Back to Journals » Journal of Pain Research » Volume 16
Treatment of Persistent Idiopathic Dentoalveolar Pain with Venlafaxine: A Multicentric Retrospective Study on Its Effectiveness and Safety
Authors Xiao X, Chai G, Wang B, Luo F
Received 8 May 2023
Accepted for publication 14 July 2023
Published 21 July 2023 Volume 2023:16 Pages 2487—2495
DOI https://doi.org/10.2147/JPR.S420492
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Keith
Xiong Xiao,1 Guoliang Chai,2 Baoguo Wang,3 Fang Luo4
1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Imaging Department, Beijing Puhua International Hospital, Beijing, People’s Republic of China; 3Department of Anesthesia, Sanbo Brain Hospital, Beijing, People’s Republic of China; 4Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Fang Luo, Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, #119, West Road of the South Fourth Ring, Fengtai District, Beijing, 100070, People’s Republic of China, Tel/Fax +86 10 59976667, Email [email protected]
Purpose: To determine the effectiveness and safety of venlafaxine in treating persistent idiopathic dentoalveolar pain (PIDP).
Patients and Methods: A retrospective analysis was conducted on a cohort comprising 129 patients with a definite diagnosis of PIDP, who were treated with venlafaxine between May 2020 and December 2022 at three different institutions. Baseline characteristics were statistically described, and visual analog scale (VAS) scores before and during treatment were collected. The percentage of pain relief was calculated. Differences in baseline characteristics between responsive and unresponsive patients were evaluated. Additionally, side effects experienced during treatment were also summarized.
Results: Among the included patients, 4 suffered immediate side effects following the initial dose of venlafaxine and the treatment was discontinued. 104 (80.6%) patients achieved pain relief. VAS scores of responsive patients at weeks 2, and months 1, 2, and 3 were significantly lower than baseline (p< 0.001). Duration of pain was the only factor related to responsiveness (Wilcoxon rank sum test p< 0.001, logistic regression p=0.001). 64 patients (49.6%) suffered from mild side effects. No serious side effects were observed during the study.
Conclusion: Venlafaxine is potentially effective and safe in the management of PIDP. Early application of venlafaxine following the diagnoses of PIDP can result in a higher possibility of pain relief.
Keywords: venlafaxine, persistent idiopathic dentoalveolar pain, effectiveness, serotonin-norepinephrine reuptake inhibitors, pain management
Introduction
Persistent idiopathic dentoalveolar pain (PIDP) is a chronic painful condition characterized by daily recurring unilateral intraoral dentoalveolar pain lasting more than 2 hours a day, for at least 3 months.1 The term PIDP was first used in the 1st edition of the International Classification of Orofacial Pain (ICOP) in 2020 and is also known as atypical odontalgia (AO), primary persistent dentoalveolar pain disorder, or phantom tooth pain.1,2 The exact prevalence of PIDP is inconclusive due to limited research; previous studies have reported a prevalence rate of 2.1% to 6% in certain populations and an incidence of 56.7% in patients who have had dental procedures.3,4
The clinical manifestations of PIDP include persistent pain with a usually moderate intensity in the intraoral dento-alveolar area.5 The pain is typically described as deep, dull, and pressure-like but with considerable variation in manifestation across patients.1 A subset of patients may also present with sensory abnormalities to cold, touch, and have pin-prick sensations on the affected side, and some patients may experience increased pain in response to percussion and/or palpation in the affected tooth or the dentoalveolar region.6 Notably, there are no significant clinical or radiographic findings that can explain the pain.1
Despite decades of research, there is still inadequate knowledge on the pain syndrome known as PIDP. The lack of specificity in its clinical manifestations makes timely and accurate diagnosis challenging.1,7 PIDP may occur spontaneously or after dental procedures. Clinical somatosensory assessment can either show positive or negative signs.1 Durham et al developed a screening instrument for AO and persistent dentoalveolar pain, which still requires field testing.8 It is suggested by ICOP-1 that the diagnoses of PIDP should be considered after other ICOP or International Classification of Headache Disorders, 3rd edition (ICHD-3) diagnoses are excluded.1,9 As a result, despite clarifying diagnostic criteria for PIDP by ICOP-1 in recent years, accurate diagnoses in a clinical setting remains challenging; leaving patients at the risk of misdiagnosis.10
The etiological mechanisms of PIDP are also not well understood. Psychological disorders such as depression, emotional stress, anxiety, hypochondria, and somatization have been suggested as possible causes.11 Somatosensory abnormalities may also be possible causative factors since a proportion of patients showed positive results in somatosensory assessments.1,12 Other researchers have proposed additional mechanisms like increased sodium channel density in nerve endings, increased neurotransmitter release, a lack of pain regulation, and neuroinflammation.13
