Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Treatment Options for Cow’s Milk Protein Allergy: A Modeling Analysis
Authors Berktas M , Kirbiyik F , Aribal E , Aksit A, Altintas DU
Received 12 December 2019
Accepted for publication 18 March 2020
Published 17 June 2020 Volume 2020:12 Pages 307—315
DOI https://doi.org/10.2147/CEOR.S242021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Dean Smith
Mehmet Berktas,1 Feza Kirbiyik,2 Elif Aribal,2 Anil Aksit,2 Derya Ufuk Altintas3
1Blue Idea Consulting, London, UK; 2Nutricia Advanced Medical Nutrition, Istanbul, Turkey; 3Cukurova University Medical School, Allergy and Immunology Department, Adana, Turkey
Correspondence: Mehmet Berktas
Blue Idea Consulting, 42 Trafalgar House, London W5 2TJ, UK
Tel +44 7833 067120
Email [email protected]
Purpose: Cow’s milk protein allergy (CMPA) is one of the most common food allergies in early childhood. We aimed to evaluate clinical and economic outcomes of the amino-acid formula (AAF) and extensively hydrolyzed formula (eHF) based treatment of CMPA by using data available from Turkey and otherwise from literature.
Materials and Methods: A theoretical model was developed to evaluate AAF and eHF for CMPA treatment in terms of the number of children tolerating formula or experiencing an allergic reaction or withdrawing formula due to taste or other palatability features and CMPA related direct medical costs from the payer perspective.
Results: We estimated that 13,000 children are diagnosed with CMPA in 1 year in Turkey. For the children receiving AAF, it is estimated that 83.7% tolerate AAF until the 24th month, and the total cost for the children tolerating AAF is estimated at 20.6 million€. The average cost per child tolerating AAF until the 24th month is estimated at 1895€. On the other hand, 48.7% are estimated to tolerate eHF until the 24th month, and the total cost for the children tolerating eHF is estimated at 12.3 million€ and the average cost per child tolerating eHF until the 24th month is estimated at 1940€.
Conclusion: The analysis revealed that the management of CMPA is associated with the economic burden on the healthcare system in Turkey. Treatment of CMPA with AAF seems to provide better clinical outcomes (high tolerability and less withdrawal due to taste or an allergic reaction) and to be an option with economic benefits when Turkey-specific conditions are considered.
Keywords: amino-acid formula, extensively hydrolyzed formula, pharmacoeconomic modeling, cow’s milk protein allergy
Introduction
Cow’s milk protein allergy (CMPA) is one of the most common food allergies in early childhood. It is a result of a clinically abnormal reaction to specific parts of some proteins in cow’s milk.1 Current guidelines for CMPA recommend elimination of dairy product from the diet of the breastfeeding mother and removal of the dairy product from the diet of the child receiving complementary feeding and using specialized substitution formula.1–4
The most used specialized substitution formulae for CMPA are amino acid-based formula (AAF) and extensively hydrolyzed formula (eHF). AAFs are produced by combining certain amino acids; therefore, they do not contain any cow’s milk protein. On the other hand, eHFs are produced by using heat-induced thermal hydrolysis, enzymatic hydrolysis, and ultra-filtration, therefore eHFs still can contain specific parts of some proteins in cow’s milk which can be related to the allergic reaction.1,5
Formula selection for CMPA is still a controversial issue, and recommendations of the guidelines on substitute formula selection for CMPA are mostly based on economic concerns rather than an evidence-based medicine approach. AAFs are recommended as the first-line substitute formula for anaphylaxis, food protein-induced enterocolitis syndrome, allergic eosinophilic esophagitis, and milk-induced chronic pulmonary disease.1–4 For other clinical conditions, eHFs are recommended due to economic concerns. However, bitter taste of eHFs, which results in discontinuation of the formula, and their potential to cause mild-to-severe allergic reactions, including anaphylaxis, are still significant pitfalls.1,4 For many high-income western countries, AAFs are reported to be expensive for 6–8 times of the cost of eHFs; however, in many countries like Turkey, the cost difference between AAFs and eHF is remarkably small (about only 20%).1 Therefore, a generalization of the international guidelines’ recommendations, which were created based on economic concerns, is not always the best practice for the countries (ie, Turkey) in which the cost difference between AAFs and eHF is small and structure of healthcare systems differs substantially. It is recommended to develop a national approach for the management of CMPA based on local medical, demographic, and economic conditions.3,4
This modeling analysis aimed to evaluate clinical and economic outcomes of the AAF- and eHF-based CMPA managements using recommendations from international guidelines and by using data available from Turkey or otherwise from literature.
