yuanyuan Wang,3 Hu Sun,3 Jie Lv,1 Xiufeng Zhang,4 Jing Wang,4 Xiumin Ma1
1State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Clinical Laboratory Center, Tumor Hospital Affiliated to Xinjiang Medical University, Urumqi, Xinjiang, 830011, People’s Republic of China; 2The Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, 830011, People’s Republic of China; 3First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, 830011, People’s Republic of China; 4Department of Respiratory Medicine, Second Affiliated Hospital of Hainan Medical University, Haikou, Hainan, 570100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiumin Ma, State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia, Clinical Laboratory Center, Tumor Hospital Affiliated to Xinjiang Medical University, Urumqi, Xinjiang, 830011, People’s Republic of China, Email [email protected] Jing Wang, Department of Respiratory Medicine, Second Affiliated Hospital of Hainan Medical University, Haikou, Hainan, 570100, People’s Republic of China, Email [email protected]
Background: Macrophage play a significant work in the development of tuberculosis. This study aims to investigate the relationship between TREM2 and macrophage polarization, as well as the related cytokines.
Methods: This study involved 43 pulmonary tuberculosis patients and 37 healthy controls. Enzyme-linked immunosorbent assay (ELISA) was used to detect the expression levels of M1/M2 macrophage-related cytokines IL-10 and IL-12 in the peripheral blood of pulmonary tuberculosis patients. The relative mRNA expression levels of TREM2, IL-10 and IL-12 were detected using quantitative real-time PCR (qRT-PCR). Additionally, Spearman rank correlation analysis was used to preliminarily assess the correlation between TREM2 and M1 / M2 macrophages. Hematoxylin-eosin (HE) staining was performed to observe the pathological manifestations of pulmonary tuberculosis lesions. Immunohistochemical (IHC) staining was used to observe the localization of the macrophage-specific molecule CD68, the M1 specific molecule iNOS, the M2 specific molecule CD163, and TREM2.
Results: The lesions of pulmonary tuberculosis patients showed Langhans multinucleated macrophages and tuberculous granulomas. The ELISA results indicated that the expression levels of IL-10 and IL-12 were significantly increased in peripheral blood of pulmonary tuberculosis patients. Additionally, the relative mRNA expression levels of TREM2, IL-10 and IL-12 were also significantly higher in the pulmonary tuberculosis group. Furthermore, a positive correlation was observed between TREM2 and IL-10, which are secreted by M2 macrophages. IHC revealed significant positivity of TREM2 and macrophage-related markers in tuberculous granuloma. Specifically, TREM2 and M2 macrophage marker CD163 were significantly expressed in the cytoplasm and membrane of Langhans multinucleated macrophages.
Conclusion: The role of macrophage polarization in pulmonary tuberculosis is significant, and further investigation is needed to understand relationship between TREM2 and M2 macrophages.
Keywords: tuberculosis, TREM2, macrophage
Introduce
Tuberculosis (TB) is considered one of the world’s most devastating infectious diseases globally, having evolved in humans over the past 70,000 years. Caused by infection with Mycobacterium tuberculosis (Mtb), it can colonize multiple organs throughout the body. Tuberculosis is responsible for approximately 1.5 million deaths per year.1 Under the pandemic trend of the Coronavirus disease (COVID-19), although the highly infectious and high mortality rate of the COVID-19 has attracted people’s attention, tuberculosis should not be ignored.2 In recent years, with the efforts of the Chinese government, the burden of TB epidemic has shown a downward trend throughout the country, but it’s still the top priority in the western region of China.3 Therefore, the research of tuberculosis still needs the efforts of more scholars.
