Back to Journals » OncoTargets and Therapy » Volume 9
Tremelimumab: research and clinical development
Authors Comin-Anduix B, Escuin-Ordinas H, Ibarrondo FJ
Received 1 December 2015
Accepted for publication 10 February 2016
Published 23 March 2016 Volume 2016:9 Pages 1767—1776
DOI https://doi.org/10.2147/OTT.S65802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Begoña Comin-Anduix,1,2 Helena Escuin-Ordinas,3 Francisco Javier Ibarrondo4
1Division of Surgical-Oncology, Department of Surgery, 2Jonsson Comprehensive Cancer Center, 3Division of Hematology-Oncology, 4Division of Infectious Diseases, Department of Medicine, David Geffen School of Medicine at University of California Los Angeles, Los Angeles, CA, USA
Abstract: The immune checkpoint therapy is a relatively recent strategy that aims to tweak the immune system to effectively attack cancer cells. The understanding of the immune responses and their regulation at the intracellular level and the development of fully humanized monoclonal antibodies are the pillars of an approach that could elicit durable clinical responses and even remission in some patients with cancer. Most of the immune checkpoints that regulate the T-cell responses (activation and inhibition) operate through proteins present on the cytoplasmic membrane of the immune cells. Therefore, specific antibodies capable of blocking the inhibitory signals should lead to unrestrained immune responses that supersede the inhibitory mechanisms, which are naturally present in the tumor microenviroment. The best-known and most successful targets for immune checkpoint therapy are the cytotoxic T-lymphocyte antigen-4 and programmed cell death-1 coreceptors. Tremelimumab (CP-675,206) is a fully humanized monoclonal antibody specific for cytotoxic T-lymphocyte antigen-4, which has been successfully used to treat patients with metastatic melanoma and some other cancers. Although still a work in progress, the use of tremelimumab as an immune checkpoint therapeutic agent is a promising approach alone or in combination with other anticancer drugs. Here, we review the use of this antibody in a number of clinical trials against solid tumors.
Keywords: immune checkpoint, anti-CTLA-4 blockade antibody, cancer
Introduction
In 2013, cancer immunotherapy was announced as the breakthrough of the year in oncology,1 and new treatments targeting immune checkpoints on T-cells have led to the first objective improved overall survival (OS) in patients with stage IV melanoma. After more than 30 years of clinical research, the use of monoclonal antibodies that block cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) is still rendering long-term benefits and even long-term survival in patients who otherwise would have had an OS of 6 months.2–6 The development of ipilimumab, the first US Food and Drug Administration-approved specific antibody against CTLA-4, opened the doors to the immune checkpoint therapy. The idea behind the immune checkpoint therapy is to target regulatory pathways in T-cells utilizing specific antibodies that block the natural regulatory processes that damper the immune T-cell response upon antigen stimulation, and, therefore, unleashing a sustained immune response against the tumor. To date, a handful of antibodies targeting CTLA4, PD-1, PD-L1, CD4, or natural killer receptors have been developed and tested in clinical trials against solid tumors.7–9 Antibodies against CTLA-4 and against PD-1, alone or in combination, have been shown to be the most promising. Tremelimumab (formerly known as CP-675,206 and ticilimumab; Pfizer, Inc., New York, NY, USA; and AstraZeneca from 2015) is an anti-CTLA-4 antibody that has been studied in clinical trials for melanoma, colon cancer, gastric cancer, and mesothelioma.10–12 In this review, we will discuss the use of tremelimumab in all these different clinical trials.
Regulation of the immune T-cell response, the immune checkpoint concept
The immune response relies on the presentation of tumoral antigens by antigen-presenting cells (APCs) in the lymph nodes and the infiltration of specific T-cell clones back into the tumor.7 The magnitude and duration of the cellular response depend on the balance between stimulatory and inhibitory signals generated upon T-cell receptor (TCR) engagement.7 Although the control of the T-cell activity runs via a myriad of intracellular pathways, most of the signals are generated by molecules present in the cytoplasmic membrane interacting with the extracellular milieu. Agonist and antagonist inputs arriving to the T-cell in the form of soluble (cytokines) or membrane-bound ligands regulate the T-cell behavior. Depending on the concentration of the ligand and the affinity of the T-cell for them, the T-cell response (or lack of it) will follow.