Materials and Methods
Objective
In this theoretical modeling analysis, we aimed to evaluate AAF- and eHF-based CMPA treatment approach scenarios in terms of the number of children who tolerate formula, number of children who experience an allergic reaction, number of children who withdraw formula due to taste or other palatability features, and CMPA-related direct medical costs and overall economic burden of the management of all children with CMPA by using data available from Turkey and otherwise from literature.
Target Population
CMPA symptoms are generally observed in the first 2–4 months of life, and we accepted that an infant with CMPA gets diagnosed before the 6th month of life and starts specialized formula at the 6th month of life in Turkish medical setting.6,7 Therefore, all alive children under one year of age were accepted as the population under risk of CMPA. Turkish Statistics Institute reported 1,309,771 alive-births and 13,036 deaths in the first year of life in 2016.8,9 Therefore, the size of the children population under risk of CMPA was accepted as 1.3 million (Table 1).
 |
Table 1 Parameters Used in the Analyses |
The prevalence or incidence of CMPA in Turkey has not been evaluated in the country-representative cross-sectional study, yet. However, different publications from Turkey, including consensus reports of expert opinion and a similar pharmacoeconomic analysis, indicated that CMPA incidence during early childhood in Turkey is approximately 2–3%.7,10,11 Therefore, for the analysis, the 1-year incidence of the CMPA was accepted as 3% (Table 1).
Substitute formula utilization numbers in Turkey for 2016 revealed that not all children with CMPA were diagnosed and given specialized substitution formula.12 Based on the 2016 formula sales report, we accepted that 33.3% of the children with CMPA are diagnosed and given specialized substitution formula (Table 1).
The Perspective of the Analysis
We evaluated CMPA and its medical and economic burden from the payer perspective (Turkish Social Security Institute); in other words, we assumed that the clinical effectiveness of the AAF and eHF is same and the only direct medical cost and formula prices which are reimbursed by Social Security Institute were used in the analyses.13,14 Specialized substitution formulae are reimbursed until 24 months of age, and formulae are provided by pharmacies on a monthly basis, which requires monthly prescription based on reimbursement code of Social Security Institute in Turkey (Table 1).15
Interventions Evaluated in the Analysis
We evaluated AAF- and eHF-based treatment scenarios by estimating a child is diagnosed with CMPA and starts specialized substitution formula (AAF or eHF) at the 6th month of life and continues using formula until the 24th month of age unless intolerability, allergic reaction, or withdrawal of formula due to taste or other palatability features have been observed.
We accepted that diagnosis and follow-up approaches (visit counts, visit frequency, and laboratory tests) were assumed to be the same for all children regardless of the substitution formula type used.
Structure of the Analysis
We developed a model covering an 18-month time period and accepted that each child is evaluated every four weeks from 6th to 24th month of age based on the requirements of the reimbursement code of the payer (Social Security Institute). During the 18-month time period, the children experience intolerability, allergic reaction, or withdrawal of formula due to taste or other palatability features are excluded from the analysis. The short-term or long-term clinical effects of subsequent formula after switching from the previous one are out of the scope of the analysis.
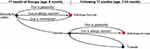
At each time point of the model, following clinical outcomes are evaluated; tolerable to formula, intolerable to formula, withdraw formula due to taste or other palatability features, experiencing mild to moderate allergic reaction leading formula withdrawal and experiencing severe allergic reaction leading formula withdrawal (Figure 1). No death is expected due to CMPA or formula or allergic reaction; therefore, the model did not include death as an outcome. We used the size of the population under risk, CMPA incidence, formula use ratio, and outcomes of the AAF- or eHF-based treatment scenarios to estimate the total economic burden of CMPA management in Turkey (Table 1).