Mtb invades the body through the respiratory tract and spreads to multiple systems via the bloodstream. It can colonize in various organs,4 leading to subsequent clinical symptoms. Mtb infection often manifests as a latent infection. Due to individual differences, about 5% to 10% of infected patients will progress to active pulmonary TB and develop clinical manifestations, such as low-grade fever, night sweats, and fatigue.4,5 Firstly, Mtb enters the lungs, and the alveolar macrophages (AMs) are among the first cells to come into contact with Mtb. Studies have shown that Mtb possesses unique cell surface lipids and secreted protein effectors that can escape the killing of innate immune cells.6 The heterogeneity and plasticity of macrophages can help limit the spread of Mtb. However, virulence factors of Mtb can affect the steady state of macrophages and thus influence the progression of the disease.6,7
Macrophages are a complex population with high plasticity. Currently, macrophages activated through the classical and alternative pathways are considered the two extremes of macrophage function plasticity modulation.8 Some studies have found that M1-type macrophages (M1) aid in the killing of Mtb by granuloma tissue, and promote the formation of granulomas. During Mtb infection, macrophages undergo foaming changes, and M2-type macrophages (M2) are thought to significantly promote the formation of foamy macrophages, leading to Mtb latent infections.9,10 Therefore, macrophages are considered key in responding to the Mtb infection process.
Macrophages detect the invasion of Mtb pathogens through various surface receptors. These receptors are critical for pathogen defense because they act as sentinels for Mtb, which can then transmit favorable signals intracellularly and trigger a powerful immune response. Current studies have shown that triggering receptor expressed oil myeloid cells (TREM) is a family of immunoglobulin receptors, expressed on the surface of myeloid cells, they play an important role in the activation of macrophages.11,12 TREM-1 amplifies inflammation through the synergistic effect of Toll-like receptor (TLR) and NOD-like receptor (NLR) signaling reaction, increasing the production of inflammatory cytokines, such as TNF-α, IL-1 and IL-6.13 TREM2 can stimulate the production of inflammatory factors, contributing to the progression of chronic inflammation and immune response. It is also related to various functions of cell maturation, such as survival, proliferation, activation, and phagocytosis.14
Recent studies have shown that patients with multiple sclerosis and other inflammatory nervous systems have a high expression level of soluble TREM2 in their cerebrospinal fluid.15,16 Additionally, increased TREM2 expression in monocytes from Alzheimer’s disease patients has been found to play an important role in the progression of the disease.17 Some scholars believe that TREM2 plays a key role in promoting the inflammatory Th1 response when infected with Mtb.18 This study aims to explore the expression level of TREM2 in pulmonary tuberculosis patients and its relationship with macrophage polarization. The results of this research may provide new insights and targets for understanding the TB infection mechanism.
Method
Research Objects
43 active pulmonary tuberculosis patients were collected; among them, 23 were male and 21 were female, with an average age of 49:51 ± 11:90 years old. They were diagnosed at the First Affiliated Hospital of Xinjiang Medical University from December 2018 to December 2020, and 5 mL EDTA anticoagulated whole blood was drawn during the active period. At the same time, a total of 37 cases of healthy control group were collected, including 21 males and 16 females, with an average age of 40:27 ± 13:61 years old, collected 5 mL EDTA anticoagulated whole blood. The healthy subjects in the control group had no infectious diseases; liver function, kidney function, and chest imaging examinations were normal; the sputum tuberculosis smear microscopy report was negative, and there was no history of tuberculosis. At the same time, 40 cases of pulmonary tuberculosis patients were treated in the First Affiliated Hospital of Xinjiang Medical University, and they undergo surgical treatment from June 2018 to June 2021; their surrounding lungs and lung stumps were collected.
The “WS288-2017 Pulmonary Tuberculosis Diagnosis” formulated from China in 2018,19 provides diagnostic criteria, inclusion and exclusion criteria for this study: There are clinical manifestations and signs of typical TB such as low fever, night sweats, and force, and at least in line with one of the following three: 1) sputum smear microscopy or sputum culture is positive for Mtb; 2) sputum examination is negative, but chest imaging examination shows typical active pulmonary TB; 3) sputum examination is negative, disease physically diagnosed TB granulomatous structural samples of lung lesions, or pleural effusion and bronchoalveolar lavage fluid were reported as TB lesions. Patients with other immune diseases, neoplastic diseases, pulmonary infection, and HIV infection were excluded.