The term immune checkpoint refers to inhibitory pathways that regulate immune responses and tolerance processes in peripheral tissues. Some of the best studied immune checkpoints are CTLA-4 and PD-17 due to their impact on tumor treatment in the clinic. In 1994, the efficacy of CTLA-4 in inducing tumor regression was demonstrated with the systematic administration of specific CTLA-4 antibodies in several mice models.13,14 The development of humanized antibodies against immune checkpoints, such as CTLA-4, opened the door to a new era in the war on cancer, where durable immune responses against tumors can be achieved.8,9
Cytotoxic T-lymphocyte antigen-4
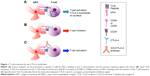
CTLA-4 (CD152) is a homolog of the coactivation receptor CD28. T-cell activation requires the concomitant interaction of TCR and CD28 with their respective ligands. Upon TCR engagement with its cognate antigen presented by HLA molecules on the APC, CD28 binds to its B7 ligand (CD86 or CB80, depending on the APC), leading to T-cell proliferation and cytokine secretion. At the same time, TCR activation induces the expression of CTLA-4, on the membrane of the T-cell, which binds to B7 ligands with a higher affinity than CD28. The binding of CTLA-4 to B7 ligands triggers intracellular transduction signals that antagonize the activation signals and terminates the T-cell response. The ratio of CD28 to CTLA-4 (or PD-1 or inducible T-cell stimulator [ICOS]), the so-called second signals, determines the output of the T-cell response. Under normal physiological conditions, the higher affinity of CTLA-4 to the B7 ligand outcompetes CD28, leading to the reduction of cell proliferation and cytokine secretion. The presence of antibodies blocking the binding of CTLA-4 to their ligands frees B7 ligands, which can now bind to CD28, thus maintaining and potentiating the T-cell response. This is correlated with tumor rejection and immunity on a secondary exposure to tumor cells15–21 (Figure 1).
The model that depicts anti-CTLA-4 antibodies directly blocking the inhibitory signals on T-cells, inactivating the brakes to enhance antitumor activity, is in collusion with the fact that B7 expression is very low in the tumor environment. Most tumor cells, with the exception of certain lymphomas, do not express the B7 costimulatory receptor and, therefore, are unable to interact and activate T-cells.9 It has been argued that inflammatory responses can partially overcome the lack of B7 expression by tumor cells. These inflammatory responses can recruit professional APCs (mainly dendritic cells and macrophages) to the inflammation site, which, in turn, can present tumor antigens to resident T-cells. However, it is still unclear whether this mechanism can lead to durable antitumoral responses. The effectiveness of anti-CTLA-4 antibodies in a microenvironment deprived of B7 stimulatory coreceptor can also be explained by an enhanced infiltration of tumor-specific T-cells from tumor-draining lymph nodes.
Tumors are populated by an army of regulatory T-cells (Tregs) and myeloid-derived suppressor cells that restrict effective antitumoral T-cell activity.10 Tregs express high levels of CTLA-4 coreceptor, and it has been shown that the administration of anti-CTLA-4 antibodies decreases the ratio of Tregs to effector T-cells,22,23 leading to an increased antitumor T-cell activity. The mechanism that mediates anti-CTLA-4-induced Treg depletion is still unknown, although the involvement of macrophage Fcγ receptor (FcγR) responses has been proposed to play a fundamental role in it.24–26
Tremelimumab and preclinical models
In the 90s, two independent in vitro studies characterized the antitumor activity of tremelimumab (CP-675,206). Hanson et al described the design of tremelimumab as an antihuman CTLA-4 antagonist immunoglobulin (Ig) G2 antibody, whose complement activation and FcγR binding is reduced compared to IgG1 antibodies. The authors showed how tremelimumab had a higher affinity for CTLA-4-Ig than for CD28-Ig and that the antibody was specific for cynomolgus monkeys and humans. Tremelimumab did not bind to human leukocyte FcγR and did not trigger the release of cytokines avoiding the cytokine release syndrome, but it increased the IL-2 production upon stimulation with the staphylococcal enterotoxin A in a concentration-dependent manner.27 At the same time, Canniff et al28 revealed how the effects of tremelimumab were independent of the disease stage or the cancer type, and that the antibody enhanced the IL-2 production in T-cells from healthy individuals and from cancer patients with solid tumors, including ovarian, renal, prostate, rectal, melanoma, and Hodgkin’s/non-Hodgkin’s lymphoma.
