Back to Journals » Infection and Drug Resistance » Volume 10
Treatment of pediatric Clostridium difficile infection: a review on treatment efficacy and economic value
Authors D'Ostroph AR, So TY
Received 10 May 2017
Accepted for publication 29 August 2017
Published 19 October 2017 Volume 2017:10 Pages 365—375
DOI https://doi.org/10.2147/IDR.S119571
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Amanda R D’Ostroph,1 Tsz-Yin So2
1UNC Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, 2Department of Pharmacy, Moses H Cone Memorial Hospital, Greensboro, NC, USA
Abstract: The incidence of Clostridium difficile infection (CDI) in pediatric patients continues to rise. Most of the pediatric recommendations for CDI treatment are extrapolated from the literature and guidelines for adults. The American Academy of Pediatrics recommends oral metronidazole as the first-line treatment option for an initial CDI and the first recurrence if they are mild to moderate in severity. Oral vancomycin is recommended to be used for severe CDI and the second recurrent infection. Additional pulsed regimen of oral vancomycin, which is tapered, may increase efficacy in refractory patients. However, there is lack of large studies evaluating the use of fidaxomicin in pediatrics to know whether it could be a safe and effective treatment option for difficult-to-treat patients. Fidaxomicin is associated with higher total drug costs compared to metronidazole and vancomycin, but the literature supports its use due to a lower rate of CDI recurrence, which may result in cost savings. Further studies are warranted to evaluate the use of fidaxomicin in patients <18 years old and to understand its role in the standard of care for pediatric patients with CDI.
Keywords: Clostridium difficile, diarrhea, fidaxomicin, vancomycin, metronidazole, pediatrics
Introduction
Clostridium difficile is a spore-forming, anaerobic, Gram-positive bacillus. Humans acquire C. difficile from their environment or via the oral–fecal route, which can lead to infection. C. difficile produces two toxins: toxin A and toxin B.1 C. difficile is the most frequent cause of antimicrobial-associated diarrhea. The disruption of the body’s normal flora, often as a result of antimicrobial treatment, leads to overgrowth of C. difficile, toxin production, and disease development.2
C. difficile testing in infants <1 year of age is not recommended due to the high rates of colonization. Studies have shown that 37% of infants who are <1 month old are colonized with C. difficile and are asymptomatic.3 The toxins produced by the organism are important for disease pathogenesis and infection. As the child ages, the rates of C. difficile colonization decrease. A recent study reported 30% and 14% colonization rates in infants aged 1–6 months and 6–12 months, respectively.3 If testing were to be completed and the results were positive, additional workup is recommended to identify other alternative causes of diarrhea due to the rare incidence of disease in this age group. By 2 years of age, normal flora in the lower intestine is established similar to that of adults.2
The exposure to antibiotics is the most important risk factor for an initial CDI.4 Penicillins, cephalosporins, clindamycin, and fluoroquinolones are associated more commonly with CDI, whereas sulfonamides, tetracyclines, vancomycin, metronidazole, and aminoglycosides are less commonly linked in children. Pediatric patients who are exposed to multiple antibiotics from different classes in the previous 30 days have been shown in recent studies to be associated with severe and recurrent CDI.5,6 Other risk factors include acid-suppressing medications, such as proton pump inhibitors, and use of gastrointestinal feeding tubes.7,8
The risk factors for an initial CDI are similar to the risk factors for recurrent infections. Retrospective studies have associated an increased risk of recurrence in patients with complex medical conditions, cancer, recent surgery, or hospitalization and in those who were treated with any non-CDI antibiotics during the time of CDI treatment.6,9,10 Another retrospective study conducted by Lo Vecchio et al11 found that metronidazole has a five-fold increase in the risk of recurrent infection (OR =5.18, p=0.03). The reasons for treatment failure with metronidazole are not well understood. It has been hypothesized that as inflammation in the colon decreases following the initial few days of treatment, the concentration of metronidazole in the stool decreases due to higher levels of systemic absorption.12
The strain of C. difficile that has been associated with causing more severe disease in the late 2000s is the NAP1.13 Studies have shown that NAP1 accounts for 10%–19% of C. difficile isolates. The epidemiology of CDI may be changing as a result of this strain because pediatric patients are developing severe CDI with no prior exposure to antibiotics or health care facilities.14 Isolate identification alone does not change care, but it can influence the therapeutic decision-making process by accounting for potential drug resistance.13 Even though the NAP1 strain may have reduced role in the USA nowadays, this strain is still found to predict clinical outcomes in patients with CDI.15,16 Further studies are needed to determine whether the NAP1 strain or other strains are the reasons for more severe disease in children.
