Back to Journals » Infection and Drug Resistance » Volume 16
Treatment of Ventriculitis and Meningitis After Neurosurgery Caused by Carbapenem-Resistant Enterobacteriaceae (CRE): A Challenging Topic
Authors Li C, Zhou P, Liu Y, Zhang L
Received 12 April 2023
Accepted for publication 8 June 2023
Published 15 June 2023 Volume 2023:16 Pages 3807—3818
DOI https://doi.org/10.2147/IDR.S416948
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Cuiling Li, Peng Zhou, Yuanqin Liu, Lei Zhang
Department of Neurosurgery, Shandong Medicine and Health Key Laboratory of Neurosurgery, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, Jinan, People’s Republic of China
Correspondence: Lei Zhang, Department of Neurosurgery, Shandong Medicine and Health Key Laboratory of Neurosurgery, The First Affiliated Hospital of Shandong First Medical University and Shandong Provincial Qianfoshan Hospital, 16766 Jingshi Road, Lixia District, Jinan, People’s Republic of China, Email [email protected]
Abstract: Post-neurosurgical infection is a common complication of neurosurgery, and serious infection can threaten the life of patients. In recent years, the increase in multidrug-resistant bacteria, especially carbapenem-resistant Enterobacteriaceae (CRE), has proved fatal to patients. Although there are a few cases of CRE meningitis and few clinical trials have been carried out, it has attracted increasing attention with the increasing probability of its occurrence, especially considering that there are few successful cases. An increasing number of studies are also looking for the risk factors and clinical symptoms of CRE intracranial infection. In terms of treatment, some new antibiotics are gradually being used in the clinic, but due to the complicated drug-resistant mechanism of CRE and the obstruction of the blood‒brain barrier (BBB), the therapeutic effect is still very poor. In addition, obstructive hydrocephalus and brain abscess caused by CRE meningitis are still important causes of patient death and are also difficult to treat.
Keywords: CRE, ventriculitis and meningitis, polymyxin, ceftazidime-avibactam, cefiderocol
Introduction
Post-neurosurgical infection is very harmful, which can not only prolong the length of stay of patients, consume medical resources and increase medical costs but also lead to poor prognosis of patients, including increased disability and mortality.1–3 Due to different surgical methods, the infection rate after neurosurgery is also different. In general, the infection rate after external ventricular drainage (EVD) is approximately 10%,4,5 also reported to be approximately 0–22% in the literature,6–8 and even 45% in individual reports, which has the highest infectious rate.9 However, it has been pointed out that the infection rate of EVD can be reduced to 0.80%4,10 under the use of strict disinfection skin preparation and strict aseptic operations, including subcutaneous tunnels and disinfection films. However, these surgical methods are very difficult to carry out in every clinical work, especially in emergency surgery. The infection rate of ordinary craniotomy, including resection of brain tumours, clearance of intracerebral haematoma, brain trauma surgery, cranioplasty, and microvascular decompression, is approximately 2.2–19.8%.11–13 The infection rate of shunt surgery represented by ventriculoperitoneal shunt (V-P shunt) is approximately 5–12%.14 Because this kind of operation is also common in children, many studies have reported that the infection rate in children is approximately 3–15%.15 Postoperative intracranial infection, especially the infection of implant surgery, has brought great harm to patients, and some severe infections can lead to death.16,17
For the types of bacteria infected after neurosurgery, most studies have noted that coagulase-negative staphylococci are the main genus of infection,18 but in recent years, an increasing number of studies have reported that the infection rate of gram-negative bacilli is increasing.17,19–21 In particular, A. baumannii has a particularly high mortality rate because of its multiple drug resistance.17,20 Because the majority of antibiotics cannot pass through the blood‒brain barrier or the blood‒brain barrier permeation rate is very low and unable to achieve effective bacteriostatic or bactericidal concentrations, intracranial infections caused by drug-resistant gram-negative bacilli have very serious consequences; these bacteria mainly include multidrug resistant (MDR), extensively drug resistant (XDR) or even pandrug resistant (PDR) A. baumannii, Pseudomonas aeruginosa and Enterobacteriaceae.22–24
Carbapenem-resistant Enterobacteriaceae (CRE) mainly refers to Enterobacteriaceae that is resistant to any kind of carbapenem and it is usually MDR, XDR or even PDR bacteria. CRE mainly includes Klebsiella pneumoniae and Escherichia coli which are also the top two CRE in China.25 More and more CRE meningitis has been reported in recent years, but few cases have been successfully cured. Briefly, CRE meningitis is one of the most difficult infection to cure because the bacteria have high drug resistance, greater toxicity, and severe systemic reactions, and it is easier to form obstructive hydrocephalus, leading to the death of patients. Our article mainly reviews the risk factors, diagnosis and treatment of CRE ventriculitis and meningitis after neurosurgery.