Tremelimumab in clinical trials
A summary of all the following clinical trials is described in Table 1, and the objective overall response (OR) in Figure 2.
Melanoma
The first published tremelimumab clinical trial included 34 melanoma, one colon cancer, and four renal cell carcinoma (RCC) patients. In this trial, six doses of tremelimumab (0.1 mg/kg, 1 mg/kg, 3 mg/kg, 6 mg/kg, 10 mg/kg, and 15 mg/kg) were tested with the possibility to re-enroll the patients of the first three cohorts (0.1 mg/kg, 1 mg/kg, and 3 mg/kg of the antibody). Four out of 29 patients with melanoma presented a positive response, and 13 of the 29 patients experienced an apparent long-term clinical benefit with an OS between 22 months and 38 months. Three patients from the 15 mg/kg cohort showed dose-limiting toxicity (DLT) and autoimmune responses consisting of diarrhea, dermatitis, vitiligo, panhypopituitarism, and hyperthyroidism. In the cohorts with doses of 10 mg/kg and 15 mg/kg, there were adverse events (AEs) consisting of grade 3 toxicities in the form of diarrhea, dermatitis, and increased serum lipase.29
The second tremelimumab clinical trial published for metastatic melanoma was a Phase I/II trial. In the Phase I, a dose-escalation of tremelimumab (3 mg/kg, 6 mg/kg, or 10 mg/kg) resulted in no response, but with some AEs such as pruritus, rash, and diarrhea. Four of the patients, dosed at 10 mg/kg, showed a delayed DLT, two of them had autoimmune hepatitis, one pruritus, skin exfoliation, rash, and leukocytoclastic vasculitis, and the last one cellulitis and edema. In the Phase II study, which included 14 extra patients enrolled on an expansion cohort treated with a dose of 10 mg/kg, four patients benefitted an OR (one complete response and three partial responses [PRs]) in each cohort. The median OS for patients treated with 10 mg/kg and 15 mg/kg of tremelimumab was 9.97 months and 11.53 months, respectively. The most common AEs in this study were diarrhea, rash, pruritus, fatigue, and nausea, all of them being more frequent on the 10 mg/kg arm.30
Kirkwood et al published a Phase II clinical trial with 251 patients with metastatic melanoma treated with tremelimumab (15 mg/kg); responses were evaluated in 241 of them. All of the evaluated patients attained PRs with a median OS of 10 months and with 16 patients who achieved OR (to note that eight [50%] of the 16 responders, started the treatment with a stage IV1c disease). OS was 40.3% at year 1. Diarrhea, colitis, and fatigue were the most common detected grade 3 AEs. Two patients died due to treatment.31
In a different single-agent Phase II, tremelimumab (15 mg/kg) was administered to 32 patients with metastatic melanoma. Four patients benefitted with an OR, where the OS fluctuated between 2 months and 41+ months, and seven patients survived >2 years. The adverse reactions included skin rash, hypophysitis, and colitis, with one patient diagnosed with immune thrombocytopenia purpura.32
The promising data from the Phase II clinical trials led to the development of a two arm Phase III clinical trial. In this trial, tremelimumab (15 mg/kg) was randomly assigned against temozolomide or dacarbazine (the standard-of-care [SOC] for melanoma in that time), with 328 patients treated with tremelimumab at the time of analysis. Patients treated with tremelimumab had an objective response of 10.7 months, with a median OS of 12.6 months. The most serious AEs of grade 3/4 toxicities were diarrhea/colitis, nausea, fatigue, and rash. Seven patients died because of toxicities related to tremelimumab. However, 21% of patients had a 3-year survival rate. Other than the increased duration of response in the tremelimumab arm (35.8 months), there were no clinical differences between the tremelimumab and the SOC arms.33
Visceral cancers
Although tremelimumab has mainly been used in the treatment of melanoma, its efficacy has also been tested with some visceral cancers.