Classifying the severity of CDI based on clinical signs and symptoms is essential to treatment selection. Patients with mild CDI have mild diarrhea, which is defined as having three to five unformed stools per day, in addition to being afebrile and lacking notable laboratory abnormalities.17,18 Moderate CDI is often associated with nausea and vomiting, dehydration, white cell count >15,000/mm3, and elevated blood urea nitrogen and serum creatinine levels. Patients with severe CDI have severe or bloody diarrhea, pseudomembranous colitis, severe abdominal pain, fever, white cell count >20,000/mm3, albumin <2.5 mg/dL, and acute kidney injury. Complications of severe CDI include toxic megacolon, peritonitis, ileus, respiratory distress, hemodynamic instability, and end organ failure.17,18 However, these disease complications are not seen as often in children as in adults.19 In addition, it is important to note that disease severity can be overestimated if adult criteria for severe disease are used in children.20
The incidence of CDI in children has increased in the USA over the past few decades. Kim et al21 conducted a 5-year retrospective cohort study of pediatric patients hospitalized with CDAD in the USA. From 2001 to 2006, there was an increase in cases per 1,000 admissions (from 2.6 to 4.0; 53%; p=0.04) and cases per 10,000 patient-days (from 4.4 to 6.5; 47%; p=0.06). Nylund et al22 demonstrated a similar upward trend in cases of CDI, from 3,565 cases in 1997 to 7,779 cases in 2006 (14.9% per year, p<0.01). Recent studies, however, did not find any increases in severity of CDI and outcome measures such as colectomy or mortality.21–23 In order to help contain the spread of C. difficile and minimize the cases of CDI, it is recommended that clinicians should use gloves when caring for patients, wash hands with soap after caring for patients, and use germicidal wipes with 10% sodium hypochlorite for cleaning the contaminated environment.24
Standard of care
The AAP published a policy statement in 2013 giving providers guidance on how to effectively treat patients with CDI; however, only oral vancomycin is approved by the US FDA for use in pediatric CDI.25 Many of the recommendations made by the AAP are extrapolated from adult guidelines published by the SHEA and the IDSA.18 For all types of CDI, the AAP recommends stopping offending antimicrobial agents, if possible. For treatment of initial occurrence and first recurrence of mild-to-moderate disease, oral metronidazole 30 mg/kg/d in four divided doses (maximum dosage of 2 g/d) is the drug of choice over oral vancomycin and oral fidaxomicin.18 Metronidazole is the preferred agent because of its efficacy against VRE and its lower cost for the patients.26,27 If a child has severe CDI or has a second recurrence of CDI, the treatment of choice is oral vancomycin 40 mg/kg/d in four divided doses (maximum dosage of 500 mg/d).28 If the patient has not improved after 24–48 hours, the oral vancomycin dose may be increased to a maximum dosage of 2 g/d. Intravenous metronidazole (30 mg/kg/d) may be added to intracolonic vancomycin dosed every 6 hours for severely ill patients with ileus, who may not be able to tolerate oral antibiotics.29,30 According to expert opinions, enema volumes should be age dependent for pediatric patients (250 mg of vancomycin in 50 mL of normal saline for 1- to 3-year-old children; 375 mg in 75 mL for 4- to 9-year-old children; and 500 mg in 100 mL for ≥10-year-old patients).31 This regimen may help achieve a clinical cure in difficult-to-treat patients29 (Figure 1).
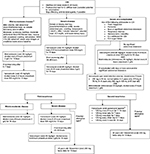  | Figure 1 Treatment algorithm for Clostridium difficile infection in pediatric patients. Notes: aThe European guideline only recommends treatment in patients with moderate infection; bif single dose is ≥125 mg based on the 40 mg/kg/d divided-four-times dosing; cbased on limited clinical data and case reports in pediatric patients. Data from previous studies.2,17,18,31,33 Abbreviations: C. difficile, Clostridium difficile; CDI, Clostridium difficile infection; ICU, intensive care unit; WBC, white blood cells. |
Some patients may develop another CDI infection after their initial clinical cure. Patients who have recurrent CDI that is not severe can be treated with metronidazole, and this decision should not be influenced by their previous drug exposure.18 Vancomycin can be selected over metronidazole if the recurrent CDI episode appears to be clinically more severe than the initial episode. Oral fidaxomicin can potentially be an alternative treatment option for the first recurrence of nonsevere CDI, but major studies evaluating its use in pediatric patients are lacking.25
Patients who are having multiple recurrences of CDI may benefit from tapering and pulsed oral vancomycin.32 In adults, patients initially receive 125 mg four times daily for 7–14 days. The taper then proceeds as follows: 125 mg twice daily for 7 days, 125 mg once daily for 7 days, 125 mg every other day for 7 days, and finally, 125 mg every 3 days for 14 days. This dosing scheme has not yet been validated in pediatric patients, but most experts will usually extrapolate it to their patients when the single dose is ≥125 mg based on the 40 mg/kg/d divided-four-times dosing (Figure 1). Oral fidaxomicin may also have a role in patients who are having multiple recurrences because studies have reported that it provided a low recurrence rate compared to the standard regimen of vancomycin.32 Table 1 summarizes the recommended medication doses for CDI treatment in pediatric patients.