The lack of understanding of the cause of PIDP makes it challenging to develop targeted treatments. Currently, there is a lack of high-quality clinical trials to establish standardized treatment modalities for PIDP, and treatment approaches are based on expert recommendations and clinical experiences.7,14 Tricyclic antidepressants (TCAs) and antiepileptics have been used to treat PIDP, with TCAs often being the first line of treatment, although their effectiveness is limited.15,16 Local anesthetics and Onabotulinumtoxin A injections are alternative options, but the evidences are from studies with smaller sample sizes and are rather inconsistent.17,18 Serotonin-norepinephrine reuptake inhibitors (SNRIs) are also used to treat various pain syndromes, including chronic orofacial pain, such as burning mouth syndrome and AO.19,20 Jia et al reported that duloxetine, as a SNRIs, can significantly improve chronic pain of PIDP patients, with tolerable side effects, which added to the evidence for the potential of SNRIs.7 However, some patients do not respond well to duloxetine or experienced intolerable side effects; therefore, the effects of other SNRIs should also be studied.
Venlafaxine and duloxetine are both SNRIs, with venlafaxine being the first SNRI to be marketed in the United States.21 A recent systematic review that included 13 studies confirmed that venlafaxine is a safe and well-tolerated analgesic drug used for the treatment of neuropathic pain.22 Although the review did not include PIDP, it did confirm the potential of venlafaxine in managing various pain syndromes.22 Since duloxetine has been proven to be effective in PIDP, venlafaxine may also have promising clinical significance. However, there have been few reports on the use of venlafaxine for the treatment of PIDP or AO. Therefore, we conducted this study to retrospectively analyze clinical data from patients diagnosed with PIDP, and to investigate the safety and effectiveness of venlafaxine in treating PIDP across multiple study centers.
Materials and Methods
Study Design and Participants
This retrospective study was approved by the Institutional Review Boards (IRBs) of Beijing Tiantan Hospital, Capital Medical University (BTH-CMU), Beijing Puhua International Hospital and Sanbo Brain Hospital, Capital Medical University, and is in compliance with the Declaration of Helsinki. Informed consent was waived by the IRBs of the respective institutions because of the retrospective nature of this study. The medical records of patients who were diagnosed with PIDP and had been prescribed venlafaxine for their treatment at these hospitals between May 2020 and Dec 2022 were collected retrospectively. The collection of medical records was performed in a de-identified manner. Afterwards, patients with clear diagnoses of PIDP were included in this study based on the inclusion and exclusion criteria.
Inclusion Criteria
- Patients who were diagnosed with PIDP according to ICOP;
- Patients not younger than 18 years old;
- Venlafaxine was prescribed to patients for the treatment of PIDP.
Exclusion Criteria
- Patients with other painful conditions, intracranial or maxillofacial diseases;
- Patients with a previous history of antidepressants;
- Patients with a history of mental illness or narcotic drug abuse;
- Patients who had received craniofacial surgical treatments before the prescription of venlafaxine;
- Patients who had been given other treatments including physical therapy or other medications for pain during the treatment of venlafaxine;
- Patients whose medical information were not completely and/or accurately recorded or required variables were not available from data acquisition;
- Patients who were lost to follow-up within 3 months of treatment except for those discontinued treatment of venlafaxine because of side effects or treatment dissatisfactory.
Medication Regimen
During the study period, all participating hospitals followed the same standardized dosage protocol for the prescription of venlafaxine. The starting dose was 37.5 mg per day, and patients were instructed to adjust their dosage weekly. If the pain was not completely controlled and there were no significant side effects, the dose was increased by 37.5 mg per day, until the maximum dose reached 225 mg per day, if necessary. If patients experienced intolerable side effects at the starting dose, they were advised to discontinue venlafaxine and seek alternative treatment options. In patients who achieved complete pain relief, the current dose was maintained. If side effects occurred during dose escalations, the dose was reduced to the last side-effect-free dose. Patients were instructed to return to the clinic every two weeks during the first month of treatment and every four weeks thereafter, to assess treatment effectiveness, report side effects, receive further dosage instructions, and obtain prescriptions for subsequent medications.