Forty-four patients with non-small-cell lung cancer (NSCLC) were treated with 15 mg/kg of tremelimumab; however, only two out of 44 patients (4.5%) showed a PR. Adverse effects, including grade 3 diarrhea and colitis, were present in the tremelimumab-treated patients but not in the SOC.34
Seventeen patients diagnosed with hepatocellular carcinoma and chronic hepatitis C virus received 15 mg/kg of tremelimumab. Only three of those patients experienced a confirmed PR. At the beginning of the trial, 43% of the enrolled patients presented deteriorated liver function. Only a few of those patients experienced related grade 3 toxicities due to treatment, consisting, mainly, of skin rash, diarrhea, and syncope, but all resolved without the use of steroids. The novelty of this clinical trial was the study of tremelimumab as an antiviral: tremelimumab decreased the median values in viral load in all the patients; however, six patients with lower viral load showed mutations in the hypervariable region 1 of hepatitis C virus.35
Tremelimumab was also investigated as a second-line treatment for patients with gastric and esophageal adenocarcinomas. Only one out of 18 patients treated with tremelimumab (15 mg/kg) achieved a PR >32 months. Although the majority of toxicities were mild, one patient died of intestine perforation.36
The response rate achieved in patients with refractory metastatic colorectal cancer, treated with tremelimumab at 15 mg/kg, was also disappointing, with only one out of 47 patients (2.2%) achieving a PR until relapse at 14.5 months. Seven patients presented grade 3 toxicities consisting of diarrhea, fatigue, and colitis.12
Tremelimumab in combination therapy
After the good results observed with tremelimumab as monotherapy against melanoma, the next logical step was to test it in combination with different anticancer therapies, including immune therapies. Ribas et al carried out the first published clinical trail with 16 patients with metastatic melanoma treated with tremelimumab (once a month or every 3 months) in a dose escalation (3 mg/kg, 6 mg/kg, 10 mg/kg, or 15 mg/kg) combined with autologous MART-1 (melanoma antigen recognized by T-cells) peptide-pulsed dendritic cells (1×107 cells). Four out of 16 patients achieved a positive response >2 years. DLTs were grade 3 diarrhea and grade 2 hypophysitis.37
In a different study for patients with advanced breast cancer, tremelimumab was combined with exemestane, an aromatase inhibitor, at 25 mg/d. Eleven out of 26 patients benefitted with a stable disease (SD) at 12 weeks, the most common grade 3 toxicities consisted of diarrhea, pruritus, constipation and fatigue.38
Patients with metastatic melanoma were treated with tremelimumab plus the immune agonist anti-CD40 antibody (CP-870,893) in a Phase I open-label, single-arm dose-escalation trial for both agents. The trial achieved an OR rate of 27.3% in a total of 24 patients, with a median OS of 26.1 months. In the 24 patients included in the toxicity analysis, uveitis, colitis, and hypophysitis were the DLT. Additionally, grade 1/2 cytokine release syndrome due to anti-CD40 administration was observed in 79.2% (19 patients) of the cases.39
High dose of IL-2 or interferon (IFN)-α-2b immunotherapy has been the traditional treatment for RCC. Consequently, tremelimumab therapy was the next compound introduced in the clinical trials for patients with RCC. Twenty-one patients were treated with 50 mg sunitinib (a pan inhibitor of receptor tyrosine kinase) daily for 4 weeks with 2 weeks off or 37.5 mg/kg sunitinib plus tremelimumab (6 mg/kg, 10 mg/kg, or 15 mg/kg) daily. The combination therapy resulted in an unexpected acute renal failure, although nine out of 21 patients presented a PR, at the time of the presentation of the data.40
The autologous dendritic cells therapy (sipuleucel-T) for patients with advanced, castrate-resistant metastatic prostate cancer opened the doors to immunotherapy treatments for those malignances.41 McNeel et al carried out a clinical trial with stage D0 prostate cancer patients utilizing tremelimumab (3 mg/kg and 6 mg/kg) in combination with 150 mg bicalutamide (an antiandrogen): three out of eleven patients presented a reasonable delay in prostate-specific antigen doubling time. Grade 3 diarrhea and rash were the maximum DLTs observed in those patients.42