In the analysis, calculated monetary values in February 2019 Turkish Lira, and these values were converted to Euro by using 5.0 TRY/Euro currency change rate. No discounting was applied to costs or outcomes.
Sensitivity Analyses
Deterministic sensitivity analyses were performed to evaluate the effects of ±10% change of each parameter in the model on the outcomes, and results were presented as a tornado graphic.
We performed probabilistic sensitivity analyses (100,000 iterations of the model) by randomly varying the probabilities (regarding relevant distribution pattern) of clinical outcomes, costs, and resource use frequencies to assess the effect on uncertainty in the model. For probability variation of the rate values used in the model, beta distribution (by using mean and a standard deviation, which equals to 10% of mean) was used whereas gamma distribution (by using mean and a standard deviation, which equals 15% of mean) was used.
The model was constructed using Microsoft Office Excel (2018).
Analysis Inputs
The severity of CMPA has a substantial effect on the clinical outcomes; therefore, we classified the children into uncomplicated CMPA versus complicated/severe CMPA sub-groups at the time of diagnosis. The ratio of the children suffering from complicated/severe CMPA was accepted to be 40% based on previous reports from Turkey, which is also consistent with international literature (Table 1).16–20
Intolerability to formula was estimated to be observed in 4-week after the formula had been started. The percentage of children who do not tolerate eHF was reported up to 29% and 40% for those with uncomplicated CMPA and with complicated/severe CMPA, and 0% for AAF in both CMPA severity sub-groups, respectively.21,22 In the base analysis, the percentage of children who do not tolerate the formula was accepted to be 29% and 40% for eHF and 1% and 1% for AAF in uncomplicated and complicated/severe CMPA subgroups (Table 1). Since no Turkey-specific data are available as stated in a consensus report of expert opinion from Turkey, we performed sensitivity analyses to evaluate a wide range of intolerability rates.7
The annual rate for or withdrawal of formula due to taste or other palatability features was accepted at 8% and 12% for AAF and eHF, respectively. The rate of eHF was selected higher than AAF, as indicated in the CMPA guideline (Table 1).4
The rate of an allergic reaction during AAF or eHF has not been reported for Turkey, but it is estimated that allergic reaction incidence during AAF usage is lower than eHF due to manufacturing methodology.5 In the analysis, annual mild to moderate allergic reaction incidence was accepted to be 1.5% and 4.0% for AAF and eHF, whereas annual incidence for the severe allergic reaction was estimated at 0.5% and 1.0%, respectively (Table 1).
Daily energy need was calculated for the weight of 50th weight-to-age percentile at each time point by using the Food and Agriculture Organization (FAO) daily energy need formula. The weighted daily energy need was calculated by gender and source energy.23–25
Monthly formula need was calculated by using daily energy need, the source of energy (breast milk or solid food or substitution formula), and total energy amount in each formula box. For energy amount in a box and box price, approved summaries of product characteristics of Nutricia Neocate were used for AAF and Abbott Similac Alimentum for eHF.15
In the analysis, number of children using formula (AAF or eHF) until the 24th month of age unless they experience intolerability, allergic reaction, or withdrawal of formula due to taste or other palatability features was calculated, and if a child using eHF experiences a withdrawal for any reason, AAF is accepted as the next formula alternative; however, the cost of AAF after switch, except during the month when the switch happens, was excluded from the cost analysis due to aim of the analysis.
