Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Treatment Outcomes in Patients with Advanced Fibrolamellar Hepatocellular Carcinoma Under Systemic Treatment: Analysis of Clinical Characteristics, Management, and Radiomics
Authors Da Fonseca LG , Yamamoto VJ , Trinconi Cunha M , Torre GS , Araujo RL , Fonseca GM , Chen ATC, Chagas AL, Herman P, Alves VAF, Carrilho FJ
Received 31 July 2023
Accepted for publication 24 October 2023
Published 1 November 2023 Volume 2023:10 Pages 1923—1933
DOI https://doi.org/10.2147/JHC.S428741
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Leonardo G Da Fonseca,1,* Victor Junji Yamamoto,1,* Mateus Trinconi Cunha,1 Giovanna Sawaya Torre,2 Raphael LC Araujo,3 Gilton Marques Fonseca,4 Andre Tsin Chih Chen,5 Aline Lopes Chagas,6 Paulo Herman,4 Venancio Avancini Ferreira Alves,7 Flair Jose Carrilho6
1Department of Medical Oncology, ICESP - Instituto do Cancer DO Estado de Sao Paulo, Hospital das Clinicas, University of São Paulo School of Medicine, São Paulo, Brazil; 2Department of Radiology, ICESP - Instituto do Cancer do Estado de Sao Paulo, Hospital das Clinicas, University of São Paulo School of Medicine, São Paulo, Brazil; 3Digestive Surgery Division, Department of Surgery, Universidade Federal de São Paulo, São Paulo, Brazil; 4Digestive Surgery Division, Department of Gastroenterology, Hospital das Clinicas, University of São Paulo School of Medicine, São Paulo, Brazil; 5Radiation Oncology Department - Instituto do Cancer do Estado de Sao Paulo, Hospital das Clinicas, University of São Paulo School of Medicine, São Paulo, Brazil; 6Division of Clinical Gastroenterology and Hepatology, Department of Gastroenterology, Hospital das Clinicas, University of São Paulo School of Medicine, São Paulo, Brazil; 7Department of Pathology, LIM-14, Hospital das Clinicas, University of São Paulo School of Medicine, São Paulo, Brazil
*These authors contributed equally to this work
Correspondence: Leonardo G Da Fonseca, Department of Medical Oncology, ICESP - Instituto do Cancer do Estado de Sao Paulo, Hospital das Clinicas, University of São Paulo School of Medicine, Avenida Dr Arnaldo, 251, 5th Floor ZIP: 01246-000, São Paulo, Brazil, Tel/Fax +55 11 3893-2000, Email [email protected]
Purpose: Fibrolamellar hepatocellular carcinoma (FLHCC) is a rare primary liver malignancy often diagnosed at advanced stages. While there are limited data on the efficacy of specific agents, we aim to report outcomes of patients treated with systemic therapies and explore prognostic factors.
Patients and Methods: Medical records of patients treated between 2010 and 2022 were reviewed. Treatments were defined after multidisciplinary assessment. Descriptive statistics were used for baseline demographics. Time-to-event outcomes were estimated using the Kaplan–Meier method, compared by log-rank and adjusted by a regression model. Radiomic features (including size, shape, and texture) of the primary lesion were extracted and dimensionality reduced. An unsupervised Gaussian Mixture Model (GMM) clustering was performed, and survival was compared between clusters.
Results: We identified 23 patients: 12 males, with a median age of 23.6 years. At diagnosis, 82.6% had metastases, most frequently to the lungs (39.1%), lymph nodes (39.1%), and peritoneum (21.7%). Patients received a median of three lines (1– 8) of treatment, including different regimens. Sorafenib (39.1%), capecitabine (30.4%), and capecitabine/interferon (13%) were the most used first-line regimens. The median time-to-failure was 3.8 months (95% CI: 3.2– 8.7). Capecitabine + interferon (42.1%) and platinum combinations (39.1%) were the most used second-line regimens, with a time-to-failure of 3.5 months (95% CI: 1.5– 11.6). Median overall survival was 26.7 months (95% CI: 15.1– 40.4). A high baseline neutrophil-to-lymphocyte ratio (NLR) was associated with worse survival (p=0.02). Radiomic features identified three clusters, with one cluster (n=6) having better survival (40.4 vs 22.6 months, p=0.039). Tumor sphericity in the arterial phase was the most relevant characteristic associated with a better prognosis (accuracy=0.93).