HE Staining
Paraffin embedded tissue sections were dewaxed, stained with hematoxylin for 1 min, stained with eosin for 3 min and 1% hydrochloric acid ethanol for 1 s, and returned to blue with PBS. Dehydrated, dried, and sealed with neutral gum, and the pathological manifestations of the lesions were observed under a microscope.
IHC Staining
In this study, the lesion tissues of 43 patients with pulmonary TB were collected. The expression and localization of TREM2, CD68, iNOS and CD163 were observed by IHC, for initial clarification the relationship between TREM2 and M1/M2 macrophages was preliminarily determined. After dewaxing, the tissue sections were repaired with sodium citrate solution for 15 min and endogenous peroxidase blocker to avoid light conditions at 37°C for 10 minutes. Goat serum was sealed for 20 min and incubated with primary antibody in refrigerator at 4°C for 14 h (Table 1). Combined with secondary antibody at 37°C for 90 min, the staining was performed with diaminobenzidine in peroxide substrate solution for 2.5 min ± 10s. Image-Pro Plus version 8.0.1 is used to process the collected images of immunohistochemistry results; after quantitative processing (IOD (sum)/Area(sum)), the positive rate is calculated.
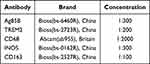 |
Table 1 Immunohistochemical Antibody |
Elisa
The kit used in this experiment was provided by Shanghai Jiang Lai Biotechnology Co., Ltd. According to the instructions of the kit, measure the OD value of the standard and samples, draw the standard curve of the four-parameter logistic function, and detect the expression levels of IL-10 (NOJL19246) and IL-12 (NO.JL18332) in blood serum.
qRT-PCR
RNA was extracted from the peripheral blood of patients, using TransZol Up plus RNA kit (Beijing, China), requiring RNA concentration (>80ng/μL) and purity (OD260/OD280=1.8–2.0). Synthesize cDNA according to the kit (Takara. NO.RR037, Japan), and then prepare the PCR reaction system according to the kit instructions (Takara. NO.RR820, Japan). The above reaction system was mixed thoroughly, centrifuged for 10s, placed in a PCR instrument, and amplified according to the following reaction conditions: pre-denaturation (1 cycle) 95 °C for 30s, PCR reaction (40 cycles) 95 °C for 5 s and 60 °C for 34s.For the obtained Cycle threshold (Ct) value, 2−ΔΔt value was represented the relative expression level for every indicator.
Statistical Analysis
The GraphPad Prism (v8.0.1) and SPSS (v26.0) software were used for calculation and visualization. For quantitative data, the mean and standard deviation (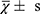 ) were used for normal distribution data, t-test was used to compare the differences between the two groups. The median and interquartile range [M (P25, P75)] were used for non-normal distribution data, the Wilcoxon test was used to analyze the differences. Count data with sample number (n) represent, The Chi-square test analysis differences between the two groups. Pearson correlation coefficient was used to evaluate the correlation between numerical variables. P value < 0.05 was considered statistically significant.
) were used for normal distribution data, t-test was used to compare the differences between the two groups. The median and interquartile range [M (P25, P75)] were used for non-normal distribution data, the Wilcoxon test was used to analyze the differences. Count data with sample number (n) represent, The Chi-square test analysis differences between the two groups. Pearson correlation coefficient was used to evaluate the correlation between numerical variables. P value < 0.05 was considered statistically significant.