In this analysis, we accepted that diagnosis and follow-up visits are performed by pediatric allergy or pediatric gastroenterology departments at tertiary healthcare setting to minimize the effects of non-optimal management of CMPA on the outcomes as previously described for Turkish medical practice.10 The cost of the diagnosis, follow-up, and additional procedures and treatment related to formula withdrawal was estimated based on a methodology previously described in a similar analysis from Turkey; it is estimated that 50.0% of the visits are related to diagnosis whereas 33.3% of those are related to intolerability or withdrawal due to any other reason.10 Due to reimbursement criteria, we accepted that children are evaluated each month, and in case of withdrawal of formula (AAF or eHF), an additional set of visits and laboratory tests is performed. The cost of an extra set of visits, laboratory tests, and treatments was estimated based on the same previously described methodology.10
We also accepted that two additional visits are required for a mild to moderate allergic reaction, whereas four visits for severe allergic reaction in tertiary health settings. Visit costs are extracted from the Social Security Institute Health Implication Code.15
Outcomes of Analysis
The model calculated the number of children who tolerate formula and the number of children who withdraw formula due to intolerability, allergic reaction or taste, or other palatability features for the 18-month period between the 6th and 24th months of life. In addition to the number of children, visit costs and formula costs were also calculated. We also calculated an average cost for each child who tolerates the formula until the 24th month of life.
Results
It is estimated that 13,000 children are diagnosed with CMPA and receive specialized substitution formula in 1 calendar year based on the size of the population under risk, annual CMPA incidence, and diagnosis rate.
In the scenario of 13,000 children with CMPA receiving AAF, it was estimated that 83.7% (10,885) tolerate AAF until 24th month of life (Supplementary Figure 1), whereas 1.0% (130) withdraw AAF due to intolerance, 3.0% (387) withdraw due to any kind of allergic reaction, and 12.3% (1598) withdraw due to taste or other palatability features (Table 2). Moreover, it was calculated 18-month total cost of 20,630,342 Euro (103,151,710 TRY) including visit costs of 3,755,625 Euro (18,778,125 TRY), formula cost of 16,864,861 Euro (84,324,303 TRY), and allergic reaction-related cost of 9856 Euro (49,282 TRY) for 10,885 children who tolerate AAF. The average cost of a child who tolerates AAF until the 24th month of life is estimated at 1895.28 Euro (9476.42 TRY) (Table 3).
 |
Table 2 Estimated Number of Children with Outcomes of the Analysis by Formula Type |
 |
Table 3 Cow’s Milk Protein Allergy-Related Estimated Cost by Formula Type |
In another scenario of 13,000 children with CMPA receiving eHF, it was estimated that 48.7% (6326) tolerate eHF until 24th month of life (Supplementary Figure 1), whereas 33.4% (4342) withdraw eHF due to intolerance, 5.8% (756) withdraw due to any kind of allergic reaction, and 12.1% (1575) withdraw due to taste or other palatability features (Table 2). Moreover, it was calculated 18-month total cost of 12,274,807 Euro (61,374,034 TRY) including visit costs of 3,200,460 Euro (16,002,299 TRY), formula cost of 9,055,103 Euro (45,275,515 TRY), and allergic reaction-related cost of 19,244 Euro (96,220 TRY) for 6326 children who tolerate eHF. The average cost of a child who tolerates eHF until the 24th month of life is estimated at 1940.26 Euro (9701.29 TRY) (Table 3).
The deterministic sensitivity analyses revealed that results of AAF- and eHF-based scenarios are the most sensitive to formula price followed by annual formula withdrawal rate due to taste or other palatability features and cost of diagnosis visits and related laboratory tests. The changes in these three parameters have greater effects on the average cost of a child who tolerates eHF until the 24th month of the life, whereas other parameters in the analyses have relatively smaller effects (Figure 2).
Probabilistic sensitivity analysis revealed that 95% confidence interval of the mean cost of a child who tolerates formula until the 24th month of the life is 1893.08 Euro–1902.26 Euro for AAF whereas it is 1935.13 Euro–1943.43 Euro for eHF (Figure 3), and percentage of children who tolerate formula until the 24th month of the life is higher for the AAF-based scenario compared to eHF-based scenario in all iterations (Figure 3). In addition to that possibility of being cost advantageous from the AAF-based scenario is higher than the eHF-based scenario for each willingness-to-pay value between 1000 and 3000 Euros in all iterations (Supplementary Figure 2).