Conclusion: FLHCC has unique features compared to conventional HCC, including young onset, gender balance, and absence of hepatopathy. Systemic therapies can provide encouraging survival, but lack of uniformity precludes defining a preferable regimen. Radiomics and NLR were suggested to correlate with prognosis and warrant further validation.
Keywords: liver cancer, systemic treatment, radiomics, fibrolamellar carcinoma, rare tumors
Introduction
Fibrolamellar hepatocellular carcinoma (FLHCC) is a rare liver malignancy that was first identified in 1956.1 FLHCC is recognized as a separate entity rather than a variant of hepatocellular carcinoma, although it is defined as a subtype of hepatocellular carcinoma according to the fifth edition of the World Health Organization (WHO) Classification of Tumours.2 This type of tumour often presents in the absence of cirrhosis or chronic hepatopathy and is mostly diagnosed in pediatric and young adult population.3,4 The fusion kinase DNAJB1-PRKACA is presumed to be an oncogenic driver of FLHCC. DNAJB1 encodes Hsp40 and PRKCA encodes a protein kinase A subunit, which regulates cell proliferation, differentiation, and apoptosis. This fusion is detectable in virtually all cases of FLHCC.5
Because the occurrence of FLHCC concentrates in an otherwise healthy population, clinical suspicion is low, and diagnosis is frequently based on tumor-related symptoms. Liver resection and transplantation are potentially curative modalities for patients diagnosed with localized disease and are associated with favorable prognosis.6–8 Nevertheless, around 50% of the cases are diagnosed at a late stage, when no curative modality is feasible. In these cases, systemic therapies are commonly indicated, but the prognosis is limited.9
Prospective clinical trials enrolling patients with FLHCC are scarce mainly due to its low incidence. Therefore, there is no standard systemic treatment, and management is extrapolated from other liver malignancies and case series. Targeted therapies10,11 and immunotherapy12 have shown modest activity, whilst retrospective cohort studies suggest divergent results with the use of chemotherapy-based regimens.13–16 Radiologic response rates are generally low, but some patients may experience long-term disease control, which highlights the need for tools to identify prognostic subgroups. Thus, in addition to clinical and laboratory variables, there is also a growing interest in exploring imaging resources such as radiomics. Radiomics explores a variety of quantitative characteristics from medical images that can be modeled by artificial intelligence algorithms to make predictions and may serve as a promising tool for treatment decisions.17,18
Due to the lack of robust evidence to guide the management of advanced FLHCC, it is relevant to report outcomes in relation to therapeutic regimens, sequences, radiological features, and prognostic factors. Therefore, the aim of the present study was to report baseline characteristics and management in a cohort of patients with advanced FLHCC.
Materials and Methods
Patients
A database of patients with primary liver malignancies consecutively treated at our institution from July 2010 to December 2022 was retrospectively analyzed. We selected adult patients with confirmed diagnosis of advanced FLHCC who were referred for systemic treatment due to unresectable or metastatic disease (de novo or recurrent disease after curative-intent treatments). We obtained approval from the institutional Ethics Committee (Hospital das Clinicas, University of Sao Paulo School of Medicine Ethics Committee – Comissão de Ética para Análise de Projetos de Pesquisa: Cappesq – Report: 3807496). Written informed consent was waived due to retrospective design and the fact that a significant proportion of patients was no longer under follow-up (death or lost to follow-up). The data was anonymized and maintained confidential to guarantee the privacy of the participants. The study complies with the Declaration of Helsinki.
Demographics, comorbidities, performance status according to the Eastern Cooperative Oncology group classification (ECOG PS), family history, clinical stage, laboratory parameters, treatment outcomes, and death were collected from medical records. Data were last updated on 14th-February 2023.