Risk Factors of Carbapenem-Resistant Enterobacteriaceae (CRE) Ventriculitis and Meningitis
Postsurgery infection is a common complication. Due to different surgical methods in neurosurgery, the infection rate is also different, and the risk factors for surgical infection are not the same. Many literatures from 2016 to 2022 have pointed out the risk factors for infection after EVD, including CSF leak, insertion site dehiscence, catheterization time and many other factors.5,8,21,26–35 We summarized the literature in recent years in Table 1. For craniotomy, we also summarized many studies. The risk factors for postoperative infection include the length of operation, reoperation, external drainage and other risk factors.18,36–44 We have also summarized the literature in recent years in Table 2.
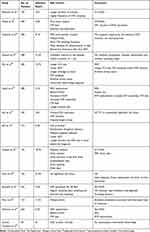 |
Table 1 Risk Factors of EVD Infections in Previous Literatures |
 |
Table 2 Risk Factors of Postcraniotomy Infection in Previous Literature |
Among these postoperative infections, we found that the proportion of gram-negative bacilli is increasing, and there are increasingly more reports of Enterobacteriaceae ventriculitis and meningitis. Shi et al45 analysed 2416 patients with postoperative craniocerebral infection from 2014 to 2019 and found that Enterobacteriaceae infection accounted for 7.3%. A total of 164 cases had a statistical prognosis, including 77 patients with Klebsiella pneumoniae infection, accounting for 47%. The risk factors for death caused by Enterobacteriaceae meningitis/encephalitis infection include comorbidities, Glasgow Coma Scale (GCS) score, sepsis, intensive care unit (ICU) admission, extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae, and ventilation. An updated literature counted 90 patients with iatrogenic meningitis caused by multidrug-resistant Enterobacteriaceae after neurosurgery, of which 46 were Klebsiella pneumoniae, and 40 of them were resistant to carbapenem. In this paper, univariate analysis showed that the rates of EVD, assisted mechanical ventilation (AMV), GCS scores ≤ 8, ICU admission, sepsis, and hospital-acquired pneumonia (HAP) were significantly different between patients in the survival and nonsurvival groups, and Multivariate Cox survival analysis showed that EVD and a GCS score ≤ 8 were independent mortality risk factors for patients with MDR Enterobacteriaceae meningitis.46 In addition, the literature also suggested that the risk factors for CRE infection were surgical wound classification, ventilation rate, craniotomy, bacteraemia, ICU admission, hospital-acquired pneumonia and mortality attributed to infection. Among them, hospital-acquired pneumonia and mortality attributed to infection were identified as independent risk factors for CRE meningitis/encephalitis.47 We summarize these studies in Table 3.
 |
Table 3 The Risk Factors of Enterobacteriaceae Meningitis in Previous Studies |
Diagnosis of Iatrogenic Ventriculitis and Meningitis
There are a variety of clinical manifestations of postoperative intracranial infection, and sometimes it is challenging to diagnose the infection, especially in patients after craniocerebral surgery. The majority of patients have clear clinical signs and laboratory changes, such as new headache, nausea, fever, somnolence, and decreased GCS score. In particular, fever, in the absence of another clear source of infection, indicates central nervous system (CNS) infection during recent head trauma or neurosurgery. Of course, the emergence of new meningeal irritation signs after neurosurgery also suggests the possibility of infection. During shunt surgery, erythema tenderness in the subcutaneous shunt and new abdominal tenderness in peritonitis indicate the possibility of infection.Laboratory examination is also an important index for the diagnosis of iatrogenic ventriculitis and meningitis, especially cerebrospinal fluid (CSF) examination, which plays an important role in the diagnosis of meningitis. Cerebrospinal fluid culture is the most important index, and it also provides a basis for further targeted treatment. However, cerebrospinal fluid culture and smears do not have positive results in most cases. Therefore, the number of leukocytes in cerebrospinal fluid and changes in sugar and protein play an important role in the diagnosis of meningitis. However, low glucose, elevated neutrophils and protein changes in cerebrospinal fluid tests cannot determine intracranial bacterial infections. Similarly, a normal cerebrospinal fluid test cannot rule out intracranial bacterial infections. In addition, positive blood culture can also indicate the types of bacteria in intracranial infections.Imaging examination is necessary for patients with suspected intracranial infection, and contrast-enhanced MRI and diffusion weighted imaging (DWI) are necessary for patients with abscess. MRI can not only make a definite diagnosis of hydrocephalus caused by severe infection, especially obstructive hydrocephalus but also provide a basis for further operation.
In recent years, some new specific tests have been incorporated into the diagnosis of iatrogenic ventriculitis and meningitis, such as CSF lactate48 or CSF procalcitonin49 for the diagnosis of bacterial intracranial infection, but its clinical application is not clear. In addition, nucleic acid amplification detection of infected CSF using polymerase chain reaction (PCR), which is used to determine pathogenic bacteria, is also widely used in clinics, but its accuracy is worth deliberating. The pathogens detected need to be consistent with the clinic before further treatment can be carried out.
Treatment of Carbapenem-Resistant Enterobacteriaceae (CRE) Ventriculitis and Meningitis
The treatment of carbapenem-resistant Enterobacteriaceae is very difficult, with a poor prognosis and high mortality of patients.50 Particularly in CRE ventriculitis and meningitis, most antibiotics cannot reach their bacteriostatic concentration in CSF due to the blood‒brain barrier (BBB). Carbapenem-resistant Enterobacteriaceae are generally extensively drug resistant (XDR) or pandrug resistant (PDR), and consequently, it is very difficult to treat CRE ventriculitis and meningitis. Next, we will review the treatment of CRE meningitis drugs and surgical selection.
