Back to Journals » Biologics: Targets and Therapy » Volume 17
Treatment Persistence of Apremilast Among Patients with Psoriatic Arthritis
Authors Haddad A, Stein N, Lavi I, Shynkar L, Bergman I, Feldhamer I, Cohen AD, Saliba W, Zisman D
Received 4 July 2023
Accepted for publication 27 September 2023
Published 4 October 2023 Volume 2023:17 Pages 129—136
DOI https://doi.org/10.2147/BTT.S425693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Shein-Chung Chow
Amir Haddad,1 Nili Stein,2 Idit Lavi,2 Lisa Shynkar,3 Irina Bergman,3,4 Ilan Feldhamer,5 Arnon Dov Cohen,5,6 Walid Saliba,2,4 Devy Zisman1,4
1Rheumatology Unit, Carmel Medical Center, Haifa, Israel; 2Department of Epidemiology, Clalit Health Services, Haifa, Israel; 3Internal Medicine Department, Carmel Medical Centre, Haifa, Israel; 4Ruth and Bruce Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel; 5Chief Physician’s Office, Central Headquarters, Clalit Health Services, Tel Aviv, Israel; 6Siaal Research Center for Family Medicine and Primary Care, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheba, Israel
Correspondence: Amir Haddad, Rheumatology Unit, Carmel Medical Centre, 7 Michal Street, Haifa, Israel, Tel +972-4-8250486, Email [email protected]; [email protected]
Introduction: Persistence in drug therapy reflects treatment effectiveness and tolerability. We aim to estimate the persistence of apremilast prescribed to patients with psoriatic arthritis (PsA) and to identify characteristics associated with treatment discontinuation in a real-world setting.
Methods: Patients with PsA treated with apremilast from January 2016 were identified from a large health database and followed until medication stop date (using 3-months grace period), death or the end of observation period (June 2021). Demographic data, Charlson comorbidity index and concomitant and previous use of conventional and biologic DMARDs were extracted. The reasons for drug discontinuation were manually retrieved from patient charts. Time to discontinuation was estimated using survival analysis using Kaplan–Meier functions.
Results: Overall, 568 PsA patients treated with apremilast were identified. The mean age was 55.3± 14.0 years, of whom 332 (58.5%) were females, 38.4% were obese (BMI> 30), 75.2% had a Charlson comorbidity index> 1, 24.1% were on concomitant treatment with methotrexate and 72.4% were biologic naïve. The median persistent period was 6.1,95% CI (5.2– 6.9) months in which only 16.9% remained persistent on apremilast. No difference was found with regard to age, sex, socioeconomic status, ethnicity and obesity between patients who were persistent compared to patients who discontinued apremilast. Concomitant treatment with methotrexate and prior history of biologic therapy did not affect drug persistency (log rank P=0.957 and 0.082, respectively). Causes for treatment discontinuation were due to lack of skin efficacy in 19.4%, lack of joint efficacy in 33.3%, combined skin and joint inefficacy at 2.3% and due to side effects in 24.1%.
Conclusion: In this large observational retrospective cohort of patients treated with apremilast, a relatively low drug persistence was observed with 6-month and 1-year survival rates of 50.3% and 31.3%, respectively. Treatment discontinuation was mainly due to joint inefficacy, advocating for more studies for proper patient selection to assure treatment effectiveness and persistency.
Plain language summery: This research aims to explore the role of apremilast in the real-world treatment landscape of Psoriatic Arthritis (PsA). The existing literature offers limited and inconclusive data regarding the persistence of apremilast in real-world settings. The outcomes of this investigation hold the potential to enhance patient selection criteria and establish treatment efficacy. Our study revealed that within this extensive observational cohort, apremilast exhibited relatively low levels of drug persistence. Discontinuation of treatment primarily stemmed from joint inefficacy, side effects, and inadequate improvement in skin symptoms. Notably, patient demographics, socioeconomic status, obesity, and the Charlson comorbidity index displayed no significant influence on treatment discontinuation. Intriguingly, concurrent use of methotrexate and prior utilization of biologics did not impact drug persistence either. These findings emphasize the need for further research aimed at refining patient selection protocols to ensure both treatment efficacy and prolonged persistence.