Treatment and Assessment
The treatments were conducted through a multimodal approach involving clinical oncologists, surgeons, hepatologists, interventional radiologists, radiation oncologists, and pathologists. Patients who were considered candidates to systemic therapy received one of the following regimens: 1) Sorafenib 800 mg daily; 2) oral hormone therapy with megestrol 160 mg daily, tamoxifen 20 mg daily or anastrozole 1 mg daily; 3) doxorubicin 75 mg/m2 every 21 days 4) 5-fluorouracil (5-FU) 370 mg/m2 plus leucovorin (LV) 50 mg/m2 every 7 days; 5) oxaliplatin 85 mg/m2 at weeks 1, 3 and 5, plus 5-FU 500 mg/m2 with LV 20 mg/m at weeks 1, 2, 3, 4, 5 and 6 (mFLOX regimen); 6) gemcitabine 1000 mg/m2 on days 1, 8 and 15, then every 28 days; 7) oxaliplatin 130 mg/m2 on day 1 plus capecitabine 2000 mg/m2/day on days 1–14, every 21 days (CapOx); 8) gemcitabine 1000 mg/m2 and oxaliplatin 85 mg/m2 every 14 days (GemOx); 9) capecitabine 2500 mg/m2/day on days 1–14, every 3 weeks alone or plus interferon (IFN)-alpha 3 million U three times a week; 10) cisplatin 60–80 mg/m2 every 21 days monotherapy or associated with capecitabine 2000 mg/m2 /day on days 1–14, every 21 days; 11) doxorubicin 25 mg/m2 on days 1–3 plus cisplatin 100 mg/m2 on day 1, then every 21 days. Dose adjustments were made for the management of adverse events if necessary. The choice of regimens and sequencing was defined at the discretion of the treating physicians based on individual clinical evaluation. Treatments were continued until tumor progression, limiting toxicities, or death. The follow-up comprehended physical examination, laboratory analysis before every treatment cycle. Images (computed tomography – CT or magnetic resonance – MR) were performed bimonthly, and evaluation was made on the basis of the Response Evaluation Criteria in Solid Tumors version 1.1.19
Radiomics
For radiomics analysis, only patients with a measurable primary dominant liver tumor in the baseline contrast-enhanced computed tomography were included. For patients who underwent resection prior to systemic treatment, we used baseline imaging before liver resection. In patients who received initial systemic treatment, baseline images were considered as the scans performed before the start of systemic treatment. Patients who have performed other imaging modalities at baseline (eg, MR or non-contrast-enhanced images) were not included in this analysis.
Digital Imaging and Communication in Medicine (DICOM) files from arterial and portal phases were extracted and anonymized, and the segmentation of the dominant liver tumor was made by a certified radiologist for both phases. The segmentation was performed using 3D Slicer (slicer.org) in a semi-automated fashion, using thresholding to define tumor boundaries with posterior manual correction.
A total of 111 radiomic features were extracted from the arterial and portal phases using PyRadiomics® software.20 Features categories included size (eg, area, volume, axis lengths, maximum 3D diameter, etc…), shape (eg, elongation, sphericity, flatness, etc.), and texture (eg, gray level matrices). A delta radiomics analysis was also performed, with a simple subtraction of arterial minus portal features.
We embedded the radiomics features in two dimensions using Uniform Manifold Approximation and Projection (UMAP) and defined the optimal quantification of clusters using the Silhouette Score with a Gaussian Mixture Model (GMM).21
Finally, we trained XGBoost,22 a gradient boosting decision tree machine learning model, in a fivefold cross validation in the entire dataset to classify each patient into the cluster assigned by the GMM. After measuring the model’s accuracy, we extracted the features with the highest importance for the model. The survival outcomes of the clusters were expressed as median survival from systemic treatment initiation.
Statistical Analysis
The overall survival (OS) was measured from the initiation of the first line systemic treatment until death. Time to treatment failure was calculated from initiation of treatment to disease progression defined by imaging or clinical interpretation or limiting toxicity requiring treatment withdrawal. Time to events were estimated using the Kaplan–Meier method and were expressed in terms of median and 95% confidence interval (CI). Survival times were compared using the Log rank test, and Hazard ratios (HR) were calculated from a Cox regression model including the variables with p<0.010 in the univariate analysis. A p value < 0.05 was considered statistically significant in the regression model. Data were analyzed with the Stata software version 15.1 (StataCorp, College Station, Texas).
Results
Baseline Features
A total of 446 patients with primary liver tumors were screened. Twenty-eight patients had a confirmed diagnosis of FLHCC, but five patients did not receive systemic therapy and were not included (two were treated with resection, one was treated with resection plus ablation, and two were managed with palliative supportive care). Finally, 23 patients were treated with systemic treatment and were enrolled in the analysis. The median age was 23.6 years (Interquartile range [IQR]: 21.8–28.6), 12 (52.2%) patients were male, and 20 (86.9%) had ECOG-PS 0. Only 1 (4.3%) patient had cirrhosis caused by hepatitis B virus and 18 (78.3%) had no comorbidities. The most frequent comorbidities were dyslipidemia (n=2; 8.7%) and hypothyroidism (n=2; 8.7%). At the time of systemic treatment indication, most of the patients had metastatic disease (n=19, 82.6%), and the most frequent sites were lungs (n=9; 39.1%), and lymph nodes (n=9; 39.1%). Additionally, 7 (30.4%) patients had macrovascular invasion. Regarding previous treatments before systemic treatment, 13 (56.5%) patients had been submitted for surgical resection, 2 (8.7%) patients to surgery followed by percutaneous ablation, and 1 (4.3%) patient had received surgery and stereotactic body radiotherapy (SBRT). Seven (30.4%) patients received systemic treatment as the first treatment. None received systemic treatment as adjuvant treatment after surgery. Only one patient had raised alpha-fetoprotein (33.2 ng/mL, reference value: <10 ng/mL), while the median alpha-fetoprotein was 2.9 ng/mL (IQR: 1.1–5.2). Baseline characteristics are detailed in Table 1.