Thirty-seven patients with melanoma (including cutaneous, uveal, or mucosal) were treated with tremelimumab (15 mg/kg) plus a high dose of IFN-α-2b. Nine of those patients presented responses, lasting a minimum of 3 months, while 14 patients showed SD up to 21 months. The most AEs included grade 3 or 4 consisting of neutropenia, diarrhea/colitis, liver enzyme increase, rash, fatigue, and depression/anxiety; all of them treatable and all of them presented at the same frequencies as in each single drug treatment.43
Millard et al44 carried out a study with 17 patients with melanoma and one patient with mesothelioma, prostate, non-small-cell lung, and pancreatic cancer, with a combination of tremelimumab plus 6 mg/kg, 10 mg/kg, or 15 mg/kg PF-3512676, an oligonucleotide agonist of the toll-like receptor-9.45,46 DLTs were observed in the 10 mg/kg or 15 mg/kg tremelimumab dosage, with grade 3 or 4 AEs, in seven patients, consisting of diarrhea, hypophysitis, colitis, nausea, vomiting, pruritus, and rash.44
Gemcitabine, a nucleoside analog, alone or in combination, is the most common anticancer drug used for metastatic pancreatic cancer.47–49 Usually chemotherapy does not improve the survival of patients with pancreatic cancer, and the pancreatic microenvironment produces signals that make the immune cells work for cancer progression instead of killing the tumor.49 Aglietta et al combined tremelimumab (6 mg/kg, 10 mg/kg, or 15 mg/kg) with gemcitabine (100 mg/m2 on days 1, 8, and 15 of each 28-day cycle). Two patients under 15 mg/kg dosage group had a PR with an OS at 7.4 months. Asthenia and nausea were the most frequent grade 3 and 4 toxicities, with one patient presenting DLT.50
Mesothelioma
The team of Prof Maio11,51–53 has been working on the use of tremelimumab for the treatment of malignant mesothelioma. In the first published study (published MESOT-TREM-2008), 29 patients were treated with tremelimumab (15 mg/kg). Two out of the 29 patients obtained a partial response with 13% (three patients) of them presenting grade 3/4 toxicities (colitis or diarrhea), peripheral neuropathy, increased hepatic transaminases, or increased amylase or lipase; all treatable.51
In a second Phase II clinical trial, the investigators changed the schedule of the compound, and the results were similar to the previous study as only one patient of 29 obtained a PR and two patients presented grade 3/4 toxicities (fever, dermatological, and gastrointestinal).52
A new randomized, double-blinded, placebo-controlled Phase II study where the authors are recruiting up to 564 patients is still in progress.11 A review on tremelimumab and mesothelioma has been recently published.53
Tremelimumab on the road
Currently, there are some clinical trials registered to study the effect of tremelimumab alone or in combination in certain cancer types (clinicaltrials.gov). Among these ongoing trials, two of them are in Phase III, with tremelimumab alone or in combination with the SOC in NSCLC or patients with squamous cell carcinoma of the head and neck (not yet recruiting). The combination of two of the most studied immune checkpoints, PD-1 and CTLA-4, is also being explored in two different clinical trials on patients with mesothelioma and NSCLC.
Lessons learned from tremelimumab clinical trials
As Ascierto54 commented, the surprising results of the metastatic melanoma Phase III clinical trials not achieving its primary end point, was potentially attributable to two factors. The first, possibly, related to the schedule of treatment in the tremelimumab arm, and the second associated with the enrollment of patients with better prognosis in the control arm, and also some of them were using ipilimumab. The majority of the ipilimumab users were treated in the US.
On the other hand, Blank and Enk10 suggested that the failure of tremelimumab Phase III clinical trial in melanoma was mainly due to tremelimumab since as an IgG2 immunoglobulin, it binds to FcγR with lower affinity, making the blocking of CTLA-4 to the T-cells less effective.