Drug Therapy
Since the first case of CRE infection was reported, researchers have studies the mechanism of drug resistance, which is very complicated.51,52 The main reason is the production of carbapenemase, which is a class of β-lactamase that can hydrolyse carbapenem drugs and mainly includes three types of enzymes according to the Ambler molecular classification: Class A enzymes, mainly Klebsiella pneumonia carbapenemase (KPC); Class B metal enzymes, including IPM, VIM and New Delhi metallo-β-lactamase (NDM); and Class C enzymes, mainly OXA-23 and OXA-48. These drug resistance genes are mainly carried on the plasmid. The main reason for the drug resistance of CRE in China is the production of KPC, followed by NDM, and these bacteria can carry other drug resistance genes at the same time, such as qnr, OXA, ESBLs, and AmpC, thus forming XDR or PDR to all conventional antibiotics.25
Due to the extensive drug resistance of CRE, few therapeutic drugs are available in the clinic. Currently, these medications mainly comprise polymyxin, tigecycline, ceftazidime-avibactam and the new antibiotics meropenem/vaborbactam, cefiderocol, plazomicin, and eravacycline. Previous studies evaluated more than 1800 patients with CRE infection in China from 2012 to 2016 and found that the sensitivity rate of polymyxin was 96.9% and that of tigecycline was 89.7%.25 For CRE ventriculitis and meningitis, Shi et al45 reported 164 cases of intracranial Enterobacteriaceae meningitis from 2014 to 2019, of which 25 cases were CRE, which is sensitive to polymyxin, and most cases were sensitive to chloramphenicol and amikacin. Similarly, Zheng et al46 counted 90 patients with multidrug resistant Enterobacteriaceae meningitis, of which 40 were CRE. These cases were also all sensitive to polymyxin, and most were sensitive to trimethoprim/sulfamethoxazole and amikacin. Another report47 counted 133 cases of Enterobacter meningitis after neurosurgery, including 26 cases of CRE. These CRE cases were all sensitive to polymyxin; therefore, polymyxin + meropenem or polymyxin + meropenem + tigecycline was used in the follow-up treatment of patients, of whom 8 survived and 18 died. Through the report of Enterobacteriaceae meningitis after neurosurgery, we can conclude that CRE accounts for approximately 15% and 25% of intracranial Enterobacteriaceae infections, which is higher than that of other infections.53 All intracranial CRE are highly sensitive to polymyxin but not sensitive to tigecycline (the resistance rates are 35%, 60%, and 34.1% according to the three studies above); consequently, we still use polymyxin as an important drug in the treatment of CRE ventriculitis and meningitis. Some case reports have also shown that polymyxin is effective in the treatment of CRE ventriculitis and meningitis.54,55 In addition, some intracranial infections caused by CRE are sensitive to certain traditional antibiotics, such as amikacin56,57 and gentamicin,58 which have successfully saved the lives of some patients. According to retrospective studies, single use of tigecycline or polymyxin did not significantly increase mortality from pulmonary infection caused by CRE, while combination of drugs (carbapenem combined with polymyxin or tigecycline or gentamicin) reduced mortality.52,59 Similar studies have not been carried out in cases of intracranial CRE infection. But most cured intracranial CRE infection cases were used by multiple antibiotics.55–57,60
In recent years, a new antibiotic ceftazidime-avibactam has been used in the clinical treatment of patients with CRE infection. Avibactam is a novel synthetic β-lactamase inhibitor that inhibits a wide range of β-lactamases, including Class A (KPC), and some Class D (OXA-48) β-lactamases and it does not inhibit Class B (IMP, VIM, VEB, and NDM) β-lactamases. Since 2016, a series of cases have been reported that ceftazidime-avibactam alone or in combination with intraventricular administration of other drugs has achieved partial success in the treatment of CRE meningitis.60–66 We have summarized the reports in Table 4 and ceftazidime-avibactam is an important choice for the treatment of intracranial CRE producing KPC or OXA-48 β-lactamase. What’s more, Gatti et al66 has shown that ceftazidime-avibactam can penetrate BBB and reach an effective bactericidal concentration by increasing drug dosage (up to 2.5g q6h). In addition, ceftazidime -avibactam has no bactericidal activity against carbapenem-resistant A. baumannii, which is more common than CRE in intracranial infections.
 |
Table 4 Ceftazidime-Avibactam Treatment for CRE Ventriculitis and Meningitis |
A new drug Meropenem/Vaborbactam is approved in August 2017 by the United States Food and Drug Administration (FDA) for the treatment of complicated urinary tract infections (cUTIs), including pyelonephritis. Vaborbactam is a new type of β-lactamase inhibitor that mainly acts on class A carbapenemases, such as KPC, to fight CRE. In many studies, it can also be seen that its antibacterial effect is similar to that of ceftazidime-avibactam.67 However, its application in intracranial CRE infection is not clear because meropenem can partially pass through the blood‒brain barrier in the case of intracranial infection, but the distribution of vaborbactam in the brain is unknown. Whether it can pass through the BBB needs further study. In addition, it also had no killing activity against carbapenem-resistant A. baumannii.
