Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Treatment of Cystic-Solid Thyroid Nodules with Ultrasound-Guided Radiofrequency Ablation and Enhancement of Thyroid Function
Received 7 June 2023
Accepted for publication 7 September 2023
Published 20 September 2023 Volume 2023:16 Pages 2773—2779
DOI https://doi.org/10.2147/JMDH.S424801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Qin Lou,1,* Yan-Feng Zhu,1,* Mei-Li Ye2
1Department of Ultrasonic Medicine, Affiliated Xiaoshan Hospital, Hangzhou Normal University, HangZhou, People’s Republic of China; 2SHU LAN(QU ZHOU)HOSPITAL, Quzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mei-Li Ye, SHU LAN(QU ZHOU)HOSPITAL, No. 2 Zhong Tower Floor, Fushang Street, Kecheng District, Quzhou, 324000, People’s Republic of China, Tel +8615167059261, Email [email protected]
Objective: To investigate the efficacy of ultrasound-guided radiofrequency ablation and its effect on thyroid function in patients with cystic-solid thyroid nodules.
Methods: We enrolled 90 patients with cystic-solid thyroid nodules and randomly assigned to either a control group (n = 37) or an observation group (n = 53). Patients in the observation group underwent ultrasound-guided radiofrequency ablation, while those in the control group were treated with ultrasound-guided lauromacrogol. Thyroid function was monitored, and complications were recorded for both groups, while nodule reduction rates were compared across a range of volumes and time periods.
Results: One month after surgery, the observation group had a larger volume of nodules than the control group, while at 12 months, the volume of nodules in the observation group was smaller. (P < 0.05). Thyroid-stimulating hormone (TSH), free thyroxine 4 (FT4), and free triiodothyronine (FT3) levels were all within normal ranges after treatment in both groups and showed no significant differences from pre-treatment levels. (P > 0.05). There was no statistically significant difference between the total incidence of adverse reactions in the control group (8.11%) and the observation group (5.66%) (P > 0.05).
Conclusion: With a low incidence of postoperative adverse reactions, the ultrasound-guided radiofrequency ablation protocol in the clinical treatment of patients with cystic-solid thyroid nodules can effectively reduce the volume of solid thyroid nodules without affecting the thyroid function of patients and can achieve more ideal treatment effectiveness, and is deserving of promotion.
Keywords: cystic-solid, radiofrequency ablation, thyroid function, thyroid nodules, ultrasound
Introduction
The thyroid gland, consisting of two connected lobes, is one of the largest endocrine glands in the human body, weighing 20–30 g in adults.1 Thyroid lesions are often found on the gland, with a prevalence of 4%–7%.1 Most of them are asymptomatic, and thyroid hormone secretion is normal. The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) is currently the commonly used thyroid nodule classification system, which uses a cytological examination method of fine needle aspiration biopsy of the thyroid gland to describe the cytological characteristics and malignant risk of thyroid nodules in thyroid tumors.2,3 Among them, type II and type III are the most common. Studies have shown that type II nodules are mostly benign with a malignancy risk of 0–3%, and type III nodules have a malignancy risk of 10–30%, which is recommended to repeat puncture or lobectomy.4–6 In clinical practice, cystic-solid nodules of the thyroid are quite common. Most nodules are harmless, but even so, they can still be unpleasant and disturbing for patients. Plus, they may develop into tumors if left unchecked. As a result, it is essential that patients receive prompt and effective care.7 The primary approach in the clinical treatment of cystic-solid thyroid nodules is surgical resection, which falls short of producing the desired therapeutic effect for patients because of the size of the incision and the length of recovery time.8 With few side effects and easy implementation, lauromacrogol effectively sclerosis cysts.9 As ultrasound-guided interventional therapy gains traction in clinical practice, more and more patients are being treated for cystic-solid thyroid nodules with ultrasound-guided radiofrequency ablation. The risks associated with this treatment are low because it involves minimal invasion and leaves behind no visible scarring. Clinical preference exists for both lauromacrogol and radiofrequency ablation, but a direct comparison of their respective benefits is rarely reported.10 Accordingly, the purpose of this study was to evaluate the clinical effectiveness of ultrasound-guided lauromacrogol versus ultrasound-guided radiofrequency ablation in the management of patients with cystic-solid thyroid nodules.
Materials and Methods
General Information
From October 2018 to September 2020, 90 patients who were treated for cystic-solid thyroid nodules at this hospital were enrolled and they were divided into a control group (n = 37) and an observation group (n = 53) based on the clinical data. In the control group, there were 10 males and 27 females, aged between 18–70 with an average age of (45.7 ± 4.7) years; 15 patients had a single thyroid nodule, while 12 patients had multiple nodules, with the longest nodule measuring 0.72–5.26 cm and the average nodule of (2.27 ± 0.53) cm. There were 9 males and 44 females, aged between 19–71 in the observation group, with a mean age of (45.2 ± 4.4) years and a standard deviation of 4.4 years; 29 patients had a single thyroid nodule and 24 patients had multiple nodules, with the largest nodule measuring 0.75–5.19 cm and an average of (2.28 ± 0.57) cm. There was no statistically significant difference between the groups in terms of age, gender, or the length of the largest nodule (P > 0.05). This study was reviewed and approved by the Ethics Committee of this hospital.