 |
Figure 3 Estimated mean cost per child who tolerates the formula and percentage of the children who tolerate the formula at the end of the 24th month (probabilistic sensitivity analysis). |
Discussion
In this theoretical modeling analysis, we aimed to estimate the number of children who tolerate formula, number of children who experience an allergic reaction, number of children who withdraw formula due to taste or other palatability features, and CMPA related direct medical costs for the children with CMPA in Turkey who are diagnosed at 6th month of life, started AAF or eHF, and followed up to 24th month of life. Our analysis revealed that 83.7% of children receiving AAF and 48.7% of children receiving eHF can tolerate the formula until 24th month of life and the average cost of a child who tolerates formula until the 24th month of the life is estimated 1895.28 Euro (9476.42 TRY) and 1940.26 Euro (9701.29 TRY) for AAF and eHF, respectively.
CMPA is commonly observed in early childhood, and its management may cause an economic burden on the health system. A BIA, including 4382 children from the Netherlands shown that the annual healthcare cost (excluding formula) of CMPA management was around 11.2 million Euros. Annual eHF cost varied 8.7 to 14.2 million, whereas AAF ranged from 9.5 to 19.4 million regarding the specialty of the physician following the child.26 The annual average cost per child diagnosed with CMPA was estimated at 2567 Euro (95% confidence interval: 1794 Euro–3365 Euro) per patient.26 Although there are methodological differences between the analysis from the Netherlands and our analysis, the management of CMPA seems to cost less in Turkey compared to the Netherlands regardless of formula type used.
A similar analysis from Australia estimated that the cost of managing 6150 newly diagnosed infants with CMPA for 6 months was 6.5 million Australia dollars in 2006/2007, and 62% to 65% of the total cost was driven by nutrition preparations followed by physician visits (28%). Moreover, the authors found that if all children diagnosed with CMPA are treated with AAF, fewer visits to hospital-based pediatric gastroenterology or allergy departments would be required.27 In our analysis, we found that children using AAF may cost more than eHF in terms of physician visits, but this is due to more children tolerate the AAF (83.7% vs 48.7) and stay under physician control for a more extended period.
Another analysis from the United Kingdom compared the AAF versus eHF for first-line treatment of CMPA from the payer perspective, and the authors estimated that 12 months management of CMPA would cost 1853 GBP and 3161 GBP for eHF and AAF treated children in 2008/2009, respectively. The authors concluded that starting eHF would be a cost-effective option for the United Kingdom, as there were no significant clinical outcome differences between AAF and eHF.28 However, the details of the analysis revealed than AAF cost almost 4-fold higher than eHF in the United Kingdom, and the difference between 2 approaches was the main driver of the total cost difference between AAF and eHF. Interestingly, the difference between annual cost excluding formulae was estimated so small.28 Our analysis showed that AAF might have clinical benefits (less withdrawal due to taste or an allergic reaction) and have economic benefits due to the small price difference between AAF and eHF.
A previous analysis for Turkey evaluated the economic outcome of practice patterns of ideal management in CMPA children presenting proctocolitis or eczema and reported that a 2-year total cost from payer perspective would be 2116 USD and 4002 USD (with 2016 prices) for proctocolitis and eczema, respectively. It was reported that the cost was mainly driven (89% to 92% of total cost) by formulae, and first-line AAF treatment may be associated with the incremental cost of 1848 USD and 3445 USD in the management of proctocolitis and eczema, respectively.10 Although it was a well-designed analysis, it evaluated the ideal management of the selected medical conditions, and the analysis assumed that almost all children with CMPA would tolerate AAF or eHF during the entire treatment period. Therefore, the results from this analysis may not reflect the real-life conditions which physician managing CMPA patients face every day. In our analysis, we included all possible outcomes which physicians may face in their daily practice, ie, intolerability, withdrawal of formula due to taste or other palatability features, or allergic reaction. When all these outcomes are considered, we estimated that more children with CMPA tolerate the AAF (83.7%) compared to eHF (48.7%) and the average cost of a child who tolerates AAF until the 24th month of the life is lower than EHF (1895 Euro vs 1940.26 Euro).