  | Table 1 Dosing of medications for CDAD in pediatric patients Notes: *US FDA-approved adult dosing; based on data from a pharmacokinetic study41 in pediatric patients. Data from previous studies.18,28–32,41 Abbreviations: CDAD, Clostridium difficile-associated diarrhea; US FDA, United States Food and Drug Administration; IV, intravenous; N/A, not applicable. |
Unlike the recommendation by the AAP, the guidelines for the management of acute gastroenteritis in children, developed by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition as well as the European Society for Pediatric Infectious Diseases, only recommend antibiotic therapy for moderate and severe cases of CDI.33 For patients who have mild antibiotic-associated CDI, their recommendation is to discontinue the antibiotic being used. Oral metronidazole is still the first-line treatment option, reserving vancomycin as an alternative when resistant strains to metronidazole are known or anticipated.33
Pharmacology
Metronidazole is an antibiotic used in the treatment of anaerobic bacterial infections. It acts by diffusing into the organism and causing DNA strand breakage and a loss of its helical structure, altering the organism’s DNA.34 These effects on DNA inhibit protein synthesis and lead to cell death. When given orally, metronidazole is well absorbed, with a bioavailability of >90%. It distributes widely in the body, so that small quantities reach the colon, which may be a disadvantage pharmacodynamically for CDI.35 Poor blood flow from the systemic circulation to the colonic mucosa is a hypothesized reason for why metronidazole can be less effective at treating severe CDI. The liver metabolizes the parent compound to five metabolites, including the active hydroxyl metabolite, which has ~30%–65% activity and a longer half-life than the parent drug. Dose adjustments should be made for patients with liver disease due to a decrease in clearance of metronidazole.35 The drug is eliminated from the body through both urine and feces, with a half-life between 6 and 10 hours.34 Therefore, metronidazole should be given at higher doses less frequently due to its concentration-dependent bactericidal activity, prolonged half-life, and sustained activity.35
Vancomycin is a glycopeptide antibiotic that acts by inhibiting bacterial cell wall synthesis by tight binding to the d-alanyl-d-alanine portion of the cell wall precursor and blocking glycopeptide polymerization.36 When given orally, the systemic absorption of vancomycin is poor, and maximum drug concentrations remain localized to the gut, which is a major pharmacokinetic advantage compared to metronidazole.37 Oral vancomycin is eliminated from the body primarily through the feces.36 Serum vancomycin concentrations are typically undetectable if vancomycin is dosed orally at 125 mg every 6 hours.37 Higher doses, such as 500 mg every 6 hours, have been shown to result in higher serum concentrations (ie, 1.0–5.1 µg/mL).37 The use of pulsed vancomycin therapy may make the spores susceptible to the antibiotic when it is readministered, and prolonged treatment duration may also be important for clinical cure.32
Fidaxomicin is a novel macrolide antibiotic that acts on susceptible organisms, including C. difficile, by inhibiting the sigma subunit of RNA polymerase and, thereby, protein synthesis.38 Fidaxomicin is bactericidal and has postantibiotic activity, which may result in lower rates of CDI recurrence.39 This medication is administered orally and remains largely confined to the GI tract, resulting in minimal systemic absorption. The parent drug is metabolized by intestinal hydrolysis to a less-active metabolite (ie, OP-1118). The first pharmacokinetic study with fidaxomicin in children confirmed the minimal systemic absorption of fidaxomicin following oral administration, with desired plasma concentrations of fidaxomicin (mean: 13.36 ng/mL) and OP-1118 (mean: 60.16 ng/mL at 1–2 hours postdose) at therapeutic doses.40,41 These plasma concentrations were similar across all pediatric ages. Fidaxomicin is eliminated primarily through the feces, which was confirmed in children by the high fecal concentrations of the parent drug (mean: 3,227.93 µg/g) and OP-1118 (mean: 865.49 µg/g) after multiple dosing. There was a trend toward higher fecal concentrations in patients <6 years of age.40,41 The pharmacokinetics of fidaxomicin in children was similar to that reported in adults (ie, elimination half-life, t1/2 =0.94–2.77 hours; maximum observed serum concentration, Cmax =5 ng/mL; time taken to reach Cmax, Tmax =2 hours; area under the concentration–time curve, AUC =48.3 ng-h/mL).41,42
Safety and tolerability
The safety and tolerability of metronidazole and vancomycin in pediatric patients is not well documented in the literature. Metronidazole and vancomycin have been well studied in adult patients, and the safety and tolerability data are extrapolated from adults to support their use in pediatric patients. A 2015 meta-analysis and systematic review included 17 studies from 13 articles (n=2,501) assessing the safety of oral metronidazole monotherapy with oral vancomycin monotherapy and combination therapy in adult patients with mild or severe CDI.43 The studies did not show any statistically significant difference in the incidence of adverse effects between oral metronidazole and vancomycin (OR =1.18, p=0.41). The studies that compared monotherapy regimens to combination therapy regimens reported a significantly lower amount of adverse effects for either monotherapy option (OR =0.30, p<0.0001). Limitations of this analysis include a relatively small sample size of each subgroup when the total sample size was divided for defined comparisons, and follow-up times also varied among studies (3–12 weeks), which could have affected the results.43 Otherwise, common side effects of oral metronidazole include dose-dependent peripheral neuropathy and metallic taste as a result of systemic absorption.34 Oral vancomycin has minimal systemic absorption, which leads to a higher incidence of gastrointestinal symptoms such as nausea, abdominal pain, and vomiting.36
Fidaxomicin has not been studied extensively in pediatric patients. In 2014, an open-label study treated children aged 6 months to <18 years with fidaxomicin.40,41 This was the first study of fidaxomicin in children. Children <6 years of age were given 32 mg/kg/d of fidaxomicin divided twice daily (up to the adult dose, which is 200 mg twice daily). Children 6 years or older were given the recommended adult dose. There is a warning associated with fidaxomicin for patients who have a known allergy to macrolides because they may be at increased risk for sensitivity to fidaxomicin.39 Fidaxomicin has been found to be well tolerated in children and has very low rates of systemic side effects, which may positively affect treatment by increased compliance.44 However, 73.7% of the participants had at least one adverse event, mostly mild and moderate (44.7% and 21.1%, respectively). Adverse events reported include upper abdominal pain, constipation, diarrhea, nausea, and vomiting. This incidence of adverse events is more likely and expected in pediatric patients with an underlying moderate-to-severe CDI, and it is difficult to determine whether the adverse effects are from the drug or are the symptoms of the CDI. One limitation of the study is that fecal concentrations were only evaluated on the last day of therapy, which did not allow clinicians to determine whether fecal concentrations were at the desired levels throughout the duration of treatment.40,41