Result
Analysis Clinical Data of TB Patients
This study included 43 active tuberculosis patients in TB group, with average age of 49.51±11.90 years old. There were 37 cases in control group, with average age of 40.27±13.61 years old. In TB group, the expression of Albumin (ALB) was 41.76±3.14(u/L), the expression of C-reactive protein (CRP) was 42.70 (9.31, 104.30) (mg/L), and the expression of White blood cells (WBC) was 8.96±2.83 (109/L), there are significant differences. Among them, ALB in the peripheral blood of TB patients was significantly lower than control group patients, while CRP and WBC were significantly increased with statistical significance. However, the expressions of Age, Hematocrit (Hct)and Mean corpuscular volume (MCV) were not prompt obvious difference (P>0.05), see Table 2.
 |
Table 2 Basic Clinical Data |
Histopathological Manifestations of Lesions
There were typical tuberculous granulomas in the pulmonary tuberculosis after HE staining, caseous necrosis accompanied by Langerhans multinucleated macrophage accumulation. Microscopically, the cytoplasm was pink, and the blue-purple nuclei gathered at the edge of giant macrophage like a horseshoe (Figure 1B). Inflammatory cell infiltration was occasionally seen at distal sites, and no obvious granulomatous structure (Figure 1A).
Ag85B is a unique virulence protein expressed by Mtb. After IHC staining, it was dark brown under microscope in the pulmonary tuberculosis lesions, in the cytoplasm and nucleus of Langhans cells were obviously positive (Figure 1D). However, no positive expression area was found in the distal tissue (Figure 1C). There was a statistically significant difference in expression between the lesion site and the distal site (Figure 1E, P < 0.001).
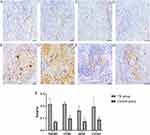
Expression Localization of TREM2 and M1/M2 Type Macrophage
The IHC staining results of TREM2 and M1/M2 type macrophage indicators are shown in the figure (Figure 2). In the pulmonary TB lesion tissue, TREM2 was mainly expressed in the cell membrane and cytoplasm, especially in Langhans multinucleated macrophages, and occasionally in the nucleus in the control group (Figure 2A and B). As a typical marker of macrophages, CD68 was significantly positive expressed in large areas of the lesions, mainly located in the nucleus and cytoplasm. In the control group, scattered brown areas were also seen, mainly only located in the nucleus (Figure 2C and D). However, iNOS and CD163, as markers of M1 and M2 type macrophage, were positively expressed in the cytoplasm of multinucleated macrophages in TB patient’s lesions (Figure 2F–H). In the TB lesion tissues and distal side tissues, the expression difference was statistically significant (Figure 2I).
Expression of TREM2, IL-10 and IL-12 in Peripheral Blood
The expression levels of IL-10 and IL-12 in the peripheral serum of research objects were detected by ELISA. The expression level of IL-12 was 105.80(65.36, 230.51) (pg/mL), and the expression level of IL-12 was 25.35(12.52, 110.08) (pg/mL), the difference was statistically significant. Table 3, Figure 3.
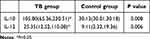 |
Table 3 Cytokine IL-10 and IL-12 Expression Levels(Pg/Ml) |
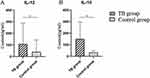 |
Figure 3 ELISA results. (A) IL-10 expression levels. (B) IL-12 expression levels.(*P < 0.05). |
The Relative Expression and Correlation Analysis of TREM2, IL-10 and IL-12 mRNA
We used qRT-PCR to detect the mRNA relative expressions of TREM2, IL-10 and IL-12. The results showed that TB group relative expression levels were significantly higher than control group. In the TB group, the TREM2 mRNA was 5.49 (4.01, 6.33), IL-10 mRNA was 32.43 (22.56, 36.73), the two subunits mRNA of IL-12 were 15.52 (13.74, 17.26) and 23.56±6.42. Table 4, Figure 4.
 |
Table 4 TREM2, IL-10 and IL-12 mRNA Expression Levels |
 |
Figure 4 qRT-PCR results. (A) TREM2 mRNA relative expression. (B and C) IL-12 mRNA relative expression. (D) IL-10 mRNA relative expression.(**P < 0.01). |
Subsequently, to further explore the relationship between TREM2 and macrophages in TB group, the results of Spearman correlation analysis indicated that there was a significant positive correlation between TREM2 and IL-10, and the correlation coefficient R value was 0.444, but there was no correlation between TREM2 and the two subunits of IL-12. Figure 5.