Limitations
When evaluating the result of this analysis, certain limitations should be considered. Since this is a model-based analysis that aims to reflect real-life conditions, there is a possibility to under or overestimate the inputs and outcomes. We performed a set of sensitivity analyses to evaluate the effect of uncertainty on each parameter in the model on the results.
We aimed to use Turkey-specific inputs in the analysis; however, not all the inputs were available for Turkey. We used estimations based on consensus reports of expert opinion from Turkey or international literature when no local data are available and performed sensitivity analyses to evaluate the effects of variability in these inputs on the outcomes. This approach may probably decrease the generalizability of our results in Turkey.
Our analysis included only direct medical costs and excluded non-medical and indirect costs. Therefore, the results of the analysis reflect the outcomes only from the payer perspective. The real burden of CMPA, including other burdens from the social perspective, may be higher than estimated.
Conclusion
In conclusion, the analysis confirmed that the management of CMPA is associated with an economic burden on the healthcare system in Turkey. Treatment of CMPA with AAF seems to provide better clinical outcomes (high tolerability and less withdrawal due to taste or an allergic reaction) and to be an option with economic benefits when Turkey-specific financial conditions are considered.
Acknowledgments
MB provided substantial contributions to conception and design of the study and was responsible for literature review, acquisition of data, analysis and interpretation of data. He drafted the manuscript and revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AA, EA and FK provided substantial contributions to conception of the study and revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DUA provided substantial contributions to conception and design of the study and was responsible for interpretation of data. She revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Numil Turkey provided unconditional financial support for the project; however, as the financial supporting source, Numil Turkey was not involved in design of the study, literature review and acquisition of data, analysis, and interpretation of data, decision to publish analysis results and drafting the manuscript. The preliminary results of this analysis were presented as poster at the ISPOR Europe 2018 Congress on 10–14 November 2018 in Barcelona, Spain. The poster’s abstract was published in Value in Health Journal (DOI: https://doi.org/10.1016/j.jval.2018.09.2445).
Disclosure
Dr Mehmet Berktas reports personal fees from Numil Turkey, Gilead Sciences, and Amgen, during the conduct of the study. Dr. Anil Aksit and Dr. Elif Aribal are employee of Nutricia. Dr. Feza Kirbiyik was an employee of Nutricia during the conduct of the study. Prof. Dr. Derya Ufuk Altintas declares no conflicts of interest in this work.
References
1. Fiocchi A, Dahda L, Dupont C, Campoy C, Fierro V, Nieto A. Cow’s milk allergy: towards an update of DRACMA guidelines. World Allergy Organ J. 2016;9(1):1–11. doi:10.1186/s40413-016-0125-0
2. Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221–229. doi:10.1097/MPG.0b013e31825c9482
3. Fiocchi A, Brozek J, Schünemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol. 2010;21:1–125. doi:10.1111/j.1399-3038.2010.01068.x
4. Vandenplas Y, Brueton M, Dupont C, et al. Guidelines for the diagnosis and management of cow’s milk protein allergy in infants. Arch Dis Child. 2007;92(10):902–908. doi:10.1136/adc.2006.110999
5. Agostoni C, Terracciano L, Varin E, Fiocchi A. The nutritional value of protein-hydrolyzed formulae. Crit Rev Food Sci Nutr. 2016;56(1):65–69. doi:10.1080/10408398.2012.713047
6. Host A, Halken S. Cow’s milk allergy: where have we come from and where are we going? Endocr Metab Immune Disord Drug Targets. 2014;14(1):2–8. doi:10.2174/1871530314666140121142900
7. Guler N, Cokugras FC, Sapan N, et al. Diagnosis and management of cow’s milk protein allergy in Turkey: region-specific recommendations by an expert-panel. Allergol Immunopathol (Madr). 2019. doi:10.1016/j.aller.2019.05.004