Plazomicin is a new generation of semisynthetic aminoglycoside antibiotics and a structural derivative of sisomicin.68 It has antibacterial activity against CRE, which produces Class A and Class D β-lactamases. However, its antibacterial activity against Class B, especially NDM, is very poor, mainly because most NDM CRE carry 16S rRNA methyltransferase, which methylates nucleotides G1405 and A1408 on the 16S site of the ribosomal RNA 30S subunit, resulting in a significant decrease in drug affinity for the A site.68 Plazomicin was approved in June 2018 by the FDA for the treatment of adults with cUTIs, including pyelonephritis. However, its application in CRE meningitis has not been reported. Considering that most aminoglycosides cannot easily pass through the BBB, it is unlikely that plazomicin can do so.
Eravacycline is a newly synthesized tetracycline antibiotic with a wide antibacterial spectrum. Similar to tigecycline, it has no antibacterial activity against P. aeruginosa.69 It was approved by the FDA in August 2018 for the treatment of complicated intra-abdominal infections (cIAIs). Eravacycline has broad-spectrum antibacterial activity against Class A and B and D β-lactamases and thus has certain bactericidal activity against CRE. However, the application of eravacycline in intracranial CRE infection lacks clinical experience, and its BBB permeability is still unclear.
Cefiderocol is a novel siderophore cephalosporin that has a unique antibacterial mechanism in which it forms a bactericidal complex that is transported into bacteria via iron transporters.70 In addition, it can also resist carbapenemase and therefore has broad-spectrum antibacterial activity against Class A, B and D β-lactamases.71 In addition, many case reports also show that it has good bactericidal activity against intracranial CRE infection,72 but also has good effect on carbapenem-resistant A. baumannii (CRAB)73 and carbapenem-resistant P. aeruginosa (CRPA)74 meningitis. Considering that most cases are cured intravenously, cefiderocol should partially pass through the inflammatory blood‒brain barrier, but its effect still needs to be supported by further clinical trials.74
Surgical Treatment
EVD and lumbar drainage (LD) are important methods for the treatment of CRE ventriculitis and meningitis. On the one hand, EVD can drain infected cerebrospinal fluid and treat hydrocephalus. On the other hand many antibiotics, such as polymyxin, tigecycline, amikacin, and gentamicin, cannot pass through the BBB, whereas antibiotics that pass through the BBB are not effective against CRE. Therefore, it is necessary to inject antibiotics through EVD or LD. The dosages of intraventricular antimicrobial drugs should be selected based on CSF concentrations to 10–20 times the MIC of the pathogenic bacteria, ventricular volume, and daily drainage volume of CSF. When antibiotics are administered by intracerebroventricular injection, the drain is clamped for 15–60 minutes to allow the agent to equilibrate throughout the CSF.75 Of course, intrathecal injection of antibiotics has a serious of side effects, which mainly depends on the type of antibiotic used. For example, polymyxin can lead to chemical meningitis, seizures, and cauda equina syndrome.76 While aminoglycosides can cause transient hearing loss, seizures, chemical meningitis, and radiculopathy.24,77 In addition, there are few studies on whether EVD or LD is more suitable for intracranial drug injection, but there is no doubt that EVD is better than LD for postinfection obstructive hydrocephalus.
The treatment of obstructive hydrocephalus caused by CRE infection is extremely complex and difficult. Because one or two tubes of EVD cannot make CSF aseptic and isolated ventricles without drainage are the medium for bacterial reproduction, this results in poor infection control. Therefore, antibiotics with high BBB permeability are needed to control CRE ventriculitis and meningitis. However, in many cases, although ceftazidime-avibactam and cefiderocol can be used intravenously to control intracranial infection, they most often need to be combined with other antibiotics (such as polymyxin) via intraventricular injection. The treatment of obstructive hydrocephalus sometimes requires ventriculoscopy to turn obstructive hydrocephalus into communicating hydrocephalus, and then aseptic treatment of CSF is needed. However, the operation is sometimes very difficult, there are many serious postoperative complications, and the survival rate of the patients is not high. Sometimes craniotomy is needed to remove brain abscesses and subdural abscesses, which is undoubtedly another fatal blow to certain patients under the circumstances of poor infection control.
Conclusion
Infection after neurosurgery is still an important cause of death and disability in patients. Among the intracranial infections caused by various bacteria, although the incidence of CRE meningitis is low, it is difficult to treat and has high mortality, which has posed difficulties in the treatment of NICU patients. Considering that the mechanism of CRE resistance is complex, it is easy to form XDR and PDR pathogens, and most antibiotics have low capacity to pass through the BBB, it is difficult to control intracranial CRE infection by intravenous administration alone. There is an urgent need for new antibiotics with good blood‒brain barrier permeability and good antibacterial activity against CRE. In addition, obstructive hydrocephalus caused by CRE remains a difficult problem in the field of neurosurgery.