Efficacy
Efficacy data on the use of standard therapy for CDI in pediatric patients are limited, whereas the efficacy of metronidazole and vancomycin in the treatment of CDI in adults has been reported in the literature for decades.28,45 It is, however, important to evaluate some of these adult data in order to derive how pediatric patients with CDI are being treated. Early studies were conducted to establish optimal dosing to be used in clinical practice. Fekety et al45 conducted a randomized trial that compared two dosing regimens of vancomycin in hospitalized patients with nonsevere CDI (n=46, mean age: 54 years). The primary objective was treatment response, and the secondary objective was CDI recurrence. Patients were stratified according to probable colitis and definite colitis diagnosis and were randomized to one of two oral vancomycin dosing regimens. Oral vancomycin dosed at 125 mg every 6 hours was found to be noninferior to 500 mg every 6 hours for the treatment of nonsevere CDI (87.5% vs 95.4%, respectively, c2=1.36, p>0.05). Interestingly, one infant (unknown weight) was included in this study and responded well to 20 mg of oral vancomycin dosed at every 6 hours (an equivalent dose to the adult dosing based on body surface area). Because both regimens are equally effective and the 500 mg regimen is more expensive, the 125 mg regimen is preferred. One limitation of the study is the small sample size, which might have prevented the higher dose regimen from being seen as superior. Another limitation could be the lack of stratification for the severity of illness and the inability to determine whether the higher dose would have been more effective in the severely ill patients.45
Metronidazole is used as an effective, first-line treatment option for initial and first recurrence of mild-to-moderate CDI and is preferred over oral vancomycin. There have been reports of treatment failure in patients treated with oral metronidazole, which presents the need for further evaluation of the most appropriate first-line agent. Zar et al28 conducted a prospective, randomized, double-blind, placebo-controlled trial comparing oral metronidazole to oral vancomycin. Hospitalized patients were enrolled from October 1994 through June 2002 and stratified according to mild or severe disease based on clinical criteria. Thus, 172 patients (mean age: 58 years) were randomly assigned to receive oral metronidazole (250 mg four times daily) or oral vancomycin (125 mg four times daily) for 10 days. The primary outcomes were cure, treatment failure, and relapse after 21 days. In patients with mild CDI, the cure rates with metronidazole and vancomycin were 90% and 98%, respectively (p=0.36). In patients with severe CDI, the cure rates with metronidazole and vancomycin were 76% and 97%, respectively (p=0.02). One limitation of the study is that the participants had a 12.5% dropout rate, which could present attrition bias, but the study still achieved its defined power. The results demonstrated that both treatments can be used effectively for mild CDI; however, the superiority of vancomycin supports its use as the drug of choice for treating patients with severe CDI.28
Published data on the efficacy of fidaxomicin in children with mild or severe CDI are very limited. A 2013 case report demonstrated successful treatment with fidaxomicin in a 10-year-old child (unknown weight) with recurrent CDI.46 The patient had exposure to multiple antibiotic treatments for recurrent pneumonia and, as a result, had five previous episodes of CDI over a 1-year period. Previous recurrent infections were treated with oral vancomycin. The providers decided to treat this recurrent CDI with 200 mg of fidaxomicin twice daily for 10 days, and the tablets were crushed, mixed with water, and given via a gastrostomy tube. After 24 hours of treatment with fidaxomicin, the patient’s diarrhea improved, and he was able to be discharged home on the third day of his admission. Clinical cure was achieved and maintained for several months. The patient was treated with clarithromycin for a recurrent pneumonia, which resulted in another CDI. Fidaxomicin treatment was used a second time and once again had an effective response.46
Fidaxomicin is a potential treatment option for recurrent infections and for patients with severe CDI. Additional studies are needed to help guide providers to select between oral fidaxomicin versus oral vancomycin. In adults, fidaxomicin was compared to vancomycin for the treatment of CDI in a prospective, multicenter, double-blind, parallel-group trial.47 Patients (n=629) who were 16 years or older with a confirmed diagnosis of CDI (ie, presence of diarrhea and C. difficile toxin A, B, or both in stool specimen) were stratified by their first or second episode of CDI. Those participants were also randomized to receive fidaxomicin (200 mg twice daily) or vancomycin (125 mg four times daily) orally for 10 days. Severity of CDI was not a criterion for inclusion. The primary end point was clinical cure, which was defined as a resolution of symptoms and no need for additional therapy for CDI as of the second day after the end of the treatment course. Secondary end points were recurrence of CDI (diarrhea and positive stool toxin test within 4 weeks after treatment) and global cure. A total of 548 out of the 629 patients enrolled were evaluated for the per-protocol analysis. Fidaxomicin was found to be noninferior to vancomycin (92.1% and 89.8%, respectively) in the rates of clinical cure. In addition, fidaxomicin resulted in a significantly lower rate of recurrence (13.3% vs 24.0%, p=0.04), including the patients with non-NAP1 strain, and improved rate of global cure (77.7% vs 67.1%, p=0.006). These conclusions were confirmed in both the per-protocol and the modified intention-to-treat analysis.47
Economics
CDI is associated with a high monetary cost to the health care system and is a cause of significant morbidity and complications that can affect mortality. In 2013, the US Centers for Disease Control and Prevention reported that C. difficile poses an immediate public health threat. The average cost for CDI management from hospitalizations, follow-up care, and drug treatment between 2005 and 2015 was ~US$63,000. The attributable cost of hospital-onset CDI was 1.5 times the cost of community-onset cases (US$34,157 vs US$20,095).48 On the other hand, CDI is estimated to cost Europe €3 billion/y in direct costs from health care services and has the potential to almost double over the next 4 decades.49 A recent study by Asensio et al50 showed that the length of stay attributable to CDI was about 5 days in children living in Spain and Italy, and the total attributable cost was €3,545 per patient.