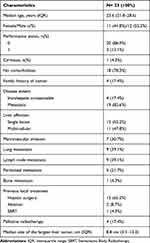 |
Table 1 Baseline Characteristics of the Patients Included in the Analysis |
Treatment Responses
Patient received a median of three lines of systemic treatment (range: 1–8) and the median time under systemic treatment was 18.4 months (IQR: 12.1–37.1). The most commonly used first-line therapy was sorafenib (n=9; 39.1%), followed by capecitabine (n=7; 30.4%), and IFN plus capecitabine (n=3; 13%), with a median of 3.8 months (95% CI: 3.2–8.7) to first-line progression. Three patients discontinued the first line due to limiting toxicities and 19 patients due to tumor progression, while 1 patient was still under treatment at the last follow-up. Nineteen patients received second line treatment, mostly capecitabine+ IFN (n=8; 42.1%), mFLOX (n=3; 15.8%) and GemOx (n=3; 15.8%), with a median of 3.5 months (95% CI: 1.5–11.6) to progression. Six patients discontinued the second-line due to adverse events and 11 patients because of disease progression, while 2 patients were under treatment at the last follow-up. Twelve patients received third-line treatment, with a median time to disease progression of 2.5 months (95% CI: 1–22). Five (21.7%) patients received conventional transarterial chemoembolization (TACE) using doxorubicin after systemic treatment initiation, 1 patient (4.3%) received SBRT aiming at local disease control and 4 (17.4%) patients received palliative radiotherapy.
Treatment, radiological responses, and time-to-treatment failure are highlighted in Table 2 and Figure 1.
 |
Table 2 Response Assessment and Time to Treatment Failure in the First, Second, and Third Lines |
 |
Figure 1 Plot illustration representing the duration of each line of systemic treatment and systemic treatment free interval individually. |
Survival and Prognostic Markers
At the last update, 6 (26.1%) were alive, 16 (69.6%) died, and 1 (4.3%) was lost to follow-up. The median follow-up from the diagnosis was 27.9 months (IQR: 22.0–41.4) and 21.4 months (IQR: 13.1–39.1) from the first-line systemic treatment. The median OS was 31.4 months (95% CI: 23.2–41.4) from the diagnosis and 26.7 months (95% CI: 15.1–40.4 months) from the first-line systemic treatment. The survival rates were 86.5% (95% CI: 63.7–95.5) at 12 months, 67.3% (95% CI: 43.3–82.9) at 18 months, and 51.5% (95% CI: 28.4–70.1) at 24 months.
Subgroup analyses were restricted by the small sample size. The presence of peritoneal metastases showed a non-significant trend towards worse survival, with a median OS of 19.8 months (95% CI: 4-not reached) versus 39.1 months (95% CI: 15.1–40.4) for patients without peritoneal metastases (p=0.07; HR: 1.7; 95% CI: 0.8–4.8). Similarly, patients with lung metastasis showed a non-significant trend towards worse survival with a median OS of 19.8 months (95% CI: 12.7–58.5) versus 39.1 months (95% CI: 10 – not reached; p=0.09). Nevertheless, a significant difference in OS according to the baseline neutrophil–lymphocyte ratio (NLR) was observed. Patients with NLR < 3.5 had a median OS of 39.1 months (29.6-not reached) versus 22.7 (2.1-not reached) for patients with NLR ≥ 3.5 (log-rank p=0.02, adjusted HR: 1.8; 95% CI: 1.1–4.3; p=0.02). (Table 3 and Figure 2)
 |
Table 3 Median Survival and Univariate Analysis |
Patients treated with sorafenib in the first line had a median survival of 38.1 months (95% CI: 17.9-not reached), and patients treated with capecitabine had a median OS of 29.6 months (4-not reached). Patients who received IFN plus capecitabine at some time point, including three in the first line and nine in the second line, had a median OS of 38.4 months (95% CI: 19.8–58.7). Two patients presented hyperammonemic encephalopathy late during the treatment course. Due to the restricted sample size and retrospective nature, no inferential comparisons between regimens were performed, and survival times are only illustrative.