 |
Figure 5 Spearman correlation analysis results. |
Discussion
Mycobacterium tuberculosis (Mtb) is an intracellular parasite that poses a significant threat to global public health. Tuberculosis caused by Mtb is a highly infectious disease with a high mortality rate in infected hosts.20 When Mtb infects the host, alveolar macrophages are the primary innate immune cells that phagocytose the droplets entering the alveolar space in the lung tissue. This leads to the early inflammation and the formation of granuloma-like structures in the lung tissue4,21。Simultaneously, it provides a site for Mtb retention and pathogenic effect, regulates the initiation of adaptive immune responses, and ultimately enables the immune system suppress or clear Mtb infection.22–24 Both M1 and M2 macrophages play an important roles in the body’s resistance to Mtb. M1 is believed to primarily kill Mtb and promote the formation of granulomas, while M2 is mainly involved in the formation of TB granulomas and promotes macrophage foaming changes.6,25
M1 macrophages are triggered and activated by IFN-γ or Lipopolysaccharide (LPS). They are characterized by the high expression of iNOS and the secretion of various pro-inflammatory cytokines, including TNF-α, IL-12, CXCL10, and others. Under the induction of IL-4, IL-13 and other cytokines, macrophages can be activated to M2, with high expression CD206 and CDl63, secrete high levels of cytokines, such as IL-6, IL-10 and CXCLl7 and others.26 In this study, IL-12 and IL-10 were used in subsequent studies as cytokines secreted by M1 and M2, respectively. And iNOS and CD163 were explored as M1/M2 cell surface markers, respectively. TREM2 expression is regulated by various pathogens and disease conditions to modulate host-triggered innate immune responses.27 TREM2 senses bacterial or fungal infections and suppresses the secretion of induced pro-inflammatory factors.28 Recently, myeloid cells have been recognized as playing a central role in various diseases, with TREM2 being a major immune signaling hub induced by pathology. This study aims to investigate the relationship between TREM2 and macrophage polarization.
Immunohistochemical experiments were conducted to clarify the relationship between TREM-2 and macrophages of different polarization types. The expression levels of IL-10 and IL-12 in the peripheral blood of patients were detected using ELISA. Finally, qRT-PCR was used to count the mRNA relative expression levels of TREM2, IL-10 and IL-12. Statistical analysis was then performed to investigate the correlation between TREM2 and the macrophage polarization index, revealing the relationship between TREM2 and macrophage polarization.
TREM2 expression was initially discovered on monocyte-derived dendritic cells (MODCs) and later on the surface of various myeloid cells, Such as osteoclasts, microglia, neutrophils, and macrophages.29,30 TREM2 is a novel immunoregulatory receptor that has been implicated in the pathophysiology of inflammatory reactions, such as sepsis and inflammatory bowel disease, by regulating cytokine secretion by macrophages.31,32 In this study, IHC results showed that TREM2 was mainly expressed in the cell membrane and cytoplasm, especially in the TB granulomas formed by Langhans multinucleated macrophages, where it exhibits significant positive expression. CD68, as a typical marker of macrophages, was significantly positive expressed in large areas of the lesions, which once again to verifies the important role of macrophages in resistance to Mtb. In addition, iNOS and CD163 were used as markers for M1 and M2 type macrophages, respectively. Both markers showed positive expression. It is noteworthy that CD163 expression was more prominent in the cytoplasm and membrane of multinucleated macrophages, which is more consistent with the expression localization of TREM2. This suggests that TREM2 may be involved in the process of promoting the foaming process of macrophages near granuloma in M2 macrophages. Therefore, we further explored the expression levels of TREM2 and macrophage-related factors, in the peripheral blood. The relative expression levels of TREM2 mRNA were significantly higher in the TB group than in the control group, indicating an important biological role for TREM2 in both the lesion site and in the peripheral blood circulation system. The experimental results of the qRT-PCR and ELISA experiments showed significant expression of IL-12 and IL-10 in the TB group. Additionally, the cytokine IL-10 secreted by M2 appeared to have higher expression levels in the peripheral blood of TB patients. Further statistical analysis indicated a higher positive correlation between TREM2 and IL - 10. Therefore, we speculation that TREM2, as a kind of transmembrane receptor, may mediated the process of M2 type macrophages during Mtb infection to exert biological effects.