Keywords: psoriatic arthritis, drug persistence, therapy, apremilast
Background
Persistence in drug therapy represents real-world treatment effectiveness and tolerability. Psoriatic arthritis (PsA) is a chronic immune mediated disease requiring long-term treatment. As there are now more PsA treatment options available on the market, it is becoming more crucial to describe treatment persistence from actual experience because it helps clinicians make more informed decisions about the best therapeutic options for PsA patients in order to maximize symptom remission, promote functional recovery and lower health-care costs.
Apremilast, an oral phosphodiesterase 4 inhibitor, is licensed to treat moderate-to-severe plaque psoriasis in adults who are not candidates for phototherapy or systemic therapy. It is also indicated for adult patients who have active PsA.1 By inhibiting phosphodiesterase 4, apremilast effectively reduces the production of multiple cytokines involved in the pathogenesis of psoriatic disease. This includes TNFα, IL-23, and interferon-γ. Unlike biologic agents that focus on targeting a single inflammatory mediator, apremilast acts on a broader spectrum of cytokines.2–4
In Phase 3 clinical trials, apremilast has exhibited effectiveness and a satisfactory safety profile when used to treat patients with active PsA. These trials have shown significant improvements in PsA disease severity and physical function, which are considered clinically meaningful5,6.
Data from real-world on treatment effectiveness and tolerability for apremilast has been reported in psoriasis but in psoriatic arthritis, it is relatively limited. The purpose of this study was to evaluate treatment persistence in a real-world setting in PsA patients receiving apremilast, as well as to identify causes and factors related to treatment discontinuation in a real-world setting.
Methods
Study Dataset
Clalit Health Services (CHS), the largest health-care provider in Israel, serves approximately 4.4 million members, which accounts for 52% of the country’s population. CHS maintains a comprehensive database that continuously receives real-time data from pharmaceutical, medical, and administrative digital systems (IF, AC).7 While primarily designed for administrative and clinical management purposes, it is also available for clinical research. The register is known for its high quality and accuracy. Another study evaluating the reliability of PsA diagnoses8 found a positive predictive value of 90.5% and a sensitivity and specificity of 88.7% and 88.1%, respectively, further affirming its credibility.
Study Population and Design
All patients diagnosed with PsA and aged 18 or above, who received prescriptions for apremilast as indicated for PsA under the Israeli National Healthcare Drug Plan, were retrospectively identified for the period of January 1st, 2016, until June 30th, 2021. They were followed until the medication stop date, death, or the end of the observation period. Under the Israeli National Healthcare Drug Plan, treatment with apremilast was approved in 2016 and indicated following the failure of two conventional disease-modifying anti-rheumatic drugs (cDMARDs).
Study Outcome
Patients were considered non-persistent if a 90-day interval run out of the original apremilast prescription refilling was exceeded, to prevent classifying short absences of treatment due to reasons such as infection or surgery as non-persistence. The 3-month time interval was chosen (AH, DZ, WS) after analyzing the data to allow for a reasonable grace period for medication refills, as a shorter grace period of 1 or 2 months had resulted in a higher misclassification rate of non-persistence.
Study Variables
Demographic data, including age, sex, ethnicity (Jewish or Arab), smoking history (current or past smoking) and socioeconomic status (SES) at the start of the study, were collected. SES was determined based on the CHS categories of low, medium, and high, which have shown a strong correlation with SES assigned by the Israel Central Bureau of Statistics.9 The patients’ BMI and the Charlson comorbidity index were calculated from the CHS database. Information regarding the concurrent use of glucocorticosteroids (GC) and cDMARDs such as methotrexate (MTX), leflunomide (LEF), sulfasalazine (SSZ) and hydroxychloroquine (HCQ) was also extracted from the database. History of previous biologic DMARDs was also recorded. At the time of the study, eight biologic agents were approved and available on the Israeli market for PsA, including adalimumab, etanercept, infliximab, golimumab, certolizumab, ustekinumab secukinumab and ixekizumab.
Treatment changes were made based on both physician and patient decisions and preferences. Causes of treatment discontinuation for apremilast were manually retrieved by reviewing each patient’s medical record in the database (LS, IB and AH).
Statistical Analysis
Descriptive statistics, such as means (standard deviations) for continuous variables and frequencies (%) for categorical variables, were utilized. Baseline characteristics between persistent and non-persistent patients were compared using the chi-square test for categorical variables and the independent samples t-test for continuous variables (IL, NS, IF).
Time to discontinuation was estimated using Kaplan–Meier curves and compared among patient subgroups, including those who were biologic-naïve or failures and those who received either apremilast monotherapy or concomitant treatment with methotrexate, using the Log rank test(NS).