Radiomics
The radiomic analysis included 15 patients, while 8 were excluded due to a lack of available baseline contrast-enhanced computed tomography scans or a lack of measurable primary liver lesions. Seven patients were treated with initial systemic treatment and underwent baseline scans before starting systemic treatment. Eight patients underwent surgical resection before systemic treatment. For these patients, the scans performed before resection were analyzed. Survival was calculated from the start of systemic treatment. Radiomic features extracted from the arterial phase identified three distinct clusters (1a, 2a, and 3a) with median OS of 23.2 months (95% CI: 4.4–34.3) for cluster 1a (n=7), 28.2 months (27.1-NR) for cluster 2a (n=3) and 41.4 months (9.1-NR) for cluster 3a. Cluster 3a had superior OS compared to cluster 1a, with a log rank p-value of 0.048.
Radiomic features extracted from the portal phase were unable to yield clusters with statistically different survival outcomes. Radiomic features from delta radiomics (arterial – portal phases) identified four clusters (1d, 2d, 3d, and 4d) with median OS of 16.4 months (95% CI: 4.4–34.3) for cluster 1d (n=4), 27.1 months (95% CI: 23.2–28.2) for cluster 2d (n=3), 23.5 months (95% CI: 22.6-NR) for cluster 3d (n=3), and 40.0 months (95% CI: 9.8-NR) for cluster 4d (n=5).
We identified five clusters (1ad, 2ad, 3ad, 4ad, and 5ad) based on integrated data from both arterial and delta phases, with median OS of 4.4 months (95% CI: 4.4-NR) for cluster 1ad (n=2), 28.2 months (95% CI: 27.1-NR) for cluster 2ad (n=3), 40.0 months (95% CI: 9.8-NR) for cluster 3ad (n=6), 22.6 months (95% CI: 22.5–23.5) for cluster 4ad (n=2), and 16.4 months (16.4-NR) for cluster 5ad (n=2).
After performing Min-Max scaling of the arterial phase and delta features, we trained XGBoost with all features from both datasets in a fivefold cross-validation. The model achieved an accuracy of 0.73 (95% CI: 0.61–0.85) to identify the cluster with a better prognosis while identifying sphericity, surface-to-volume ratio, low gray-level emphasis, and small area high gray-level emphasis in the arterial phase as important features. We then repeated UMAP embedding with only these features and identified three clusters: cluster 1 (n=5) with a median OS of 22.6 months (95% CI: 4.4–34.6), cluster 2 (n=7) with a median OS of 40.0 (95% CI: 9.8-NR), and cluster 3 (n=3) with a median OS of 28.2 months (95% CI: 27.1-NR). Cluster 2 had better survival compared to cluster 1 (log rank p=0.039).
Lastly, using normalization instead of Min-Max scaling, XGBoost achieved an accuracy of 0.93 (95% CI: 0.81–1.0), and only tumor sphericity in the arterial phase was considered a relevant feature, meaning that a round-shaped tumor was associated with a better prognosis. Tumor sphericity was defined as the index between the measured area of the tumor and the area of a sphere of the same volume, to quantify the similarity of tumor morphology to a sphere.
Discussion
This study reports a cohort of patients with FLHCC treated at a tertiary center, focusing on the management of advanced-stage disease. Our findings confirm that FLHCC has unique features compared to conventional HCC, regarding young onset, gender balance, and low alpha-fetoprotein. Only one case occurred in a patient with cirrhosis caused by chronic hepatitis B. Fifty-one percent of the cohort were alive at 2 years with sequential lines of systemic treatment, mainly sorafenib or chemotherapy-based regimens. Data suggest a potential prognostic role for radiomics and baseline NLR.
FLHCC is a rare disease, and information on its management is largely based on case reports, retrospective studies, and database analysis. Most of the series describe 40–50% of potentially resectable cases at diagnosis, but 50–80% of them relapse.23 Additionally, around 60% of the cases are not amenable to curative resection at first presentation due to widespread disease or high tumor burden, with a limited prognosis.7,9,24 Our cohort was enriched with patients who presented high tumor burden, mostly metastatic. The most common sites of metastasis were the lungs, lymph nodes, and peritoneum, which is in line with other reports on FLHCC.7,25,26 Peritoneal and lungs spread appeared to have a limited prognosis in the present study. Other studies suggested that macrovascular invasion, nodal spread, and multinodularity also have a negative prognostic impact.7,27–29 Data remain conflicting in relation to prognostic impact of gender,7,30,31 and the present study did not show survival difference between genders.