Recent studies have found that TREM2 specifically binds to Mtb infection and plays a role in defending against it by disabling the antimicrobial response of macrophages. Turnbull et al reported that the binding of TREM-2 to its ligand inhibits macrophage activation and the secretion of pro-inflammatory cytokines, such as TNF-α and IL-6. The expression of TREM-2 was significantly increased on the membrane surface of M2-type macrophages and was almost absent on the membrane surface of M1-type macrophages, which is consistent with the results of the present study.33 This is basically consistent with our research results. TREM2 and CD163 were positively expressed in the cytoplasm and membrane of Langhans multinucleated macrophages, and the expression patterns are very similar. TREM2 suppresses the macrophage inflammatory response by inhibiting the TLR signaling pathway.34,35 In addition, some studies have shown that macrophages overexpressing TREM2 can produce more IL-10 after exposure to LPS.36 This was also verified in this study, the expression levels of both IL-10 and IL-12 were significantly increased, while the expression level of IL-10 was a little higher when the TB group expressed more TREM2. Especially, in our further correlation analysis, the statistical results suggest that the relative expression levels of TREM2 and IL-10 have a higher correlation, showing a significant positive correlation. In fact, some scholars have proposed that, when Mtb infects macrophages, TREM2 acts as a gatekeeper, which can block the production of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and reactive oxygen species (ROS), at the same time, it can enhance the expression of interferon-β (IFN-β) and IL-10.37 This is consistent with our findings. In tuberculosis, the positive relationship between TREM2 and M2 is more closely, which lays a foundation for us to further reveal the action mechanism of TREM2 on M2 type macrophages surface, and we hope to provide new ideas for other scholars’ research.
Conclusion
In conclusion, the present study has shown a positive correlation between TREM2 and M2 macrophage-specific cytokines. This suggests that TREM2 may play a critical role in mediating macrophage polarization to M2 during the development of TB.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
This study was approved by the Ethics Committee of First Affiliated Hospital of Xinjiang Medical University and the trial conformed to the principles outlined in the Declaration of Helsinki. All adult subjects provided written informed consent, and no children were included in the study. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for Publication
Informed consent for publication was obtained from all participants.
Funding
State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia (SKL-HIDCA-2021-53), Scientific Research Project of Science Department of Xinjiang Autonomous Region (2023D01C237) and Key research and development project in Hainan Province (ZDYF2021SHFZ228).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships.
References
1. Cobelens F, Suri R, Helinski M, et al. Accelerating research and development of new vaccines against tuberculosis: a global roadmap. Lancet Infect Dis. 2022;22(4):e108–e120. doi:10.1016/S1473-3099(21)00810-0
2. Sarınoğlu R C, Sili U, Eryuksel E, Olgun Yildizeli S, Cimsit C, Karahasan Yagci A. Tuberculosis and COVID-19: an overlapping situation during pandemic. J Infect Dev Ctries. 2020;14(7):721–725. doi:10.3855/jidc.13152
3. Wang Y, Zhu W, Li T, Chen W, Wang W. Changes in newly notified cases and control of tuberculosis in China: time-series analysis of surveillance data. Infect Diseases Poverty. 2021;10(1):16. doi:10.1186/s40249-021-00806-7
4. Khan A, Singh V, Hunter R, CJJolb J. Macrophage heterogeneity and plasticity in tuberculosis. J Leukoc Biol. 2019;106(2):275–282. doi:10.1002/JLB.MR0318-095RR
5. Albors-Vaquer A, Rizvi A, Matzapetakis M, et al. Active and prospective latent tuberculosis are associated with different metabolomic profiles: clinical potential for the identification of rapid and non-invasive biomarkers. Emerging Microbes. 2020;9(1):1131–1139. doi:10.1080/22221751.2020.1760734
6. Ahmad F, Rani A, Alam A, et al. Macrophage: a cell with many faces and functions in tuberculosis. Front Immunol. 2022;13:747799. doi:10.3389/fimmu.2022.747799
7. Guirado E, Schlesinger L, Kaplan G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol. 2013;35(5):563–583. doi:10.1007/s00281-013-0388-2
8. Tardito S, Martinelli G, Soldano S, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmunity Rev. 2019;18(11):102397. doi:10.1016/j.autrev.2019.102397
9. Marino S, Cilfone N, Mattila J, et al. Macrophage polarization drives granuloma outcome during mycobacterium tuberculosis infection. Infection. 2015;83(1):324–338.