All statistical analyses were performed using IBM SPSS Statistics 28.0 (IBM, New York, NY). A significance level of P < 0.05 for the 2-tailed tests was considered statistically significant.
Study Ethics
The study was reviewed and approved by the Institutional Review Board of Carmel Medical Center, Haifa (CMC0014-14). The data accessed complied with the relevant data protection and privacy regulations.
Results
Overall, 568 PsA patients treated with apremilast were identified. The mean age of the study population was 55.3±14.0 years, of whom 332 (58.5%) were females, 38.4% were obese (BMI>30), 75.2% had a Charlson comorbidity index>1, 24.1% were on concomitant treatment with methotrexate, and 72.4% were biologic naïve. Socioeconomic status was estimated as low, medium and high at 29.8%, 43.0% and 27.2%, respectively.
The median persistent period (grace period 90 days) was 6.1, 95% CI (5.2–6.9) months in which only 16.9% remained persistent on apremilast as is shown in Figure 1.
 |
Figure 1 Kaplan-Meier analysis of apremilast persistence in patients with psoriatic arthritis. |
Concomitant treatment with methotrexate and prior history of biologic therapy did not affect drug persistency (log rank P=0.957 and log rank P=0.082, respectively) as shown in the survival curves in Figures 2 and 3.
 |
Figure 2 Kaplan-Meier analysis of apremilast persistence in patients with monotherapy and on concomitant methotrexate therapy. |
 |
Figure 3 Kaplan-Meier analysis of apremilast persistence in biologic-naïve patients and with prior history of biologic therapy. |
Causes for treatment discontinuation were due to lack of skin efficacy in 19.4%, lack of joint efficacy in 33.3%, combined skin and joint inefficacy at 2.3%, side effects in 23.9%, other reasons in 12.4% as is shown and detailed in Table 1.
 |
Table 1 Summary of Studies in the Literature |
 |
Table 2 Causes for Drug Discontinuation |
We investigate for factors associated with treatment discontinuation as shown in Table 2. No difference was found with regard to age or sex. Socioeconomic status, ethnicity, obesity, and Charlson score above 1 among patients who were persistent compared to patients who discontinued apremilast.
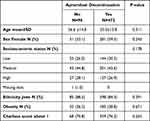 |
Table 3 Factors Associated with Treatment Discontinuation |
Discussion
This study utilized a vast and inclusive national dataset comprising a diverse population of PsA patients in Israel, who were treated with apremilast from 2016 until mid of 2021.
Using the 90 days grace period, we report a relatively low drug persistence with 6-month and 1-year survival rates of 50.3% and 31.3%, respectively. Studies on drug survival of apremilast in psoriatic disease are limited. Reviewing the literature on patients with psoriasis. Graier et al10 have reported an overall drug survival rate at 12 months at 57.3%, and the median survival was 15.7 months on 367 patients with psoriasis. In this study, concomitant psoriatic arthritis (n=89) was not associated with an increased risk of drug discontinuation. Similar 12-months survival rates were reported in retrospective observational studies from Spain (54.9%)11 and Japan (53.4%)12 on patients with psoriasis. Spidian et al13 reported a 1-year discontinuation rate for apremilast at 69% based on a French insurance claims database of 14,147 non-selected patients with psoriasis. A study from the Slovenian psoriasis registry has reported a 12-months survival rate of only 20.0%.14
Of note, a very lower 12-month survival rate of 2.1% for apremilast was detected in insurance claims database from the United States.15
In addition, reviewing literature on patients with PsA, we have found two database/registry-based studies and three cohort-based studies as summarized in Table 3. In a recently published study from the Finnish registry in 2023 on 318 patients with PsA treated with apremilast, the median time to apremilast discontinuation was higher for patients with psoriasis than for those with PsA (14 vs 11 months) using a grace period of 6 months for defining drug discontinuation, 42% (134/318) of those with PsA remained on apremilast until the end of the observation period. The median follow-up time of apremilast use was 18 months16 compared to our 57.5% survival rate at 12 months.
Moreover, in another study on 381 biologic-naïve PsA patients from the IBM MarketScan® Commercial and Medicare database, the reported persistence rate for patients initiating apremilast was 42.5% at 12 months post-index and was similar to patients initiating biologics [42.5% vs 48.0%]; [p = 0.082]20.