Furthermore, the investigators from all the clinical trials with CTLA-4 blocking antibodies observed that immunotherapeutic agents do not follow a strict Response Evaluation Criteria In Solid Tumors since this immune treatment induces some tumor expansion (seen by radiography) due to the inflammatory response associated with the treatment. Besides, the immune-related response criteria (irRC) were introduced in 2009, after the design of the majority of tremelimumab clinical trials. In summary, the irRC present the OR as a consecutive evaluation of the total measurement of the tumor burden at least 4 weeks from baseline.55 Additionally, the categorization of PR or SD is still a gray area for immunotherapy.56 Recently, Shimonura et al reported two case reports of an ongoing Phase I trial in Japan with tremelimumab alone given every 4 weeks. One of the cases was initially diagnosed as mild bile duct cancer, later diagnosed as adenocarcinoma. The second one was presented an unknown primary cancer, later diagnosed, as squamous cell carcinoma. Both patients experienced a remarkable tumor shrinkage following an initial disease progression, without any immune-related AE. As the author suggested, these cases could open a new era for tremelimumab on solid tumors.57
Two publications summarize the events of delayed responses in follow-up studies. One of them showed the new irRC with a small cohort of ipilimumab-treated patients (as described in the previous paragraph).55,58 The long-term analysis with ipilimumab showed a median OS of 11.4 months. Twenty-two percent of the 1,861 patients had an OS of 3 years.5 In a second study, a 12-year follow-up done in 141 patients previously treated with tremelimumab (dosage from 0.01 mg/kg to 15 mg/kg) showed 22 patients (15.6%) benefiting with a response, with a median OS of 13 months.29,30,32,37 The authors of this report estimated a 5-, 10-, and 12.5-year survival rate of 20%, 16%, and 16%, respectively. Besides, the authors showed how sex or age does not correlate with OR or OS.59
Biology learned from tremelimumab clinical trials
The major source of knowledge about the effect of tremelimumab comes from patients with melanoma. Analyzing immunological parameters present in peripheral blood, Reuben et al categorized patients treated with tremelimumab depending on the presence or absence of immune-related adverse event (IRAE) antitumor responses (ATRs) and/or objective ATRs. Patients who were double-negative IRAE/ATR presented a good correlation between the transcripts of CTLA-4 and PD-1 receptor, whereas the double-positive IRAE/ATR patients presented a positive correlation with the glucocorticoid-induced tumor necrosis factor receptor and CTLA-4 transcripts. Patients with only ATR presented a significant decrease in constitutive IL-10 production and T-regulatory cells defined by the authors as CD4+CD25high cells.60
Other investigators reported augmented T-cell activation and memory cell markers, but no difference on the frequency of Tregs (CD3+CD4+CD25+FoxP3+) was found in response to tremelimumab. However, the peripheral blood of those OR patients presented upregulation of transcripts for B-cells at baseline.61 Fifteen biopsies from seven of the patients described before showed diffused intratumoral infiltrates (TILs) of CD8+ T-cells in responder patients or regressing lesions; sparse, patchy CD8+ TILs were found on nonregressing lesions. On the other hand, the levels of CD4+ and FoxP3 or indoleamine 2,3-dioxygenase TILs presented only marginal changes in postdosing samples.62 Another study done with paired biopsies of patients with metastatic melanoma, found an increase in CD8+ TILs independently of the clinical or tumor response, with comparable expression of memory (CD45RO), activation (HLA-DR), cell replication (Ki67), and suppressor/effector cell (FoxP3) immune markers.32 Furthermore, that cohort not only presented a reduction in phoshoproteins downstream of TCR in T-cells, such as pAKT in T-cells and monocytes,63 but also an expansion of the T-cells repertoire in peripheral blood.64 Moreover, there were also interactions between tremelimumab and the invariant natural killer cells (iNKTs) implicated in both innate and adaptive immune responses: the iNKT-CD4+ and iNKT-CD8+ presented a higher effector memory phenotype than the iNKT-double-negative iNKT cells (iNKT-DN) after treatment.65
The study that investigated the combination of tremelimumab with IFN-α showed that patients who had autoimmune disorders with a baseline of higher absolute lymphocytes count of 1,000/μL and a baseline ≤2.7 times the upper limit of normal donors for C-reactive protein presented a slight association with clinical benefit after therapy.43
The combination of tremelimumab with MART-1 peptide-pulse DC studies led to positive clinical responses in patients who had higher frequency and number of iNKT-CD8+ cells present in peripheral blood before treatment. Cell signaling of monocytes was highly altered after treatment, and the proximal signal of TCR (pZAP70 and pLAT) was decreased. However, the immune monitoring done by tetramer and ELISPOT analysis showed no differences.65
When used in combination with anti-CD40α, tremelimumab seemed to increase the ability of the T-cells to replicate and enhance lysis (high frequency of double positive for Gramzyme B and Ki67); and PD-1 plus eomesodermin in CD8+ T-cells after treatment.39