The cost of medications also plays into the overall cost of treating pediatric patients with CDI. Commercially available oral vancomycin costs ~US$71–US$143/d (depending on the dosing regimen chosen) compared to only US$2/d with oral metronidazole.24,51 Even though oral vancomycin can be compounded extemporaneously, which can reduce cost, there is still a significant price difference compared to metronidazole, which can affect compliance.24 Oral fidaxomicin costs ~US$335/d, which makes it the most expensive currently available CDI treatment.51 However, economic calculations often include additional factors besides the cost of the drug treatment when assessing the cost-effectiveness of a medication.
Cost-effectiveness data in pediatrics are lacking and the following are some adult data. Thomas et al52 compared the drug costs of oral vancomycin and oral metronidazole in a model that factored in a resistance rate to metronidazole of 20%. The study calculated the average cost of metronidazole treatment to be less than that of vancomycin (US$561 vs US$910, respectively). Costs would be equivalent between the two treatments only once metronidazole resistance rates reached 75%. In addition, vancomycin would achieve economic superiority if drug costs were able to be reduced by 88%. Limitations of this study are that the cost-effectiveness model did not define or account for disease severity and that the study did not include all direct and indirect costs for CDI treatment.52
A 2011 meta-analysis conducted in Canada included direct costs of CDI to the health care system (ie, hospital costs, physician payments, and diagnostic tests) and direct costs to the patients (ie, copayments).53 The calculated cost assumed that both metronidazole and vancomycin were given as the commercially available oral capsules. The average cost per case was Canadian dollar (CAD) 36,018 with metronidazole and CAD 36,250 with vancomycin, favoring metronidazole by CAD 232. An additional sensitivity analysis was completed to assess more difficult-to-treat isolates, which factored in estimated effectiveness (metronidazole 0.42 vs vancomycin 0.60) and the cost of the compounded vancomycin formulation. This analysis found the average cost of metronidazole to be CAD 36,464 and vancomycin to be CAD 33,465, which they attributed to a decreased length of hospital stay in patients treated with vancomycin. One limitation of this meta-analysis is that the studies that were included had small sample sizes, which could have influenced the calculated cost differences.53
After fidaxomicin received approval for the treatment of CDI, many payers and providers were interested to see how its costs compared to the costs of other standard-of-care medications. Gallagher et al54 evaluated patients (n=95, mean age: 72.1 years) who had received treatment for an initial CDI with vancomycin or fidaxomicin and calculated the average cost per patient. The total cost for each medication accounted for drug costs, hospital admission charges, and insurance reimbursements for any readmissions. The total drug costs for fidaxomicin and vancomycin were US$62,112 and US$6,646, respectively. Compared to vancomycin, fidaxomicin had a lower rate of recurrence and readmissions and saved the hospital ~US$142,507. By looking only at the considerable decrease in readmissions and cost savings, fidaxomicin was recommended to be used as first-line treatment over vancomycin.54
The British Society of Antimicrobial Chemotherapy55 also conducted a cost-effectiveness analysis of fidaxomicin versus vancomycin, which included patients with severe CDI and patients with their first CDI recurrence.55 A 1-year time horizon Markov model utilized effectiveness, resource use, direct costs, and utilities from published sources and a Scottish expert panel. The main model outcome for the two treatments was the ICER, which was expressed as cost per QALY. Patients with severe CDI had similar total treatment costs with fidaxomicin and vancomycin (£14,515 and £14,344, respectively). In patients with a first recurrence, treatment with fidaxomicin was less expensive than with vancomycin (£16,535 and £16,926, respectively). Fidaxomicin was cost-effective in severe CDI (ICER £16,529/QALY) and dominant in effectiveness and cost in patients with a first recurrence due to improvement in clinical outcomes. Interpreting cost-effectiveness using a willingness-to-pay threshold of £30,000/QALY showed that fidaxomicin was cost-effective for 60% of severe CDI and 68% of first recurrences.55
A recent decision analysis compared four different treatment options, namely, metronidazole, vancomycin, fidaxomicin, and FMT, for recurrent CDI in adults. This study found that FMT colonoscopy was the most cost-effective.56 FMT colonoscopy is not very well studied in pediatric patients and is not without risk. For settings where FMT is not applicable, the authors concluded that the preferred strategy to treat an initial recurrent infection is with oral vancomycin. The decision analysis results suggested that fidaxomicin is not currently a cost-effective option and would require a 35%–51% decrease in drug cost (ie, <US$1,359) to be cost-effective.56
All the current data on the cost attributable to pediatric CDI failed to account for the parental cost of loss of earning, in addition to the cost of child care for the rest of the family. This speculation or assumption can surely add to the overall cost of treating pediatric patients with CDI in the hospital setting. Even though some of the therapies mentioned earlier are more costly than the others, the cost should be minor compared with effective clinical outcomes and the ability to prevent recurrence; so using an agent that has been shown to combat this potentially life-threatening condition should have the utmost priority.