In another Italian retrospective study on 131 PsA patients reported a higher 6-months retention rate of 72.1% with Inefficacy (n=7), diarrhea (n=10), nausea (n=3) and headache (n=7) were the most frequent reasons for discontinuation.17
Another observational non-interventional 52-week prospective study on 167 consecutive PsA patients with early disease, who were biologic naïve treated with apremilast reported a higher 52-week drug survival rate of 75%18. Moreover, in another study on 62 PsA patients has reported an even higher retention rate on apremilast of 88% after 6 months and 73% after 1 year; however, it was attributed to a selection bias.19
When viewing the results of our study in contrast to other apremilast studies, differences in the design, study population, definition of drug persistency and analytical methods should be taken into consideration. Nonetheless, our study provides valuable real-world data on a large PsA diverse patient population, from different sites in Israel that could possibly have reinforced the generalizability of the results across diverse health-care settings as is summarized in Table 3.
Overall, the reported persistency of apremilast in the other observational studies in the literature was higher than the reported rate in our study as is shown in Table 3. However, if we consider the ACR20 response rates in the pivotal trials of apremilast, the palace 1 and 2 trials,5,6 that was at only 40% in 52 weeks, as well as the high discontinuation rates reported in these trials that was about 10%, we could imply and conclude that data presented in our study, which is also the largest real-world study available so far, are more representative on the persistence of apremilast in the real world.
Our study indicates that apremilast drug survival was not influenced by the patients’ demographics, socioeconomic status, obesity or the Charlson comorbidity index. Unfortunately, the small size group did not allow for any age group stratification. Moreover, prior biologic therapy and the concomitant use of methotrexate did not have a significant impact on persistency.
Of note, a previous study on our cohort of biologics in PsA21 identified female sex as an independent risk factor for treatment discontinuation; however, this was not the case for apremilast in this study.
Treatment discontinuation was due to joint inefficacy in about 33% of patients, whereas skin efficacy was in only about 22% of patients. These differences could have been confounded by the indication for treatment in each patient. Interestingly, about 24% of our non-persistent patients had discontinued the medication due to side effects, and the most commonly reported side effect was diarrhea as is known with the treatment of apremilast. Reviewing the apremilast clinical trials data, diarrhea, headache, nausea, upper respiratory tract infection, nasopharyngitis and vomiting were the most reported adverse events. Incidents of diarrhea and nausea were generally highest during the first 2 weeks of dosing, occurring in up to 20% percent of patients, with the majority resolving within 1 month despite continuing therapy and no medical intervention. Laboratory abnormalities were infrequent and transient. The discontinuation rates because of adverse events (weeks 0 to 52) were <10%. However, in our study, the reported rate of discontinuation was higher (24%) even though all of the patients were started on an escalating dose of apremilast. Moreover, the fact that the 1-month survival rate of apremilast is around 81.2%, as is shown in the Kaplan–Meier curve in Figure 1, and the lower persistence rates observed on using grace periods of less than 90 days, implies that the discontinuation was mainly driven by drug side effects as usually we would allow for more than 1-month time to assess for treatment effectiveness before announcing drug failure.
There are some limitations in our study. Caution should be exercised when interpreting our results since treatment indications and discontinuation are not guided by a specific protocol but rather based on the choices and preferences of patients and physicians, as well as clinical impressions of the physicians. Additionally, we lacked information on the extent of disease activity parameters in the joints and skin at baseline and upon discontinuation, which could have provided further insights into disease severity and patient selection for apremilast treatment.
However, our study has a most notable strength, which is the use of a large dataset of PsA patients. This dataset provides the largest real-world investigation on the use of apremilast in PsA published to date, providing an accurate representation of drug treatment patterns and persistence in real-life circumstances.
To summarize, our observational analysis of a sizable PsA cohort demonstrated a rather low persistence rate for apremilast in rheumatology practices. More research is needed to clarify the role of apremilast in the treatment of PsA, find acceptable patient selection criteria to assure treatment success and persistence, improve treatment planning, and more efficiently spend societal economic resources.
Acknowledgments
We would like to acknowledge the valuable contribution of Mrs Idit Lavi in the data analysis for this study. Unfortunately, Mrs Lavi passed away prior to the publication of this work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Otezla (apremilast) [package insert]. Summit: Celgene Corporation; 2019. Available from: https://media2.celgene.com/content/uploads/otezla-pi.pdf.
2. Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83(12):1583–1590. doi:10.1016/j.bcp.2012.01.001
3. Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40(8):495–500. doi:10.1186/1477-7525-11-82
4. Coates LC, FitzGerald O, at Al HPS. Psoriasis, psoriatic arthritis, and rheumatoid arthritis: is all inflammation the same? Semin Arthritis Rheum. Seminars in Arthritis and Rheumatism. 2016;46:291–304. doi:10.1016/j.semarthrit.2016.05.012
5. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomized, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020–1026. doi:10.1136/annrheumdis-2013-205056
6. Cutolo M, Myerson GE, Fleischmann R, et al. A Phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43(9):1724–1734. doi:10.3899/jrheum.151376
7. Vinker S, Fogelman Y, Elhayany A, et al. Usefulness of electronic databases for the detection of unrecognized diabetic patients. Cardiovasc Diabetol. 2003;2(1):13. doi:10.1186/1475-2840-2-13
8. Eder L, Cohen AD, Feldhamer I, et al. The epidemiology of psoriatic arthritis in Israel - a population-based study. Arthritis Res Ther. 2018;20(1):3. doi:10.1186/s13075-017-1497-4
9. Filc D, Davidovich N, Novack L, et al. Is socioeconomic status associated with utilization of health care services in a single-payer universal health care system? Int J Equity Health. 2014;13(1):115. doi:10.1186/s12939-014-0115-1
10. Graier T, Weger W, Sator PG, et al. Effectiveness and clinical predictors of drug survival in psoriasis patients receiving apremilast: a registry analysis. JAAD Int. 2021;2:62–75. doi:10.1016/j.jdin.2020.10.012
11. Del Alcazar E, Suarez-Perez JA, Armesto S, et al. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicenter study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34(12):2821–2829. doi:10.1111/jdv.16439
12. Kishimoto M, Komine M, Kamiya K, et al. Drug survival of apremilast in a real-world setting. J Dermatol. 2019;46(7):615–617. doi:10.1111/1346-8138
13. Sbidian E, Billionnet C, Weill A, et al. Persistence of apremilast in moderate-to-severe psoriasis: a real-world analysis of 14147 apremilast- and methotrexate-naive patients in the French National Health Insurance database. Br J Dermatol. 2020;182(3):690–697. doi:10.1111/bjd.18047
14. Lunder T, Zorko MS, Kolar NK, et al. Drug survival of biological therapy is showing class effect: updated results from Slovenian National Registry of psoriasis. Int J Dermatol. 2019;58(6):631–641. doi:10.1111/ijd.14429
15. Wu B, Muser E, Teeple A, et al. Treatment adherence and persistence of five commonly prescribed medications for moderate to severe psoriasis in a U.S. commercially insured population. J Dermatolog Treat. 2020:1–8. doi:10.1080/09546634.2019.1687828
16. Koskivirta I, Ruotsalainen J, Kurki S, et al. A Real-world registry-based study on apremilast use in psoriasis and psoriatic arthritis in Finland. Scand J Rheumatol. 2023;16:1–7. doi:10.1080/03009742.2022.2151109
17. Favalli EG, Conti F, Selmi C, et al. Retrospective evaluation of patient profiling and effectiveness of apremilast in an Italian multicentric cohort of psoriatic arthritis patients. Clin Exp Rheumatol. 2020;38(1):19–26.
18. Sfikakis PP, Vassilopoulos D, Katsifis G, et al. Apremilast for biologic-naïve, peripheral psoriatic arthritis, including patients with early disease: results from the APROACH observational prospective study. Rheumatol Int. 2023;43(5):889–902. doi:10.1007/s00296-022-05269-z
19. Foti R, Visalli E, Amato G, et al. Drug Survival of Apremilast for Psoriatic Arthritis in a Real-World Setting: a Single-Center Experience. J Rheumatol. 2023;50(3):458–459. doi:10.3899/jrheum.220333
20. Feldman SR, Pelletier CL, Wilson KL, et al. Treatment patterns and costs among biologic-naive patients initiating apremilast or biologics for psoriatic arthritis. J Comp Eff Res. 2019;8(9):699–709. doi:10.2217/cer-2019-0034
21. Haddad A, Gazitt T, Feldhamer I, et al. Treatment persistence of biologics among patients with psoriatic arthritis. Arthritis Res Ther. 2021;23(1):44. doi:10.1186/s13075-021-02417-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