For recurrent or de novo metastatic disease not amenable to surgery or transplant, the literature supports the use of systemic treatment, although there is not a definitive evidence-driven regimen due to the lack of randomized trials.15 With the young onset and normal liver function, systemic agents used for hepatoblastoma, conventional hepatocellular carcinoma, and other gastrointestinal malignancies are reported. In a pooled analysis of 26 patients with FLHCC treated with systemic treatment without surgery reported by Kaseb et al, the median OS was 20.6 months with encouraging responses seen with 5-FU plus IFN.9 A Phase II study in patients with primary liver tumors, including nine with FLHCC treated with 5-FU and IFN reported a response rate of 62.5% and a median OS of 23.1 months.16 Lamarca et al also reported favorable results with this regimen, with a median OS of 38.5 months for patients treated at some point with IFN and 5-FU.15 Our study showed similar OS of 38 months for patients who received IFN plus capecitabine, although few responses were observed with this regimen.
Data from the Fibrolamellar Consortium published by Ang et al reported 45 patients treated with systemic treatment, including fluoropyrimidine, cisplatin, oxaliplatin, irinotecan, gemcitabine, and doxorubicin, with varying results. Ten patients received sorafenib at some point during treatment, including eight patients with disease progression and one mixed response.7 In a study with 17 patients treated with systemic treatment, Chakrabarti et al reported 4 out of 9 patients treated with sorafenib showing stable disease, with duration ranging from 5 months to 5 years.25 In our study, sorafenib was associated with a 20% of DCR in the first line and provided numerically longer survival when compared to other regimens, with a median survival of 38.1 months, suggesting a time effect with sorafenib presumably appearing later. Some patients in our cohort experienced treatment-interval periods during their clinical course (shown in Figure 1), which can be used as a strategy to balance quality of life and treatment-related toxicities in selected patients with less aggressive disease behavior.
More recently, studies have addressed the search for actionable drivers and targeted therapies. The DNAJB1-PRKACA transcript, which is virtually present in all cases of FLHCC, results in overexpression of aurora-kinase A. The use of a ENMD-2076, a selective aurora-kinase A inhibitor, was reported in a phase II trial showing a median overall survival of 19 months, with 1 partial response and 20 patients with stable disease out of 35 patients included.11 Studies with everolimus and sunitinib were discontinued after showing no clinical benefit.10,32 Whether DNAJB1-PRKACA is an actionable target remains under active investigation and ongoing studies are addressing this target (NCT04248569).10,32–35
Since molecular targets are still far from clinical practice in FLHCC, novel tools are needed for prognostication. Radiomics is an emerging field that extracts quantitative data from conventional radiological imaging modalities (including size, shape, and texture) and correlates with several clinical outcomes. This field is increasingly being explored in the setting of primary liver malignancies.36–38 Despite the small sample size, we are able to identify clusters with distinct outcomes and suggest that radiomic features of the primary tumor may be explored as a prognostic tool to either predict survival or help in practical decisions. Tumor sphericity was shown to be a relevant radiomic feature in the present study, which is in line with other studies in oral,39 lung,40 and breast cancer.41
Currently, radiomics remains a promising methodology for clinical practice lacking validation in larger studies.18,42 In our study, even with a small sample, the use of radiomics was able to detect cluster patterns with different prognoses, including a detection accuracy of 0.79. We recognize the possibility of false-positive results and, here, we cautiously interpret our results as a generating hypothesis that claims for both larger prospective data and validation cohorts.
In addition, high NLR was associated with worse survival in the present study. Similar findings were also reported by our group among patients with conventional HCC, which is thought to be associated with a pro-tumoral systemic inflammatory response.43
Conducting clinical trials in FLHCC has proven challenging and unpractical since it presents putative low patients accruing, usually presenting advanced disease and unmeasurable worldwide differences in both surgical and systemic approaches. Retrospective series may inform about prognostic markers, stratification factors, and experimental regimens, in addition to helping to select subgroups to be included in prospective trials. However, retrospective studies often present selection bias by reporting a small number of patients treated in tertiary centers.
Moreover, there may be bias due to less intensive regimens, such as sorafenib or capecitabine, being chosen for patients with low tumor burden and an indolent course, while patients with more aggressive features tended to receive regimens with high cytotoxicity activity, such as platinum combinations. Finally, imaging assessments were performed in routine practice, which hinders accurately determining responses and time to treatment failure.