Inclusion and Exclusion Criteria
Patients must meet the following criteria to be enrolled in this study: ① patients whose imaging tests reveal cystic-solid thyroid nodules, as defined by clinical diagnosis criteria;11 ② patients whose pre-op tests reveal normal coagulation function and whose aspiration biopsies reveal benign lesions; ③ patients whose informed consent forms have been signed. Patients were excluded if they met any of the following criteria: ① patients with thyroid cancer;12 ② patients with serious dysfunction in important organs (heart, kidney, liver, etc.); ③ patients with neurological diseases, cognitive disorders, psychiatric diseases, and poor treatment compliance.
Methods
Both groups of patients were treated with ultrasound-guided procedures. Patients assigned to the observation group received radiofrequency ablation guided by ultrasound. Patients were placed in the supine position and given local anesthesia, then, their throats were swabbed, their nodules examined with a color Doppler ultrasound machine, and the volumes of the nodules recorded. The puncture site was marked immediately, and then a liquid isolation area was established to protect the recurrent laryngeal nerve, the trachea, the carotid artery, and the thyroid. The cyst fluid was drained using ultrasound guidance during a real-time puncture. Patients in the observation group underwent extensive radiofrequency ablation on a layer-by-layer basis (Starmed, models: 18–07s07F (0.7 cm) and 18–07s10F (1.0 cm), South Korea). Two injections of lauromacrogol at a dosage of one-third of the cyst’s fluid volume were given to patients in the control group. After 5 minutes of retention, the entire contents of the first injection were extracted, and 2–3 mL of lauromacrogol was injected and retained for the second injection.
Indexes for Observation
① Nodule volume at different time points in the two groups. At the 1st, 3rd, 6th, and 12th month after surgery, the volume of the nodules in each group was measured and compared. ② Patients had 3 mL of venous blood drawn while fasting in the morning before and after treatment, with heparin anticoagulation used to prevent excessive clotting. The supernatant was collected following a 15-minute centrifugation run and frozen at −800 °C for later use. Through the use of an automated chemiluminescent immunoanalyzer, we were able to detect the presence of free thyroxine 4 (FT4), free triiodothyronine (FT3), and thyroid-stimulating hormone (TSH) (Roche).11 ③ The occurrence of pain and other adverse reactions were observed.
Statistical Method
SPSS 22.0 software was used for the statistical analysis of the data, and P < 0.05 indicated that the difference was statistically significant. Percentages are used to describe the enumeration data, and the chi-squared test was used for statistical analysis. The t-test was used to make comparisons based on the mean ± SD (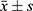 ) measurement data.
) measurement data.
Results
Comparison of Nodule Volumes Between the Two Groups at Different Time Points
Nodule volume did not differ significantly between the groups at any time point, including 3 and 6 months after surgery (P > 0.05). In the 1st post-operative month, the nodule volume in the observation group was greater than that in the control group, while it was smaller at 12 months. At certain post-operative intervals, such as 1 and 12 months, nodule volumes differed significantly between the two groups (P < 0.05, Table 1, Figures 1–2).
 |
Table 1 Comparative Analysis of Nodule Volume Between the Two Groups at Various Time Intervals (mL, |
Comparison of Thyroid Function Between the Two Groups
The levels of FT4, FT3, and TSH were all within normal ranges after treatment in both groups, showing no significant change from pre-treatment levels (P > 0.05, Table 2).
 |
Table 2 Comparison of Thyroid Function Indexes Between the Two Groups ( |
Comparison of Occurrence of Adverse Reactions Between the Two Groups
There was no statistically significant difference in the total incidence of adverse reactions between the observation group (5.66%) and the control group (8.11%) (P > 0.05, Table 3).