10. Refai A, Gritli S, Barbouche M, Essafi M. Mycobacterium tuberculosis virulent factor esat-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front Cell Infect Microbiol. 2018;8:327. doi:10.3389/fcimb.2018.00327
11. Wang L, Chen Q, Yu Q, Xiao J, Zhao H. Cigarette smoke extract-treated airway epithelial cells-derived exosomes promote M1 macrophage polarization in chronic obstructive pulmonary disease. Int Immunopharmacol. 2021;96:107700. doi:10.1016/j.intimp.2021.107700
12. Zhang X, Zhao Y, Zhu X, et al. Active vitamin D regulates macrophage M1/M2 phenotypes via the STAT-1-TREM-1 pathway in diabetic nephropathy. J Cell Physiol. 2019;234(5):6917–6926. doi:10.1002/jcp.27450
13. Molad Y, Pokroy-Shapira E, Carmon V. CpG-oligodeoxynucleotide-induced TLR9 activation regulates macrophage TREM-1 expression and shedding. Innate Immunity. 2013;19(6):623–630. doi:10.1177/1753425913476970
14. Katsel P, Haroutunian V. Is Alzheimer disease a failure of mobilizing immune defense? Lessons from cognitively fit oldest-old. Dialogues Clin Neurosci. 2019;21(1):7–19. doi:10.31887/DCNS.2019.21.1/vharoutunian
15. Filipello F, Goldsbury C, You S, Locca A, Karch C, Piccio L. Soluble TREM2: innocent bystander or active player in neurological diseases? Neurobiol Dis. 2022;165:105630. doi:10.1016/j.nbd.2022.105630
16. Li R, Qin Q, Yang H, et al. TREM2 in the pathogenesis of AD: a lipid metabolism regulator and potential metabolic therapeutic target. Mol Neurodegener. 2022;17(1):40. doi:10.1186/s13024-022-00542-y
17. Durmanova V, Javor J, Parnicka Z, et al. TREM2 coding variants in Slovak Alzheimer’s disease patients. J Integr Neurosci. 2022;21(4):105. doi:10.31083/j.jin2104105
18. Wu Y, Wu M, Ming S, et al. TREM-2 promotes Th1 responses by interacting with the CD3ζ-ZAP70 complex following mycobacterium tuberculosis infection. J Clin Invest. 2021;131(17). doi:10.1172/JCI137407
19. NHFPC. 肺结核诊断标准(WS 288—2017) [Diagnosis of tuberculosis WS288-2017]. 新发传染病电子杂志 [Chin J Infect Control]. 2018;17(7):642–652. Chinese. doi:10.3969/j.issn.1671-9638.2018.07.019
20. Macedo Couto R, Ranzani O, Waldman E. Zoonotic tuberculosis in humans: control, surveillance, and the one health approach. Epidemiologic Reviews. 2019;41(1):130–144. doi:10.1093/epirev/mxz002
21. Ngo M, Bartlett S, Bielefeldt-Ohmann H, et al. A blunted GPR183/oxysterol axis during dysglycemia results in delayed recruitment of macrophages to the lung during mycobacterium tuberculosis infection. J Infect Dis. 2022;225(12):2219–2228. doi:10.1093/infdis/jiac102
22. Chandra P, He L, Zimmerman M, et al. Inhibition of fatty acid oxidation promotes macrophage control of mycobacterium tuberculosis. mBio. 2020;11(4). doi:10.1128/mBio.01139-20
23. Laval T, Chaumont L, Demangel C. Not too fat to fight: the emerging role of macrophage fatty acid metabolism in immunity to mycobacterium tuberculosis. Immunol Rev. 2021;301(1):84–97. doi:10.1111/imr.12952