Data Acquisition
This study extracted baseline data from medical records, which included demographic variables (such as gender and age) and variables concerning the clinical conditions and treatments including: pain laterality, duration of disease, pain intensity, history of dental procedures, previous treatments, and depressive symptoms at diagnosis. Data on efficacy and safety of venlafaxine were also collected from hospital medical records of outpatient visits during treatment. These variables included: time of visit, pain intensity at visit, current dose of venlafaxine, side effects or complications, and other combined or subsequent treatments. Pain intensity was measured using a visual analog scale (VAS), with scores ranging from 0 (no pain) to 10 (worst pain imaginable). Depressive symptoms were assessed using the structured interview guide for Hamilton depression rating scale (HDRS). A decrease in VAS score of more than 50% from baseline was defined as pain relief,23 and was considered responsive to treatment, otherwise was considered as unresponsive. Treatment onset was defined as a decrease in VAS score by 30% compared to baseline.7 The time points of treatment onset and pain relief were enquired during outpatient visits and written in medical records, making it possible for this study to retrieve these variables.
Statistics Analyses
Statistical analyses were conducted using IBM SPSS Statistics Version 23. Each collected variable was analyzed, with means and standard deviations calculated for measurement data that followed a normal distribution, and quartiles calculated for those that did not. Frequencies and percentages were calculated for categorical data. Percentages of patients classified as responsive or unresponsive to treatment were described. For responsive patients, paired Wilcoxon tests were used to compare their VAS scores at 2 weeks, 1 month, 2 months, and 3 months after treatment initiation with their baseline VAS score. The percentages of side effects were also described. Univariate and multivariate binary logistic regression analyses were performed to identify the factors associated with venlafaxine responsiveness.
Results
Demographic Data
The study identified a total of 161 patients who suffered from pain syndrome that met the diagnostic criteria for PIDP, and were given venlafaxine for treatment at the participating study centers. Out of these patients, 17 had other coexisting pain syndromes, 6 were given other analgesics simultaneously, 5 had mental illnesses, 2 had a history of venlafaxine, and 2 had incomplete medical records. Eventually, 129 patients were included in the study based on the inclusion and exclusion criteria (Figure 1). All of these patients had tried taking analgesics. Table 1 summarizes the details of all included patients.
 |
Table 1 Summary of Included Patients (n=129) |
 |
Figure 1 The flow chart of patient inclusion and exclusion. |
Effectiveness of Venlafaxine
Among the included patients, 4 experienced side effects when given the lowest dose of venlafaxine, leading to immediate treatment discontinuation. Among the remaining 125 patients, 104 patients (80.6%) achieved pain relief during treatment for 3 months, while 21 patients (16.2%) quit their treatment of venlafaxine because of unsatisfactory efficacy (Figure 1).
The median dose at treatment onset was 75.0 mg per day (IQR: 75.0, 75.0 mg per day) after a median treatment duration of 9 days (IQR:8, 11 days) from treatment initiation. For patients who achieved pain relief, the median dose at pain relief was 112.5 mg per day (IQR: 75.0, 112.5 mg/d). And, the VAS score at 2 weeks, 1 month, 2 months and 3 months after the initial of treatment showed a significant decrease, compared to the baseline value (p<0.001, Figure 2). Patients who experienced pain relief had a maintenance dose of 187.5 mg per day (IQR: 150.0, 225.0 mg per day), while the maximum dose for patients who did not experience pain relief and discontinued treatment due to side effects or inadequate effectiveness was 187.5 mg per day (IQR: 150.0, 187.5 mg per day).
 |
Figure 2 The changing trend of VAS scores in patients who achieved pain relief. *p<0.001, paired Wilcoxon tests. Abbreviation: VAS, visual analog scale. |
Predictor of Efficacy
Baseline characteristics of patients who discontinued venlafaxine due to treatment dissatisfaction were statistically described and compared to patients who achieved satisfactory pain relief. It was found that the duration of pain (p<0.001) was the only significantly different variable between the two subgroups. Binary logistic regression also showed that a longer history of pain duration is a risk factor for unresponsiveness to venlafaxine (Table 2).
 |
Table 2 Statistical Tests and Logistic Regression to Identify Risk Factors for Responsiveness |
Side Effects During Treatment
A total of 64 patients (49.6%) suffered from side effects of venlafaxine including the four patients who quit treatment at the lowest initial dose (gastrointestinal disorders in 2, dizziness in 2, and drowsiness in 1). Most patients suffered from relatively mild side effects, with dry mouth (22.5%), sweating (20.2%), drowsiness (13.2%) and dizziness (13.2%) being the most commonly reported side effects (Table 3).