In the study with patients having hepatocarcinoma and chronic hepatitis C, tremelimumab induced an expansion of virus-specific IFN-γ lymphocytes, Tregs (defined as CD4+FoxP3+ cells only), and a transient expansion of peptides.35
In the advanced gastric and esophageal adenocarcinoma study, a higher transient expression of FoxP3 in CD4+CD25high T-cells was observed, with a constant increased expression of CTLA-4 in CD4+CD25−/low T-cells during treatment. No changes in the frequency of CD8 T-cells were observed.36
The majority of patients with breast cancer treated with the combination of tremelimumab plus exemestane presented T-cells expressing ICOS, the proportion of ICOS/Tregs (CD4+FoxP3+) being favorable to ICOS.38
The effect of tremelimumab on the immune system of mesothelioma patients was noticed on ICOS T-cells (CD4 and CD8), which increased during the first cycle. There was a better survival of patients with CD4+ICOS+ T-cells circulating over a value of 54 cells/μL and 26 cells/μL at days 30 and 26, respectively. Circulating T-cells were more activated (HLA-DR+) and presented higher frequency of memory (CD45RO) markers, but no changes on B or natural killer cells were observed after treatment.51
In summary, tremelimumab alone or in combination has a role in the activation and increase in the frequency of memory cells. It also has some effect on Tregs and monocytes of the immune system. The differences found in several studies regarding changes in the Treg population under tremelimumab treatment could mainly be due to how the different authors define these subpopulations. In some reports, authors used the markers in mice (CD4 and FoxP3 dual expression), which is not enough to define Tregs in humans.66
Conclusion
The milestone of long-term cancer survivals in patients treated with anti-CTLA-4 antibodies opened a door to immune therapy with immune checkpoint inhibitors. Although tremelimumab, one of the two initial anti-CTLA-4 antibodies, has demonstrated promising clinical activity in metastatic melanoma, it is still in clinical development in mesothelioma and others cancers. In other kinds of tumors, such as gastric and esophageal adenocarcinoma and NSCLC, tremelimumab, alone or in combination also presented a low antineoplastic activity, typically ~5%−10% of long-term survival rate. The use of anti-CTLA-4 antibodies, including tremelimumab, leads to strong immune-related side effects, although the majority of them are treatable. It is already known that combinations of different tumor-killing strategies give the best results, and tremelimumab will be a valuable tool in immune combinational therapies. Moreover, a deeper understanding of how tremelimumab works on the niche of the tumor microenvironment may allow a better selection of patients with cancer, who will benefit the most from this immune checkpoint therapy, and it may get tremelimumab out of the pipeline of experimental compounds.
Disclosure
The authors report no conflicts of interest in this work.
References
Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. | ||
Hodi FS, O’Day SJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. | ||
Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. | ||
Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. | ||
Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33(17):1889–1894. | ||
Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. | ||
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. | ||
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. | ||
Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. | ||
Blank CU, Enk A. Therapeutic use of anti-CTLA-4 antibodies. Int Immunol. 2015;27(1):3–10. | ||
Calabro L, Ceresoli GL, di Pietro A, et al. CTLA4 blockade in mesothelioma: finally a competing strategy over cytotoxic/target therapy? Cancer Immunol Immunother. 2015;64(1):105–112. | ||
Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28(21):3485–3490. | ||
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. | ||
Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. | ||
Azuma M, Ito D, Yagita H, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366(6450):76–79. | ||
Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. | ||
Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71(7):1065–1068. | ||
Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229(1):12–26. | ||
Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262(5135):905–907. | ||
Freeman GJ, Borriello F, Hodes RJ, et al. Uncovering of functional alternative CTLA-4 counter-receptor in B7-deficient mice. Science. 1993;262(5135):907–909. | ||
Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87(13):5031–5035. | ||
Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18(2):206–213. | ||
Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA-4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–1945. | ||
Bulliard Y, Jolicoeur R, Windman M, et al. Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210(9):1685–1693. | ||
Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92(6):475–480. | ||
Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. | ||
Hanson DC, Canniff PC, Primiano MJ, et al. Preclinical in vitro characterization of anti-CTLA4 therapeutic antibody CP-675,206. Abstract 3802. AACR. Cancer Res. 2004;64:877. | ||