Data Sharing Statement
All data generated or analyzed in this study are included in this published article.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Li Z, Wu X, Yu J, et al. Empirical combination antibiotic therapy improves the outcome of nosocomial meningitis or ventriculitis in neuro-critical care unit patients. Surg Infect. 2016;17(4):465–472. doi:10.1089/sur.2015.060
2. Pintado V, Pazos R, Jiménez-Mejías ME, et al. Staphylococcus aureus meningitis in adults: a comparative cohort study of infections caused by meticillin-resistant and meticillin-susceptible strains. J Hosp Infect. 2019;102(1):108–115. doi:10.1016/j.jhin.2018.11.008
3. Iwasaki Y, Inokuchi R, Harada S, et al. Bacterial meningitis caused by hypervirulent Klebsiella pneumoniae capsular genotype K54 with development of granuloma-like nodal enhancement in the brain during the subacute phase. Intern Med. 2017;56(3):373–376. doi:10.2169/internalmedicine.56.7384
4. Flint AC, Rao VA, Renda NC, Faigeles BS, Lasman TE, Sheridan W. A simple protocol to prevent external ventricular drain infections. Neurosurgery. 2013;72(6):993–999. doi:10.1227/NEU.0b013e31828e8dfd
5. Jamjoom AAB, Joannides AJ, Poon MT-C. Prospective, multicentre study of external ventricular drainage-related infections in the UK and Ireland. J Neurol Neurosurg Psychiatry. 2018;89(2):120–126. doi:10.1136/jnnp-2017-316415
6. Bischoff P, Schröder C, Gastmeier P, et al. Surveillance of external ventricular drainage-associated meningitis and ventriculitis in German intensive care units. Infect Control Hosp Epidemiol. 2020;41:452–457. doi:10.1017/ice.2019.367
7. Hasbun R. Healthcare-associated ventriculitis: current and emerging diagnostic and treatment strategies. Expert Rev Anti Infect Ther. 2021;19(8):993–999. doi:10.1080/14787210.2021.1866544
8. Walek KW, Leary OP, Sastry R, et al. Risk factors and outcomes associated with external ventricular drain infections. Infect Control Hosp Epidemiol. 2022;43(12):1859–1866. doi:10.1017/ice.2022.23
9. Sorinola A, Buki A, Sandor J, Czeiter E. Risk factors of external ventricular drain infection: proposing a model for future studies. Front Neurol. 2019;10:226. doi:10.3389/fneur.2019.00226
10. Flint AC, Toossi S, Chan SL, Rao VA, Sheridan W. A simple infection control protocol durably reduces external ventricular drain infections to near-zero levels. World Neurosurg. 2017;99:518–523. doi:10.1016/j.wneu.2016.12.042
11. Schipmann S, Akalin E, Doods J, et al. When the infection hits the wound: matched case-control study in a neurosurgical patient collective including systematic literature review and risk factors analysis. World Neurosurg. 2016;95:178–189. doi:10.1016/j.wneu.2016.07.093
12. Jiménez-Martínez E, Cuervo G, Carratalà J. A care bundle intervention to prevent surgical site infections after a craniotomy. Clin Infect Dis. 2021;73(11):e3921–e3928. doi:10.1093/cid/ciaa884
13. Fang C, Zhu T, Zhang P, Xia L, Sun C. Risk factors of neurosurgical site infection after craniotomy: a systematic review and meta-analysis. Am J Infect Control. 2017;45(11):e123–e134. doi:10.1016/j.ajic.2017.06.009
14. Rosenthal VD, Richtmann R, Singh S, et al. International nosocomial infection control consortium. surgical site infections, international nosocomial infection control consortium (INICC) report, data summary of 30 countries, 2005–2010. Infect Control Hosp Epidemiol. 2013;34(6):597–604. doi:10.1086/670626
15. Gibson A, Kaplan S, Vallejo J, et al. Impact of serum vancomycin trough levels in the treatment of central nervous system shunt infections caused by coagulase-negative staphylococci. Pediatr Neurosurg. 2018;53(4):243–246. doi:10.1159/000488498
16. Hussein K, Bitterman R, Shofty B, et al. Management of post-neurosurgical meningitis: narrative review. Clin Microbiol Infect. 2017;23(9):621–628. doi:10.1016/j.cmi.2017.05.013
17. Panic H, Gjurasin B, Santini M, Kutlesa M, Papic N. Etiology and outcomes of healthcare-associated meningitis and ventriculitis-a single center cohort study. Infect Dis Rep. 2022;14(3):420–427. doi:10.3390/idr14030045
18. Chen C, Zhang B, Yu S, et al. The incidence and risk factors of meningitis after major craniotomy in China: a retrospective cohort study. PLoS One. 2014;9(7):e101961. doi:10.1371/journal.pone.0101961