8. Turkish Statistics Institute. National Birth Statistics. 2016.
9. Turkish Statistics Institute. National Mortality Statistics. 2016.
10. Sekerel BE, Seyhun O. Expert panel on practice patterns in the management of cow’s milk protein allergy and associated economic burden of disease on health service in Turkey. J Med Econ. 2017;20(9):923–930. doi:10.1080/13696998.2017.1342171
11. Sackesen C, Altintas DU, Bingol A, et al. Current Trends in tolerance induction in cow’s milk allergy: from passive to proactive strategies. Front Pediatr. 2019;7:372. doi:10.3389/fped.2019.00372
12. IMS Health. National Formula Sales Report.
13. Social Security Institute. Pharmaceuticals reimbursement bylaw. Available from: https://kms.kaysis.gov.tr/Home/Kurum/22620739?AspxAutoDetectCookieSupport=1.
14. Social Security Institute. Pharmaceuticals reimbursement application guideline. Available from: https://kms.kaysis.gov.tr/Home/Kurum/22620739?AspxAutoDetectCookieSupport=1.
15. Social Security Institute. Health implication code. Available from: https://kms.kaysis.gov.tr/Home/Kurum/22620739?AspxAutoDetectCookieSupport=1.
16. Orhan F, Karakas T, Cakir M, Aksoy A, Baki A, Gedik Y. Prevalence of immunoglobulin E-mediated food allergy in 6–9-year-old urban schoolchildren in the eastern Black Sea region of Turkey. Clin Exp Allergy. 2009;39(7):1027–1035. doi:10.1111/j.1365-2222.2009.03263.x
17. Aycin GD, Altintas D. Contribution of the method of diagnosis and the range of IgE –non IgE at cow’s milk allergy in daily practice. J Turgut Ozal Med Cent. 2016;23(3):288–292. doi:10.5455/jtomc.2016.06.070
18. Asilsoy S, Ceylan O, Bekem Soylu O, Can D, Altinoz S, Agin H. Clinical findings in patients with cow’s milk allergy. J Tepecik Educ Res Hosp. 2007;17(2):83–87. doi:10.5222/terh.2007.65469
19. Senol HD, Koksal BT. The clinical characteristics of children with food allergy in Van. Van Med J. 2015;22:266–272. Available from: http://vantipderg.org/jvi.aspx?pdir=vtd&plng=tur&un=VTD-60790
20. Meyer R, Groetch M, Venter C. When should infants with cow’s milk protein allergy use an amino acid formula? A practical guide. J Allergy Clin Immunol Pract. 2018;6(2):383–399. doi:10.1016/j.jaip.2017.09.003
21. Sladkevicius E, Nagy E, Lack G, Guest JF. Resource implications and budget impact of managing cow milk allergy in the UK. J Med Econ. 2010;13(1):119–128. doi:10.3111/13696990903543242
22. Hill DJ, Murch SH, Rafferty K, Wallis P, Green CJ. The efficacy of amino acid-based formulas in relieving the symptoms of cow’s milk allergy: a systematic review. Clin Exp Allergy. 2007;37(6):808–822. doi:10.1111/j.1365-2222.2007.02724.x
23. World Health Organization. The WHO child growth standards. Available from: http://www.who.int/childgrowth/standards/en/.
24. Hacettepe University Institute of Population Studies. Turkey Population and Health Research (TNSA-2013). 2013.
25. The Food and Agriculture Organization (FAO). Report of a Joint FAO/WHO/UNU Expert Consultation/Energy and Protein Requirements. 1985.
26. Sladkevicius E, Guest JF. Budget impact of managing cow milk allergy in the Netherlands. J Med Econ. 2010;13(2):273–283. doi:10.3111/13696998.2010.482909
27. Guest JF, Nagy E. Modelling the resource implications and budget impact of managing cow milk allergy in Australia. Curr Med Res Opin. 2009;25(2):339–349. doi:10.1185/03007990802594685
28. Taylor RR, Sladkevicius E, Panca M, Lack G, Guest JF. Cost-effectiveness of using an extensively hydrolysed formula compared to an amino acid formula as first-line treatment for cow milk allergy in the UK. Pediatr Allergy Immunol. 2012;23(3):240–249. doi:10.1111/j.1399-3038.2011.01262.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.