Current clinical application
Metronidazole should continue to serve as the first-line agent for initial and first recurrence of mild-to-moderate severity in pediatric patients with CDI. For patients who have an underlying infection for which prolonged non-CDI antibiotics are required, an additional week of CDI treatment may help reduce the risk of recurrence.18 Current guidelines by the American College of Gastroenterology recommend switching to oral vancomycin therapy for patients who are not responding to oral metronidazole after 5–7 days of treatment.57 Future studies should evaluate whether metronidazole should be given every 8 hours instead of every 6 hours for CDI in pediatric patients due to its concentration-dependent bactericidal activity and prolonged half-life. If patients are on vancomycin therapy and do not demonstrate clinical improvement within 24–48 hours or for patients who develop complications, the dose of oral vancomycin can be increased to a maximum of 500 mg every 6 hours in adults, although the supportive evidence of this higher dose is lacking in pediatric patients. After 3–5 days of treatment with oral vancomycin at this higher dose, it is appropriate to complete a laboratory draw and assess the concentration of vancomycin in the serum. Some uncertainties with oral vancomycin are the use of pulse and rectal drug dosing; further studies are needed to evaluate such practices in the pediatric population.
At this time, fidaxomicin has not been approved for the treatment of moderate-to-severe CDI in children, and there are limited data that demonstrate safe and effective use of this medication in children. However, the case report previously mentioned is a great example of a type of patient who may benefit from fidaxomicin.46 Fidaxomicin could potentially be considered once a pediatric patient has failed treatment with oral metronidazole and oral vancomycin and is still having recurrence of moderate-to-severe CDI. A gap in knowledge still remains as regards the use of fidaxomicin in pediatric patients, and more studies need to be completed before using it as the standard of care for this population. A Phase IIA, multicenter, open-label, uncontrolled study has recently been performed evaluating the safety, tolerability, and pharmacokinetics of fidaxomicin in patients between 6 months and 17 years of age.41 Patients were given 16 mg/kg per dose (maximum dose of 200 mg) orally every 12 hours, whereas patients aged 6 years to <18 years were given 200 mg every 12 hours; the duration of therapy for both groups was 10 days. Thirty eight patients were enrolled into this study. The data collection of this study has been completed and is pending publication.41 Astellas Pharma Inc and Merck Sharp & Dohme Corp are also currently studying an oral suspension formulation of fidaxomicin in an ongoing clinical trial comparing the safety and efficacy of fidaxomicin and vancomycin in pediatric patients with CDI.58 All these new data will surely enhance the potential usage of fidaxomicin for CDI in the pediatric population.
More CDI-based clinical trials are being conducted in pediatric patients. Of interest, investigators are evaluating the use of a new monoclonal antibody bezlotoxumab (MK-6072) in children with CDI.59 This medication is already being used in the adult population for the prevention of recurrent CDI.60 This is a Phase III, randomized, double-blind, placebo-controlled trial evaluating the safety, tolerability, pharmacokinetics, and efficacy of a single infusion of bezlotoxumab in children aged 1 year to <18 years with a confirmed diagnosis of CDI. The investigators will evaluate the concentration–time curve and AUC as few of the primary end points. They will also look at the recurrence rate of CDI and sustained clinical response (up to 12 weeks) after being on this therapy. This study is in the recruiting process, and if the results are positive, bezlotoxumab may potentially be added to the armamentarium of clinicians for preventing or treating pediatric patients with severe, recurrent CDI.59
Conclusion
Metronidazole and vancomycin are still the standard of care for the treatment of pediatric patients with CDI. Even though the data for their use in children are lacking, their proven efficacy and tolerability are mainly extrapolated from previously reported adult data and guidelines. Fidaxomicin provides an additional therapeutic option for patients with recurrent CDI. Studies that are available thus far show that fidaxomicin is well tolerated in pediatric patients and is effective at achieving clinical cure and preventing recurrent infections. Its use should be limited to cases of drug-resistant C. difficile isolates and refractory patients at this time. Clinicians may potentially refer to the adult recommended dosing for children older than 6 years of age. Further studies are needed to get fidaxomicin approved for use in patients <18 years old and to understand its role in the standard of care for pediatric patients with CDI.
AAP, American Academy of Pediatrics; CDAD, Clostridium difficile-associated diarrhea; CDI, Clostridium difficile infection; US FDA, United States Food and Drug Administration; FMT, fecal microbiota transplant; ICER, incremental cost-effectiveness ratio; IDSA, Infectious Diseases Society of America; NAP1, North American pulsed-field type 1 isolate; OR, odds ratio; QALY, quality-adjusted life year; SHEA, Society for Healthcare Epidemiology of America; VRE, vancomycin-resistant enterococcus.
Acknowledgment
The authors do not have any financial interest in any product or service mentioned in the manuscript and did not receive any financial support, including grants, equipment, medications, employment, gifts, or honoraria.
Disclosure
The authors report no conflicts of interest in this work.