19. Rogers T, Sok K, Erickson T, et al. The comparison of gram-positive and gram-negative healthcare-associated ventriculitis and meningitis in adults and children. Intensive Care Med. 2020;46(1):128–131. doi:10.1007/s00134-019-05815-7
20. Kurtaran B, Kuscu F, Ulu A, et al. The causes of post-operative meningitis: the comparison of gram-negative and gram-positive pathogens. Turk Neurosurg. 2018;28(4):589–596. doi:10.5137/1019-5149.JTN.20575-17.1
21. Mehreen SF, Padmaja K, Sudhaharan S, Teja VD, Saradhi MV, Krishna YV. Clinical and microbiological spectrum of external ventricular drain related infections (EVDRIs) from a tertiary care center. Iran J Microbiol. 2022;14(2):168–173. doi:10.18502/ijm.v14i2.9183
22. Munari M, Franzoi F, Sergi M, et al. Extensively drug-resistant and multidrug-resistant gram-negative pathogens in the neurocritical intensive care unit. Acta Neurochir. 2022;164(3):859–865. doi:10.1007/s00701-020-04611-3
23. Pandey S, Li L, Deng XY, Cui DM, Gao L. Outcome following the treatment of ventriculitis caused by multi/extensive drug resistance gram negative Bacilli; Acinetobacter baumannii and Klebsiella pneumonia. Front Neurol. 2019;9:1174. doi:10.3389/fneur.2018.01174
24. Karvouniaris M, Brotis A, Tsiakos K, Palli E, Koulenti D. Current perspectives on the diagnosis and management of healthcare-associated ventriculitis and meningitis. Infect Drug Resist. 2022;15:697–721. doi:10.2147/IDR.S326456
25. Wang Q, Wang X, Wang J. Phenotypic and genotypic characterization of carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi:10.1093/cid/ciy660
26. Khalaveh F, Fazel N, Mischkulnig M. Risk factors promoting external ventricular drain infections in adult neurosurgical patients at the intensive care unit—a retrospective study. Front Neurol. 2021;12:734156. doi:10.3389/fneur.2021.734156
27. Dakson A, Kameda-Smith M, Staudt MD, et al. A nationwide prospective multicenter study of external ventricular drainage: accuracy, safety, and related complications. J Neurosurg. 2021;26:1–9.
28. Zhu Y, Wen L, You W. Influence of ward environments on external ventricular drain infections: a retrospective risk factor analysis. Surg Infect. 2021;22(2):211–216. doi:10.1089/sur.2019.355
29. Sweid A, Weinberg JH, Abbas R. Predictors of ventriculostomy infection in a large single-center cohort. J Neurosurg. 2020;134(3):1218–1225. doi:10.3171/2020.2.JNS192051
30. Kim J, Lee J, Feng R. Ventricular catheter tract hemorrhage as a risk factor for ventriculostomy-related infection. Oper Neurosurg. 2020;18(1):69–74. doi:10.1093/ons/opz148
31. Sam J, Lim C, Sharda P, Wahab N. The organisms and factors affecting outcomes of external ventricular drainage catheter-related ventriculitis: a Penang experience. Asian J Neurosurg. 2018;13(2):250–257. doi:10.4103/ajns.AJNS_150_16
32. Hussein K, Rabino G, Feder O. Risk factors for meningitis in neurosurgical patients with cerebrospinal fluid drains: prospective observational cohort study. Acta Neurochir. 2019;161(3):517–524. doi:10.1007/s00701-019-03801-y
33. Kohli G, Singh R, Herschman Y, Mammis A. Infection incidence associated with external ventriculostomy placement: a comparison of outcomes in the emergency department, intensive care unit, and operating room. World Neurosurg. 2018;110:e135–e140. doi:10.1016/j.wneu.2017.10.129
34. Phan K, Schultz K, Huang C. External ventricular drain infections at the Canberra Hospital: a retrospective study. J Clin Neurosci. 2016;32:95–98. doi:10.1016/j.jocn.2016.03.019
35. Atkinson R, Fikrey L, Jones A, Pringle C, Patel HC. Cerebrospinal fluid infection associated with silver-impregnated external ventricular drain catheters. World Neurosurg. 2016;89:505–509. doi:10.1016/j.wneu.2016.01.034
36. Corsini Campioli C, Challener D, Comba IY. Overview and risk factors for postcraniotomy surgical site infection: a four-year experience. Antimicrob Steward Healthc Epidemiol. 2022;2(1):e14. doi:10.1017/ash.2021.258
37. Jiménez-Martínez E, Cuervo G, Hornero A. Risk factors for surgical site infection after craniotomy: a prospective cohort study. Antimicrob Resist Infect Control. 2019;8(1):69. doi:10.1186/s13756-019-0525-3
38. Wang L-Y, Cao X-H, Shi L-K, Ma -Z-Z, Wang Y, Liu Y. Risk factors for intracranial infection after craniotomy: a case-control study. Brain Behav. 2020;10(7):e01658. doi:10.1002/brb3.1658
39. Kuwano A, Saito T, Nitta M, et al. Relationship between characteristics of glioma treatment and surgical site infections. Acta Neurochir. 2022;165(3):659–666.