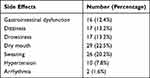 |
Table 3 Summary of Side Effects |
Discussion
As there is currently no established standard treatment protocol for PIDP and many patients have received ineffective treatments,1 it is important to assess the effectiveness of available treatments and explore potential approaches. To this end, we conducted a study using a multi-centric cohort of patients with confirmed diagnoses of PIDP who were treated with venlafaxine. Our findings indicate that this SNRI agent is both effective and safe for treating PIDP. Furthermore, we report that an early application of venlafaxine may result in better effectiveness in pain control. As our study may be the first to report on venlafaxine’s clinical value in treating PIDP, we hope that our results will provide a potential alternative treatment option for patients and support the development of a future standard treatment protocol for PIDP based on clinical evidence.
In this study, 104 patients (80.6%) experienced pain relief from venlafaxine treatment, which is comparable to a previous study that used duloxetine, another SNRI agent, and documented a 77.0% rate of pain relief.7 An earlier study reported a 65.9% efficacy in the treatment of AO with TCAs,16 and the percentage of pain relief observed with venlafaxine in this study was not lower than that value. However, a previous study reported that venlafaxine had a similar reduction in VAS score as placebo in the treatment of “persistent idiopathic facial pain” (PIFP), a neighboring entity to PIDP.1,24 But the study only included a small study cohort and the dose of venlafaxine was set at 75mg per day, whereas higher doses of venlafaxine provided better effectiveness in pain syndromes.22,25 This study provides better evidence, and based on the rate of pain relief in this study, we report that venlafaxine is considerably effective in the treatment of PIDP.
The pain-relieving mechanism of venlafaxine is still unclear, and the treatment mechanism for PIDP also remains to be studied.23 However, venlafaxine is known to inhibit the reuptake of serotonin at lower doses (<100 mg per day) and norepinephrine at high doses (100–375 mg per day), which work together alleviate pain.22,23 In our study, patients who achieved pain relief typically experienced treatment onset (a 30% decrease in VAS scores) within the first two weeks of treatment at the dose of 75.0 mg per day. This suggests that the inhibition of serotonin reuptake may play a role in venlafaxine’s analgesic effects for PIDP. Additionally, the median dose at which pain relief was achieved (a 50% decrease in VAS score) was 112.5 mg per day, indicating that the inhibition of norepinephrine reuptake may also have contributed in improving analgesia. Studies suggest that the analgesic effect of serotonin-norepinephrine reuptake inhibitors (SNRIs) may be related to their antidepressant properties, although this remains controversial.23,26 Our study found that the onset of treatment efficacy was 9 days, which is shorter than the usual time of onset for the treatment of depression, suggesting that the therapeutic effect of SNRIs on PIDP may not rely on their antidepressant properties and its mechanism is worthy of further investigation.
The duration of pain is a risk factor for unresponsiveness to venlafaxine. A similar finding is reported by Jia et al that this baseline variable is also a risk factor for failure of obtaining pain relief from duloxetine.7 PIDP is often misdiagnosed and mistreated, leading to delayed treatment due to its nonspecific symptoms and lack of awareness among physicians.1,10 In our study, NSAIDs were prescribed to 93.8% of patients before venlafaxine, but failed to provide pain relief. Since there is limited evidence that NSAIDs have a definite therapeutic effect on PIDP, it may be suggested that NSAIDs should not be prescribed alone to PIDP patients to avoid the painful experience of ineffective treatment and the decreased responsiveness caused by the delayed use of effective agents. It is also observed that aminophenol oxycodone and tramadol were also prescribed prior to venlafaxine, but failed to provide adequate pain relief. However, as literatures regarding the effectiveness of these two medications are still lacking, their clinical significance in the potential treatment of PIDP is yet to be determined. Also, these findings once again suggest that raising awareness in the field of dentistry, pain management and other related disciplines regarding PIDP is vital. Other variables were not found to have an association with responsiveness to venlafaxine. Although venlafaxine is usually prescribed as an antidepressant, however, baseline HDRS was not found to be a factor for responsiveness; this indicates again that its effect on PIDP might be independent from their effects of antidepressants.