Canniff PC, Donovan CB, Burkwit JJ, et al. CP-675,206 anti-CTLA4 antibody clinical candidate enhances IL-2 production in cancer patient T cells in vitro regardless of tumor type or stage of disease. Abstract 709. AACR. Cancer Res. 2004;64:164. | ||
Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23(35):8968–8977. | ||
Camacho LH, Antonia S, Sosman J, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27(7):1075–1081. | ||
Kirkwood JM, Lorigan P, Hersey P, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16(3):1042–1048. | ||
Huang RR, Jalil J, Economou JS, et al. CTLA-4 blockade induces frequent tumor infiltration by activated lymphocytes regardless of clinical responses in humans. Clin Cancer Res. 2011;17(12):4101–4109. | ||
Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. | ||
Zatloukal PP, Heo DS, Kang J, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC). ASCO Annual Meeting. J Clin Oncol. 2009;27(15s suppl):abstr8071. | ||
Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59(1):81–88. | ||
Ralph C, Elkord E, Burt DJ, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16(5):1662–1672. | ||
Ribas A, Comin-Anduix B, Chmielowski B, et al. Dendritic cell vaccination combined with CTLA4 blockade in patients with metastatic melanoma. Clin Cancer Res. 2009;15(19):6267–6276. | ||
Vonderheide RH, LoRusso PM, Khalil M, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–3494. | ||
Bajor DLM, Mick R, Riese MJ, et al. CT137 – Combination of Agonistic CD40 Monoclonal Antibody CP-870,893 and Anti-CTLA-4 Antibody Tremelimumab in Patients with Metastatic Melanoma. Proceedings, Part 2: Clinical Trials and Late-Breaking Abstracts. Clinical Trials Plenary Session: Combinations of Therapeutic Agents. AACR. Vol. Part 2. I. Philadelphia, PA: AACR.org; 2015. | ||
Rini BI, Stein M, Shannon P, et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2011;117(4):758–767. | ||
Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. | ||
McNeel DG, Smith HA, Eickhoff JC, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61(7):1137–1147. | ||
Tarhini AA, Cherian J, Moschos SJ, et al. Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol. 2012;30(3):322–328. | ||
Millward M, Underhill C, Lobb S, et al. Phase I study of tremelimumab (CP-675 206) plus PF-3512676 (CPG 7909) in patients with melanoma or advanced solid tumours. Br J Cancer. 2013;108(10):1998–2004. | ||
Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117(5):1184–1194. | ||
Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene. 2008;27(2):161–167. | ||
Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. | ||
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691–1703. | ||
Conroy T, Desseigne F, Ychou M, et al; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–1825. | ||
Aglietta M, Barone C, Sawyer MB, et al. A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol. 2014;25(9):1750–1755. | ||
Calabro L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14(11):1104–1111. | ||
Calabro L, Morra A, Fonsatti E, et al. Efficacy and safety of an intensified schedule of tremelimumab for chemotherapy-resistant malignant mesothelioma: an open-label, single-arm, phase 2 study. Lancet Respir Med. 2015;3(4):301–309. | ||
Guazzelli A, Hussain M, Kristic-Demonacos M, Mutti L. Tremelimumab for the treatment of malignant mesothelioma. Expert Opin Biol Ther. 2015;15(12):1819–1829. | ||
Ascierto PA. Is there still a role for tremelimumab in the treatment of cancer? Transl Cancer Res. 2013;2(1):48–50. | ||
Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. | ||
Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15(23):7116–7118. | ||
Shimomura A, Fujiwara Y, Kondo S, et al. Tremelimumab-associated tumor regression following after initial progression: two case reports. Immunotherapy. 2016;8(1):9–15. | ||
Hales RK, Banchereau J, Ribas A, et al. Assessing oncologic benefit in clinical trials of immunotherapy agents. Ann Oncol. 2010;21(10):1944–1951. | ||
Eroglu Z, Kim DW, Wang X, et al. Long term survival with cytotoxic T lymphocyte-associated antigen 4 blockade using tremelimumab. Eur J Cancer. 2015;51(17):2689–2697. | ||
Reuben JM, Lee BN, Li C, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106(11):2437–2444. | ||
Comin-Anduix B, Lee Y, Jalil J, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med. 2008;6:22. | ||
Ribas A, Comin-Anduix B, Economou JS, et al. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15(1):390–399. | ||
Comin-Anduix B, Sazegar H, Chodon T, et al. Modulation of cell signaling networks after CTLA4 blockade in patients with metastatic melanoma. PLoS One. 2010;5(9):e12711. | ||
Robert L, Harview C, Emerson R, et al. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology. 2014;3:e29244. | ||
Ibarrondo FJ, Yang OO, Chodon T, et al. Natural killer T cells in advanced melanoma patients treated with tremelimumab. PLoS One. 2013;8(10):e76829. | ||
Trzonkowski P, Bacchetta R, Battaglia M, et al. Hurdles in therapy with regulatory T cells. Sci Transl Med. 2015;7(304):304s318. | ||
Emoto M, Zerrahn J, Miyamoto M, Perarnau B, Kaufmann SH. Phenotypic characterization of CD8(+)NKT cells. Eur J Immunol. 2000;30(8):2300–2311. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.