40. Maye H, Colombo F, Bourama E, et al. Does the use of surgical adjuncts affect postoperative infection rates in neuro-oncology surgery? World Neurosurg. 2022;162:e246–e250. doi:10.1016/j.wneu.2022.02.124
41. Caruso JP, Griffin S, El Ahmadieh TY. Surgical site infection after autologous cranioplasty for decompressive craniectomy in traumatic brain injury: a retrospective review of two level 1 trauma centers. J Craniofac Surg. 2021;32(8):2728–2731. doi:10.1097/SCS.0000000000007830
42. Alkhaibary A, Alharbi A, Abbas M. Predictors of surgical site infection in autologous cranioplasty: a retrospective analysis of subcutaneously preserved bone flaps in abdominal pockets. World Neurosurg. 2020;133:e627–e632. doi:10.1016/j.wneu.2019.09.120
43. Valentini L, Chiaffarino F, Bonfanti N, et al. Incidence and risk factors of neurosurgical site infections: results of a prospective multicenter cohort study on 6359 surgeries. J Neurosurg Sci. 2021;65(1):24–32. doi:10.23736/S0390-5616.18.04322-9
44. Shi Z-H, Xu M, Wang Y-Z. Post-craniotomy intracranial infection in patients with brain tumors: a retrospective analysis of 5723 consecutive patients. Br J Neurosurg. 2017;31(1):5–9. doi:10.1080/02688697.2016.1253827
45. Shi Y-J, Zheng G-H, Qian L-Y, Qsman RA, Li -G-G, Zhang G-J. Longitudinal analysis of risk factors for clinical outcomes of Enterobacteriaceae meningitis/Encephalitis in post-neurosurgical patients: a comparative cohort study during 2014–2019. Infect Drug Resist. 2020;13:2161–2170. doi:10.2147/IDR.S252331
46. Zheng G, Shi Y, Cao Y. Clinical feature, therapy, antimicrobial resistance gene distribution, and outcome of nosocomial meningitis induced by multidrug-resistant Enterobacteriaceae-a longitudinal cohort study from two neurosurgical centers in Northern China. Front Cell Infect Microbiol. 2022;12:839257. doi:10.3389/fcimb.2022.839257
47. Guanghui Z, Jing L, Guojun Z, Hong L. Epidemiology and risk factors of neurosurgical bacterial meningitis/encephalitis induced by carbapenem resistant Enterobacteriaceae. J Infect Chemother. 2020;26(1):101–106. doi:10.1016/j.jiac.2019.07.023
48. Baheerathan A, Pitceathly RD, Curtis C, Davies NW. CSF lactate. Pract Neurol. 2020;20(4):320–323. doi:10.1136/practneurol-2019-002191
49. Velissaris D, Pintea M, Pantzaris N, et al. The role of procalcitonin in the diagnosis of meningitis: a literature review. J Clin Med. 2018;7(6):148. doi:10.3390/jcm7060148
50. Willyard C. The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543(7643):15. doi:10.1038/nature.2017.21550
51. Iovleva A, Doi Y. Carbapenem-Resistant Enterobacteriaceae. Clin Lab Med. 2017;37(2):303–315. doi:10.1016/j.cll.2017.01.005
52. Suay-García B, Pérez-Gracia MT. Present and future of Carbapenem-resistant Enterobacteriaceae (CRE) infections. Antibiotics. 2019;8(3):122. doi:10.3390/antibiotics8030122
53. Zilberberg MD, Nathanson BH, Sulham K, Fan W, Shorr AF. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis. 2017;17(1):279. doi:10.1186/s12879-017-2383-z
54. Mermer S, Aydemir S, Ozgiray E, Sipahi OR. Carbapenem-resistant Klebsiella pneumoniae meningitis: a case report. J Chemother. 2016;28(5):454–455. doi:10.1179/1973947815Y.0000000020
55. Patrial YC, Tortorelli LP, Rodrigues ACS. Post-neurosurgical meningitis caused by KPC-producing Klebsiella pneumoniae: report of two cases. Rev Inst Med Trop Sao Paulo. 2019;61:e69. doi:10.1590/s1678-9946201961069
56. He Z, Wang C, Liu B, et al. Successful treatment of serious meningitis caused by extremely carbapenem-resistant Enterobacter cloacae (MIC≥16mg/L) with i.v. meropenem and i.v. amikacin plus intraventricular amikacin. Infect Drug Resist. 2019;12:3765–3770. doi:10.2147/IDR.S224509
57. Li J, Liu Y, Wu G, et al. Intravenous plus intraventricular tigecycline-amikacin therapy for the treatment of carbapenem-resistant Klebsiella pneumoniae ventriculitis: a case report. Medicine. 2022;101(30):e29635. doi:10.1097/MD.0000000000029635
58. Zhou M, Liang R, Liao Q, et al. Lumbar cistern drainage and gentamicin intrathecal injection in the treatment of carbapenem-resistant Klebsiella Pneumoniae intracranial infection after intracerebral hemorrhage craniotomy: a case report. Infect Drug Resist. 2022;15:6975–6983. doi:10.2147/IDR.S378753
59. Sheu -C-C, Chang Y-T, Lin S-Y, Chen Y-H, Hsueh P-R. Infections caused by carbapenem-resistant Enterobacteriaceae: an update on therapeutic options. Front Microbiol. 2019;10:80. doi:10.3389/fmicb.2019.00080
60. Yue C, Lei L. The treatment of nosocomial meningitis and brain abscess by carbapenem-resistant Klebsiella pneumonia. Br J Neurosurg. 2019;25:1–3.