Venlafaxine has been found to have been well-tolerated for treating neuropathic pain. Similar results were seen in previous studies.23,27,28 Although 49.6% of patients suffered from side effects, the majority of them were not severe. Only a small number of patients had hypertension or arrhythmia. Therefore, venlafaxine seems to be relatively safe in the treatment of PIDP, although regular monitoring of blood pressure and heart rate is also necessary. Nonetheless, it is important to consider the patient’s other medical conditions and medications before using venlafaxine for PIDP. Venlafaxine should be used with caution in patients with glaucoma, persistent hypertension, hypovolemia, recent myocardial infarction, and renal insufficiency.22 Since PIDP is generally seen in elderly patients, the chances of existing comorbidities are also relatively higher,1 therefore, careful patient selection is necessary. The use of Venlafaxine is also contraindicated in patients under monoamine oxidase inhibitors, metoprolol, selective serotonin reuptake inhibitors, anticoagulants, and antiplatelet medications.
This study has several limitations. First, it is retrospective and lacks a control group, making it less convincing than a prospective controlled study, despite being conducted at multiple study centers. Second, the sample size is limited, although we have pooled up cases from three research centers. Third, this study has not examined the serum drug concentration of the patients. When exploring the effects of drug dosage on treatment effectiveness and side effects, only rank variables of dosage can be used, quantitative analyses were impossible. Forth, groups with different doses were not set up to compare their effectiveness. However, this study was able to generate the aforementioned results. In the future, prospective studies including a larger sample size, a control group and patient randomization will be conducted, to avoid these limitations. However, based on this study, it can be concluded that venlafaxine has therapeutic efficacy in the treatment of PIDP.
Conclusion
Venlafaxine is potentially effective and safe in the management of PIDP, as 80.6% of patients achieved pain relief and only 49.6% experienced side effects which were mostly mild. Early application of venlafaxine after diagnosis of PIDP may result in a higher possibility of pain relief.
Abbreviations
PIDP, persistent idiopathic dentoalveolar pain; VAS, visual analog score; ICOP, International Classification of Orofacial Pain; AO, atypical odontalgia; TCA, tricyclic antidepressants; SNRI, serotonin-norepinephrine reuptake inhibitors; HDRS, Hamilton depression rating scale.
Acknowledgments
We acknowledge our colleagues who contributed to this work but not met the criteria for authorship. This study was funded by the Capital’s Funds for Health Improvement and Research (2020-2-2046) and Capital Medical University Research and Cultivation Fund (No. PYZ22115).
Disclosure
The authors report no conflicts of interest in this work.
References
1. ICOP. International classification of orofacial pain, 1st edition (ICOP). Cephalalgia. 2020;40(2):129–221. doi:10.1177/0333102419893823
2. Benoliel R, Gaul C. Persistent idiopathic facial pain. Cephalalgia. 2017;37(7):680–691. doi:10.1177/0333102417706349
3. Marbach JJ, Hulbrock J, Hohn C, Segal AG. Incidence of phantom tooth pain: an atypical facial neuralgia. Oral Surg Oral Med Oral Pathol. 1982;53(2):190–193. doi:10.1016/0030-4220(82)90285-7
4. Miura A, Tu TTH, Shinohara Y, et al. Psychiatric comorbidities in patients with Atypical Odontalgia. J Psychosom Res. 2018;104:35–40. doi:10.1016/j.jpsychores.2017.11.001
5. Dawson A, Dawson J, Ernberg M. The effect of botulinum toxin A on patients with persistent idiopathic dentoalveolar pain-A systematic review. J Oral Rehabil. 2020;47(9):1184–1191. doi:10.1111/joor.13053
6. List T, Leijon G, Svensson P. Somatosensory abnormalities in atypical odontalgia: a case-control study. Pain. 2008;139(2):333–341. doi:10.1016/j.pain.2008.05.002
7. Jia Z, Yu J, Zhao C, Ren H, Luo F. Outcomes and predictors of response of duloxetine for the treatment of persistent idiopathic dentoalveolar pain: a retrospective multicenter observational study. J Pain Res. 2022;15:3031–3041. doi:10.2147/JPR.S379430
8. Durham J, Stone SJ, Robinson LJ, Ohrbach R, Nixdorf DR. Development and preliminary evaluation of a new screening instrument for atypical odontalgia and persistent dentoalveolar pain disorder. Int Endod J. 2019;52(3):279–287. doi:10.1111/iej.13017
9. Olesen J, Bendtsen L, Goadsby P. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
10. Xiao X, Jiang L, Liu L, Chai G, Luo F. Challenges of misdiagnosis and suboptimal treatment of persistent idiopathic facial pain and atypical odontalgia: a retrospective multi-centric cross-sectional investigation. J Pain Res. 2020;13:2853–2860. doi:10.2147/JPR.S269329
11. List T, Leijon G, Helkimo M, Oster A, Dworkin SF, Svensson P. Clinical findings and psychosocial factors in patients with atypical odontalgia: a case-control study. J Orofac Pain. 2007;21(2):89–98.