24. Thacker V, Dhar N, Sharma K, Barrile R, Karalis K, McKinney J. Mycobacterium tuberculosisA lung-on-chip model of early infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. Elife. 2020;9. doi:10.7554/eLife.59961
25. Khan A, Zhang K, Singh V, et al. Human M1 macrophages express unique innate immune response genes after mycobacterial infection to defend against tuberculosis. Commun. Biol. 2022;5(1):480. doi:10.1038/s42003-022-03387-9
26. Fuchs A, Syrovets T, Haas K, et al. Carboxyl- and amino-functionalized polystyrene nanoparticles differentially affect the polarization profile of M1 and M2 macrophage subsets. Biomaterials. 2016;85:78–87. doi:10.1016/j.biomaterials.2016.01.064
27. Cheng X, Wang X, Nie K, et al. Systematic pan-cancer analysis identifies TREM2 as an immunological and prognostic biomarker. Front Immunol. 2021;12:646523. doi:10.3389/fimmu.2021.646523
28. Hamerman JA, Jarjoura JR, Humphrey MB, Nakamura MC, Seaman WE, Lanier LL. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Iimmunol. 2006;177(4):2051–2055. doi:10.4049/jimmunol.177.4.2051
29. Carrasco K, Boufenzer A, Jolly L, et al. TREM-1 multimerization is essential for its activation on monocytes and neutrophils. Cell Mol Immunol. 2019;16(5):460–472. doi:10.1038/s41423-018-0003-5
30. Lin C, Chang T, Lu Y, et al. TREM-2 mediates dendritic cell-induced NO to suppress Th17 activation and ameliorate chronic kidney diseases. J Mol Med. 2022;100(6):917–931. doi:10.1007/s00109-022-02201-7
31. Seno H, Miyoshi H, Brown SL, Geske MJ, Colonna M, Stappenbeck TS. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci USA. 2009;106(1):256–261. doi:10.1073/pnas.0803343106
32. Correale C, Genua M, Vetrano S, et al. Bacterial sensor triggering receptor expressed on myeloid cells-2 regulates the mucosal inflammatory response. Gastroenterology. 2013;144(2):346–356.e343. doi:10.1053/j.gastro.2012.10.040
33. Turnbull I, Gilfillan S, Cella M, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Iimmunol. 2006;177(6):3520–3524. doi:10.4049/jimmunol.177.6.3520
34. Jaitin DA, Adlung L, Thaiss CA, et al. Lipid-associated macrophages control metabolic homeostasis in a Trem2-dependent manner. Cell. 2019;178(3):686–698.e614. doi:10.1016/j.cell.2019.05.054
35. Leyns CEG, Ulrich JD, Finn MB, et al. TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc Natl Acad Sci USA. 2017;114(43):11524–11529. doi:10.1073/pnas.1710311114
36. Chen Q, Zhang K, Jin Y, et al. Triggering receptor expressed on myeloid cells-2 protects against polymicrobial sepsis by enhancing bacterial clearance. American j Respiratory. 2013;188(2):201–212.
37. Dabla A, Liang Y, Rajabalee N, et al. TREM2 promotes immune evasion by mycobacterium tuberculosis in human macrophages. MBio. 2022;13(4):e0145622. doi:10.1128/mbio.01456-22
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.