61. Pektezel MY, Isikay I, Gocmen R, Inkaya AC. Carbapenem-resistant Klebsiella pneumoniae meningitis and abscess treated with ceftazidime-avibactam. Enferm Infecc Microbiol Clin. 2022;40(6):332–333. doi:10.1016/j.eimc.2021.03.014
62. Holyk A, Belden V, Lee JJ. Ceftazidime/avibactam use for carbapenem-resistant Klebsiella pneumoniae meningitis: a case report. J Antimicrob Chemother. 2018;73(1):254–256. doi:10.1093/jac/dkx358
63. Gofman N, To K, Whitman M, Garcia-Morales E. Successful treatment of ventriculitis caused by Pseudomonas aeruginosa and carbapenem-resistant Klebsiella pneumoniae with i.v. ceftazidime–avibactam and intrathecal amikacin. Am J Health Syst Pharm. 2018;75(13):953–957. doi:10.2146/ajhp170632
64. Yasmin M, Hanrahan J, Marshall S. Using therapeutic drug monitoring to treat KPC-producing Klebsiella pneumoniae central nervous system infection with ceftazidime/avibactam. Open Forum Infect Dis. 2020;7(9):ofaa349. doi:10.1093/ofid/ofaa349
65. De Santis LB, Gómez IM, Díaz BS, et al. Nosocomial meningitis caused by ESBL- and OXA-48-producing Klebsiella pneumoniae and treated with ceftazidime-avibactam. Report of one case and review of the literature. Rev Esp Quimioter. 2022;35(6):572–576. doi:10.37201/req/043.2022
66. Gatti M, Virgili G, Cojutti PG. Real-time optimization of pharmacodynamic target attainment at infection site during treatment of post-neurosurgical ventriculitis caused by carbapenem-resistant gram negatives with ceftazidime-avibactam-based regimens: a report of two cases. Microorganisms. 2022;10(1):154. doi:10.3390/microorganisms10010154
67. Ackley R, Roshdy D, Meredith J. Meropenem-vaborbactam versus ceftazidime-avibactam for treatment of carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2020;64(5):e02313–e02319. doi:10.1128/AAC.02313-19
68. Castanheira M, Davis AP, Mendes RE, Serio AW, Krause KM, Flamm RK. In vitro activity of plazomicin against gram-negative and gram-positive isolates collected from U.S. hospitals and comparative activities of aminoglycosides against carbapenem-resistant Enterobacteriaceae and isolates carrying carbapenemase genes. Antimicrob Agents Chemother. 2018;62(8):e00313–e00318. doi:10.1128/AAC.00313-18
69. Zhanel GG, Cheung D, Adam H. Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs. 2016;76(5):567–588. doi:10.1007/s40265-016-0545-8
70. Ito A, Nishikawa T, Matsumoto S. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2016;60(12):7396–7401. doi:10.1128/AAC.01405-16
71. Wright H, Bonomo RA, Paterson DL, et al. New agents for the treatment of infections with gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect. 2017;23(10):704–712. doi:10.1016/j.cmi.2017.09.001
72. Colombo F, Waheed A, Panese S. Treatment with cefiderocol in K. pneumoniae KPC nosocomial external ventricular drainage meningitis: a brief report. Infez Med. 2022;30(3):454–458. doi:10.53854/liim-3003-15
73. Kufel WD, Abouelhassan Y, Steele JM. Plasma and cerebrospinal fluid concentrations of cefiderocol during successful treatment of carbapenem-resistant Acinetobacter baumannii meningitis. J Antimicrob Chemother. 2022;77(10):2737–2741. doi:10.1093/jac/dkac248
74. Stevenson DR, Cherian BP, Kinzig M, Sörgel F, Wareham DW. Intravenous cefiderocol for neurosurgical meningitis from an extensively drug-resistant New-Delhi metallo-β-lactamase-producing Pseudomonas aeruginosa strain. J Glob Antimicrob Resist. 2023;32:29–30. doi:10.1016/j.jgar.2022.11.019
75. Tunkel AR, Hasbun R, Bhimraj A. 2017 infectious diseases society of america’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis. 2017;64(6):e34–e65. doi:10.1093/cid/ciw861
76. Bargiacchi O, De Rosa FG. Intrathecal or intraventricular colistin: a review. Infez Med. 2016;24(1):3–11.
77. Nau R, Blei C, Eiffert H. Intrathecal antibacterial and antifungal therapies. Clin Microbiol Rev. 2020;33(3):e00190–e00219. doi:10.1128/CMR.00190-19
78. Zheng G, Cao Y, Liu C. Phenotype, molecular characterisation and risk factors for postoperative meningitis caused by ESBL-producing-Enterobacteriaceae: a six years multi-centre comparative cohort study. BMC Infect Dis. 2021;21(1):85. doi:10.1186/s12879-021-05784-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