12. Zagury JG, Eliav E, Heir GM, et al. Prolonged gingival cold allodynia: a novel finding in patients with atypical odontalgia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(3):312–319. doi:10.1016/j.tripleo.2010.10.008
13. Miyauchi T, Tokura T, Kimura H, et al. Effect of antidepressant treatment on plasma levels of neuroinflammation-associated molecules in patients with somatic symptom disorder with predominant pain around the orofacial region. Hum Psychopharmacol. 2019;34(4):e2698. doi:10.1002/hup.2698
14. Abiko Y, Matsuoka H, Chiba I, Toyofuku A. Current evidence on atypical odontalgia: diagnosis and clinical management. Int J Dent. 2012;2012:518548. doi:10.1155/2012/518548
15. Tarce M, Barbieri C, Sardella A. Atypical odontalgia: an up-to-date view. Minerva Stomatol. 2013;62(5):163–181.
16. Tu TTH, Miura A, Shinohara Y, et al. Pharmacotherapeutic outcomes in atypical odontalgia: determinants of pain relief. J Pain Res. 2019;12:831–839. doi:10.2147/JPR.S188362
17. Cuadrado ML, Garcia-Moreno H, Arias JA, Pareja JA. Botulinum neurotoxin type-A for the treatment of atypical odontalgia. Pain Med. 2016;17(9):1717–1721. doi:10.1093/pm/pnw040
18. Garcia-Saez R, Gutierrez-Viedma A, Gonzalez-Garcia N, Gomez-Mayordomo V, Porta-Etessam J, Cuadrado ML. OnabotulinumtoxinA injections for atypical odontalgia: an open-label study on nine patients. J Pain Res. 2018;11:1583–1588. doi:10.2147/JPR.S169701
19. Ferreira GE, Abdel-Shaheed C, Underwood M, et al. Efficacy, safety, and tolerability of antidepressants for pain in adults: overview of systematic reviews. BMJ. 2023;380:e072415. doi:10.1136/bmj-2022-072415
20. Mico JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27(7):348–354. doi:10.1016/j.tips.2006.05.004
21. Coutens B, Yrondi A, Rampon C, Guiard BP. Psychopharmacological properties and therapeutic profile of the antidepressant venlafaxine. Psychopharmacology. 2022;239(9):2735–2752. doi:10.1007/s00213-022-06203-8
22. Aiyer R, Barkin RL, Bhatia A. Treatment of neuropathic pain with venlafaxine: a systematic review. Pain Med. 2017;18(10):1999–2012. doi:10.1093/pm/pnw261
23. Gallagher HC, Gallagher RM, Butler M, Buggy DJ, Henman MC. Venlafaxine for neuropathic pain in adults. Cochrane Database Syst Rev. 2015;2015(8):CD011091. doi:10.1002/14651858.CD011091.pub2
24. Forssell H, Tasmuth T, Tenovuo O, Hampf G, Kalso E. Venlafaxine in the treatment of atypical facial pain: a randomized controlled trial. J Orofac Pain. 2004;18(2):131–137.
25. Trouvin AP, Perrot S, Lloret-Linares C. Efficacy of venlafaxine in neuropathic pain: a narrative review of optimized treatment. Clin Ther. 2017;39(6):1104–1122. doi:10.1016/j.clinthera.2017.05.347
26. Obata H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci. 2017;18(11):2483.
27. Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy. A randomized, double-blind trial. Neurosciences. 2014;19(3):192–198.
28. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697–706. doi:10.1016/j.pain.2004.05.010
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