Conclusion
In conclusion, clinical features and survival outcomes of FLHCC in this study are consistent with literature. Although sorafenib and cytotoxic chemotherapy may provide encouraging DCR, the lack of uniformity precludes defining a preferable regimen. NLR and radiomics of the primary lesion have shown a prognostic role in advanced FLHCC and deserve further validation. FLHCC is a highly lethal tumor with little progress in recent years. Multicenter collaborations are needed to achieve a better understanding of this disease and provide better outcomes.
Disclosure
Da Fonseca LG received fees from Bayer, BMS, AstraZeneca, and Roche. The authors report no other conflicts of interest in this work.
References
1. Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–186. doi:10.1001/ARCHPEDI.1956.02060020170015
2. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182. doi:10.1111/HIS.13975
3. Pawlik T, Lafaro K. Fibrolamellar hepatocellular carcinoma: current clinical perspectives. J Hepatocell Carcinoma. 2015;2:157. doi:10.2147/JHC.S75153
4. El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology. 2004;39:798–803. doi:10.1002/HEP.20096
5. Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–1014. doi:10.1126/SCIENCE.1249484
6. Glavas D, Bao QR, Scarpa M, et al. Treatment and prognosis of fibrolamellar hepatocellular carcinoma: a systematic review of the recent literature and meta-analysis. J Gastrointest Surg. 2023;27:705–715. doi:10.1007/S11605-023-05621-Z
7. Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3.
8. Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol. 2016;14:151. doi:10.1186/S12957-016-0903-8
9. Kaseb AO, Shama M, Sahin IH, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology. 2013;85:197–203. doi:10.1159/000354698
10. El Dika I, Mayer RJ, Venook AP, et al. A multicenter randomized three-arm phase II Study of (1) Everolimus, (2) Estrogen Deprivation Therapy (EDT) with Leuprolide + Letrozole, and (3) Everolimus + EDT in patients with unresectable fibrolamellar carcinoma. Oncologist. 2020;25:925–e1603. doi:10.1634/THEONCOLOGIST.2020-0367
11. Abou-Alfa GK, Mayer R, Venook AP, et al. Phase II multicenter, open-label study of oral ENMD-2076 for the treatment of patients with advanced fibrolamellar carcinoma. Oncologist. 2020;25:e1837–45. doi:10.1634/THEONCOLOGIST.2020-0093
12. Chen KY, Popovic A, Hsiehchen D, et al. Clinical outcomes in fibrolamellar hepatocellular carcinoma treated with immune checkpoint inhibitors. Cancers. 2022;14:5347. doi:10.3390/CANCERS14215347/S1
13. Polychronidis G, Murtha-Lemekhova A, Fuchs J, Hoffmann K, Karathanasi E. A multidisciplinary approach to the management of fibrolamellar carcinoma: current perspectives and future prospects. Onco Targets Ther. 2022;15:1095–1103. doi:10.2147/OTT.S296127
14. Fonseca GM, Varella AD, Coelho FF, Abe ES, Dumarco RB, Herman P. Downstaging and resection after neoadjuvant therapy for fibrolamellar hepatocellular carcinoma. World J Gastrointest Surg. 2014;6:107. doi:10.4240/WJGS.V6.I6.107
15. Lamarca A, Frizziero M, Fulton A, et al. Fibrolamellar carcinoma: challenging the challenge. Eur J Cancer. 2020;137:144–147. doi:10.1016/J.EJCA.2020.06.035
16. Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421–427. PMID: 12560429. doi:10.1200/JCO.2003.10.103
17. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278:563–577. PMID: 26579733. doi:10.1148/RADIOL.2015151169
18. Koçak B, Durmaz EŞ, Ateş E, Kılıçkesmez Ö. Radiomics with artificial intelligence: a practical guide for beginners. Diagnostic Interv Radiol. 2019;25:485. PMID: 31650960. doi:10.5152/DIR.2019.19321
19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
20. Van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–7. doi:10.1158/0008-5472.CAN-17-0339
21. McInnes L, Healy J, Saul N, Großberger L. UMAP: uniform manifold approximation and projection. J Open Source Softw. 2018;3:862. doi:10.21105/JOSS.00861
22. Chen T, Guestrin C. XGBoost: a scalable tree boosting system. Proc ACM SIGKDD Int Conf Knowl Discov Data Min. 2016;785–794. doi:10.1145/2939672.2939785
23. Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820–830. doi:10.1016/J.JAMCOLLSURG.2012.08.001
24. Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331. doi:10.1002/CNCR.21703
25. Chakrabarti S, Tella SH, Kommalapati A, et al. Clinicopathological features and outcomes of fibrolamellar hepatocellular carcinoma. J Gastrointest Oncol. 2019;10:554–561. doi:10.21037/JGO.2019.01.35
26. Abdelhamed W, El-Kassas M. Fibrolamellar hepatocellular carcinoma: a rare but unpleasant event. World J Gastrointest Oncol. 2022;14:1103–1114. doi:10.4251/wjgo.v14.i6.1103
27. Chagas AL, Kikuchi L, Herman P, et al. Clinical and pathological evaluation of fibrolamellar hepatocellular carcinoma: a single center study of 21 cases. Clinics. 2015;70:207–213. doi:10.6061/clinics/2015(03)10
28. Herman P, Chagas AL, Perini MV, et al. Surgical treatment of fibrolamellar hepatocellular carcinoma: an underestimated malignant tumor? Hepatobiliary Pancreat Dis Int. 2014;13:618–621. doi:10.1016/S1499-3872(14)60294-0
29. Yamashita S, Vauthey JN, Kaseb AO, et al. Prognosis of fibrolamellar carcinoma compared to non-cirrhotic conventional hepatocellular carcinoma. J Gastrointest Surg. 2016;20:1725–1731. doi:10.1007/S11605-016-3216-X
30. Polychronidis G, Feng J, Murtha-Lemekhova A, Heger U, Mehrabi A, Hoffmann K. Factors influencing overall survival for patients with fibrolamellar hepatocellular carcinoma: analysis of the surveillance, epidemiology, and end results database. Int J Gen Med. 2022;15:393–406. doi:10.2147/IJGM.S338066
31. Maniaci V, Davidson BR, Rolles K, et al. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol. 2009;35:617–621. doi:10.1016/J.EJSO.2008.12.009
32. Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi:10.1016/S1470-2045(09)70171-8
33. Graham RP, Yeh MM, Lam-Himlin D, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Mod Pathol. 2018;31:141–149. doi:10.1038/MODPATHOL.2017.103
34. Kim YJ, Rhee H, Yoo JE, et al. Tumour epithelial and stromal characteristics of hepatocellular carcinomas with abundant fibrous stroma: fibrolamellar versus scirrhous hepatocellular carcinoma. Histopathology. 2017;71:217–226. doi:10.1111/HIS.13219
35. Cornella H, Alsinet C, Sayols S, et al. Unique genomic profile of fibrolamellar hepatocellular carcinoma. Gastroenterology. 2015;148:806–818.e10. doi:10.1053/J.GASTRO.2014.12.028
36. Yang Y, Fan W, Gu T, et al. Radiomic features of multi-ROI and Multi-Phase MRI for the prediction of microvascular invasion in solitary hepatocellular carcinoma. Front Oncol. 2021;11:756216. doi:10.3389/fonc.2021.756216
37. Yao W, Yang S, Ge Y, et al. Computed tomography radiomics-based prediction of microvascular invasion in hepatocellular carcinoma. Front Med. 2022;9. doi:10.3389/FMED.2022.819670
38. Ma X, Wei J, Gu D, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29:3595–3605. doi:10.1007/s00330-018-5985-y
39. Tarsitano A, Ricotta F, Cercenelli L, et al. Pretreatment tumor volume and tumor sphericity as prognostic factors in patients with oral cavity squamous cell carcinoma. J Craniomaxillofac Surg. 2019;47:510–515. doi:10.1016/J.JCMS.2018.12.019
40. Davey A, van Herk M, Faivre-Finn C, Mistry H, McWilliam A. Is tumour sphericity an important prognostic factor in patients with lung cancer? Radiother Oncol. 2020;143:73–80. doi:10.1016/J.RADONC.2019.08.003
41. Li W, Newitt DC, Yun B, et al. Tumor sphericity predicts response in neoadjuvant chemotherapy for invasive breast cancer. Tomography. 2020;6:216. doi:10.18383/J.TOM.2020.00016
42. Rajpurkar P, Lungren MP. The current and future state of AI interpretation of medical images. N Engl J Med. 2023;388:1981–1990. doi:10.1056/NEJMRA2301725
43. Da Fonseca L, Uratani L, Soares G, et al. Early variation of inflammatory indexes refines prognostic prediction in patients with hepatocellular carcinoma under systemic treatment. Mol Clin Oncol. 2023;18(4). doi:10.3892/MCO.2023.2625
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