References
Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18(2):247–263. | ||
Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013;167(6):567–573. | ||
Jangi S, Lamont JT. Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr. 2010;51(1):2–7. | ||
Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372(16):1539–1548. | ||
Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008;46(suppl 1):S19–S31. | ||
Nicholson MR, Thomsen IP, Slaughter JC, Creech CB, Edwards KM. Novel risk factors for recurrent Clostridium difficile infection in children. J Pediatr Gastroenterol Nutr. 2015;60(1):18–22. | ||
Kim J, Shaklee JF, Smathers S, et al. Risk factors and outcomes associated with severe Clostridium difficile infection in children. Pediatr Infect Dis J. 2012;31(2):134–138. | ||
Nylund CM, Eide M, Gorman GH. Association of Clostridium difficile infections with acid suppression medications in children. J Pediatr. 2014;165(5):979–984. | ||
Sandora TJ, Fung M, Flaherty K, et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J. 2011;30(7):580–584. | ||
Schwab EM, Wilkes J, Korgenski K, et al. Risk factors for recurrent Clostridium difficile infection in pediatric inpatients. American Academy of Pediatrics/Section on hospital medicine. Hosp Pediatr. 2016;6(6):339–344. | ||
Lo Vecchio A, Lancella L, Tagliabue C, et al. Clostridium difficile infection in children: epidemiology and risk of recurrence in a low-prevalence country. E J Clin Microbiol Infect Dis. 2017;36(1):177–185. | ||
Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut. 1986;27(10):1169–1172. | ||
Toltzis P, Kim J, Dul M, Zoltanski J, Smathers S, Zaoutis T. Presence of the epidemic North American pulsed field type 1 Clostridium difficile strain in hospitalized children. J Pediatr. 2009;154(4):607–608. | ||
Bryant K, McDonald LC. Clostridium difficile infections in children. Pediatr Infect Dis J. 2009;28(2):145–146. | ||
See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis. 2014;58(10):1394–1400. | ||
Bauer KA, Johnston JEW, Wenzler E, et al. Impact of the NAP-1 strain on disease severity, mortality, and recurrence of healthcare-associated Clostridium difficile infection. Anaerobe. 2017;48:1–6. | ||
Debast SB, Bauer MP, Kuijper EJ; European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(suppl 2):1–26. | ||
Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431–455. | ||
Spivack JG, Eppes SC, Klein JD. Clostridium difficile-associated diarrhea in a pediatric hospital. Clin Pediatr (Phila). 2003;42(4):347–352. | ||
Pai S, Aliyu SH, Enoch DA, Karas JA. Five years experience of Clostridium difficile infection in children at a UK tertiary hospital: proposed criteria for diagnosis and management. PLoS One. 2012;7(12):e51728. | ||
Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001–2006. Pediatrics. 2008;122(6):1266–1270. | ||
Nylund CM, Goudie A, Garza JM, Fairbrother G, Cohen MB. Clostridium difficile infection in hospitalized children in the United States. Arch Pediatr Adolesc Med. 2011;165(5):451–457. | ||
Zilberberg MD, Tillotson GS, McDonald C. Clostridium difficile infections among hospitalized children, United States, 1997– 2006. Emerg Infect Dis. 2010;16(4):604–609. | ||
Dubberke ER, Gerding DN, Classen D, et al. Strategies to prevent Clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S81–S92. | ||
Schutze GE, Willoughby RE; Committee on Infectious Diseases; American Academy of Pediatrics. Clostridium difficile infection in infants and children. Pediatrics. 2013;131(1):196–200. | ||
Wenisch C, Parschalk B, Hasenhündl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22(5):813–818. | ||
Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhea and colitis. Lancet. 1983;2(8358):1043–1046. | ||
Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45(3):302–307. | ||
Shetler K, Nieuwenhuis R, Wren SM, Triadafilopoulos G. Decompressive colonoscopy with intracolonic vancomycin administration for the treatment of severe pseudomembranous colitis. Surg Endosc. 2001;15(7):653–659. | ||
Rokas KE, Johnson JW, Beardsley JR, Ohl CA, Luther VP, Williamson JC. The addition of intravenous metronidazole to oral vancomycin is associated with improved mortality in critically ill patients with Clostridium difficile infection. Clin Infect Dis. 2015;61(6):934–941. | ||
Crews J, Kaplan SL, Torchia MM. In: Post TW, editor [webpage on the Internet]. Clostridium diffficile Infection in Children: Treatment and Outcomes. Available from: https://https://www.uptodate.com/contents/clostridium-difficile-infection-in-children-treatment-and-outcome. Accessed August 29, 2017. | ||
McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97(7):1769–1775. | ||
Guarino A, Ashkenazi S, Gendrel D, et al; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59(1):132–152. | ||
Metronidazole [prescribing Information]. Parsippany, NJ: Watson Labs; 2006. | ||
Lamp KC, Freeman CD, Klutman NE, Lacy MK. Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials. Clin Pharmacokinet. 1999;36(5):353–373. | ||
Vancomycin Hydrochloride [prescribing information]. Lake Forest, IL: Akron, Inc; 2013. | ||
Bhansali SG, Mullane K, Ting LS, et al. Pharmacokinetics of LFF571 and vancomycin in patients with moderate Clostridium difficile infections. Antimicrob Agents Chemother. 2015;59(3):1441–1445. | ||
Dificid (fidaxomicin) [prescribing information]. Whitehouse Station, NJ: Merck; 2015. | ||
Zhanel GG, Walkty AJ, Karlowsky JA. Fidaxomicin: A novel agent for the treatment of Clostridium difficile infection. Can J Infect Dis Med Microbiol. 2015;26(6):305–312. | ||
Sears P, Kaplan SL, Michaels M, Flanagan S, O’gorman M [webpage on the Internet]. Safety and Pharmacokinetic Study of Fidaxomicin in Children with Clostridium difficile-Associated Diarrhea. Philadelphia, PA: ID Week; 2014. Available from: https://idsa.confex.com/idsa/2014/webprogram/Paper48416.html. Accessed October, 2014. | ||
Optimer Pharmaceuticals LLC. Safety, tolerability, and pharmacokinetics of fidaxomicin in pediatric subjects with Clostridium difficile-associated diarrhea (CDAD). In ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine, US. 2000. Available from: https://clinicaltrials.gov/show/NCT01591863. NLM Identifier: NCT01591863. Accessed April 8, 2017. | ||
Shue YK, Sears PS, Shangle S, et al. Safety, tolerance, and pharmacokinetic studies of OPT-80 in healthy volunteers following single and multiple oral doses. Antimicrob Agents Chemother. 2008;52(4):1391–1395. | ||
Li R, Lu L, Lin Y, Wang M, Liu X. Efficacy and safety of metronidazole monotherapy versus vancomycin monotherapy or combination therapy in patients with Clostridium difficile infection: a systematic review and meta-analysis. PLoS One. 2015;10(10):e0137252. | ||
Louie T, Miller M, Donskey C, Mullane K, Goldstein EJ. Clinical outcomes, safety, and pharmacokinetics of OPT-80 in a phase 2 trial with patients with Clostridium difficile infection. Antimicrob Agents Chemother. 2009;53(1):223–238. | ||
Fekety R, Silva J, Kauffman C, Buggy B, Deery HG. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: comparison of two dosage regimens. Am J Med. 1989;86(1):15–19. | ||
Smeltzer S, Hassoun A. Successful use of fidaxomicin in recurrent Clostridium difficile infection in a child. J Antimicrob Chemother. 2013;68(7):1688–1689. | ||
Louie TJ, Miller MA, Mullane KM, et al; OPT-80-003 Clinical Study Group. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364(5):422–431. | ||
Zhnag S, Palazuelos-Munoz S, Balsells EM, Nair H, Chit A, Kyaw MH. Cost of hospital management of Clostridium difficile infection in United States – a meta-analysis and modeling study. BMC Infect Dis. 2016;16(1):447–465. | ||
Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. ESCMID Study Group for Clostridium difficile (ESGCD) and EU Member States and the European Centre for Disease Prevention and Control (ECDC). Clin Microbiol Infect. 2006;12(s6):2–18. | ||
Asensio A, Di Bella S, Lo Vecchio A, et al. The impact of Clostridium difficile infection on resource use and costs in hospitals in Spain and Italy: a matched cohort study. Int J Infect Dis. 2015;36:31–38. | ||
RED BOOK Online [database on the Internet]. Truven Health Analytics. Available from: http://www.micromedexsolutions.com. Accessed August 29, 2017. | ||
Thomas KL, Holmes K, Jackson BR, et al. A cost comparison of metronidazole and vancomycin in the treatment of Clostridium difficile associated diarrhea. Am J Gastroenterol. 2007;102(suppl 2):S268. | ||
Perras C, Tsakonas E, Ndegwa S, et al. Vancomycin or Metronidazole for Treatment of Clostridium difficile Infection: Clinical and Economic Analyses. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2011:1–56. Technology Report; No. 136. | ||
Gallagher JC, Reilly JP, Navalkele B, Downham G, Haynes K, Trivedi M. Clinical and economic benefits of fidaxomicin compared to vancomycin for Clostridium difficile infection. Antimicrob Agents Chemother. 2015;59(11):7007–7010. | ||
Nathwani D, Cornely OA, Van Engen AK, Odufowora-Sita O, Retsa P, Odeyemi IA. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother. 2014;69(11):2901–2912. | ||
Konijeti GG, Sauk J, Shrime MG, Gupta M, Ananthakrishnan AN. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis. 2014;58(11):1507–1514. | ||
Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478–498. | ||
Astellas Pharma Inc. A study to investigate the safety and efficacy of fidaxomicin (oral suspension or tablets) and vancomycin (oral liquid or capsules) in pediatric subjects with Clostridium difficile-associated diarrhea (CDAD) (SUNSHINE). In ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine, US. 2000. Available from: https://clinicaltrials.gov/show/NCT02218372. NLM Identifier: NCT02218372. Accessed August 29, 2017. | ||
Merck Sharp & Dohme Corp. Bezlotoxumab (MK-6072) versus placebo in children with Clostridium difficile infection (MODIFY III). In ClinicalTrials.gov [Internet]. Bethesda, MD: National Library of Medicine, US. 2000. Available from: https://clinicaltrials.gov/show/NCT03182907. NLM Identifier: NCT03182907. Accessed August 29, 2017. | ||
Wilcox MH, Gerding DN, Proxton IR, et al; MODIFY I and MODIFY II Investigators. Bezlotoxumab for prevention of recurrently Clostridium difficile infection. N Engl J Med. 2017;376(4):305–317. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