 |
Table 3 Comparative Analysis of Adverse Events Between the Two Groups [n (%)] |
Discussion
Most prevalent cystic-solid thyroid nodules can be either benign or malignant.13 Surgery is the mainstay treatment for benign thyroid nodules. However, open surgery has drawbacks that prevent it from satisfying patients’ needs for postoperative physical appearance. These include high trauma, excessive surgical bleeding, and a noticeable scar. Furthermore, postoperative hypothyroidism is common because the majority of surgically excised portions are larger than the volume of nodules. Due to its diminutive size and complex neighboring relationship, the thyroid is particularly vulnerable to a wide range of postoperative adverse reactions, including recurrent laryngeal nerve injury.14
Good clinical efficacy can be achieved with the two most common therapies used to treat thyroid nodules, radiofrequency ablation and lauromacrogol sclerosing, and both therapies have only a mild impact on thyroid function and surrounding tissues.15 The sclerosing effect is achieved by injecting lauromacrogol into the cyst, which directly injures the secretory cells of the cyst wall, triggering an aseptic inflammatory response that causes adhesion and fibrosis and ultimately blocks the cyst cavity.16 Ultrasound-guided radiofrequency ablation has become the standard method for treating patients with thyroid nodules in recent years. It requires much less surgical skill to implement, and it results in no subsequent scarring. A more complete therapeutic effect is achieved because it can both inactivate the solid parts and suction the cystic parts. Relevant studies have shown that the cutting-edge procedure of ultrasound-guided radiofrequency ablation is effective in the treatment of thyroid tumors by destroying the tumors with thermal energy.17 Therapeutic ultrasound-guided radiofrequency ablation relies on the probe’s high temperature to destroy tumor tissues in the thyroid. The high RF waves emitted by the probe electrode generate heat through the high-speed concussive collision and friction of polar macromolecules in the surrounding thyroid nodule tissues with ions. The increased temperature in the nodule area thus promotes the target nodule area to reach the effective treatment temperature. To induce tumor necrosis by solidification and stimulate and activate the autoimmune function to quicken the absorption rate of necrotic tissues, this high-temperature state is maintained for a certain period until dehydration and protein degeneration take place in the target lesion tissues. In the end, the cells are eradicated, and the nodules are shrunk or ablated. As previously reported, partial target areas can attain a temperature of around 95 °C during ultrasound-guided radiofrequency ablation, enabling prompt and efficient eradication of the tumor.18 In this study, we found that the nodule volume in the observation group gradually decreased in the 1st, 3rd, and 12th month after surgery, and that by the 12th month, the nodule volume in the observation group was smaller than that in the control group (P < 0.05). As thermal ablation typically inactivates tissue that extends just slightly beyond the primary nodule boundary, the volume immediately postoperatively is likely larger than expected. Unlike the control group, whose cystic cavity has already been closed and whose solid portions cannot be fully absorbed, inactivated tissues can continue to be absorbed with the passage of time. The results also showed that adverse reaction rates were lower in the observation group (P > 0.05). Consistent with the findings of He et al, ultrasound-guided radiofrequency ablation is an effective and safe method for treating thyroid nodules.19 This is because ultrasound-guided radiofrequency ablation can effectively reduce the amount of epidermal trauma, ensure the cosmetic success of the procedure, and minimize postoperative pain and other adverse reactions.
Thyroid function did not change noticeably in the observation group (P > 0.05) due to the fact that ultrasound-guided radiofrequency ablation has the potential to keep patients’ normal thyroid tissues intact and prevent any impaired thyroid function. A comprehensive operation is also possible, resulting in an efficient reduction of the nodule volume due to damage to the solid portions of the nodular cells from multiple directions. Furthermore, ultrasound-guided radiofrequency ablation can be used to achieve site-specific ablation of multiple thyroid nodules, avoiding further surgery, further reducing the risks of adverse reactions from the degree of surgical damage, and improving the ablation impact. Having a strong quasi-targeting property and minimal effect on surrounding tissues, it can shield the thyroid and ensure normal thyroid function. In addition, the surgical scope can be tightly controlled, and recovery time is minimized. The direction of the probe can be altered at any time with ultrasound guidance. The precise location of the lesion and the site of the puncture were identified with the aid of ultrasonography, as were other conditions such as the tissues surrounding the mass and the number and volume of lesions. In addition to ensuring radiofrequency precision, it can successfully avoid important high-risk vessels, critical tissues, and organs throughout the procedure, protect the thyroid and surrounding healthy tissues from damage, reduce surgical bleeding, and lessen the likelihood of postoperative pain. Additionally, a protective isolation zone can be established between nodules and nerves prior to treatment to effectively reduce nerve damage. An earlier study demonstrated that pain is a common side effect of radiofrequency ablation for patients with solid cystic thyroid nodules.20 Although two patients in the observation group experienced pain after treatment, there was no significant difference between the observation group and the control group, according to the findings of our study (P > 0.05). The pain was tolerable, and no special treatment was administered. Pain disappeared immediately following radiofrequency ablation, hence therapeutic efficacy was unaffected.
There are still limitations in this article. Firstly, the sample size we included is small and it is a single center study, and the results may have some bias; Secondly, our study did not strictly conduct randomized controls, which may result in bias in case selection; in addition, regarding the diagnosis of thyroid nodules, artificial intelligence has been widely used in ultrasound diagnosis and pathological analysis of thyroid nodules in recent years, and has achieved encouraging results.21–23 In the future, we can also introduce artificial intelligence into our hospital’s diagnosis and differentiation, in order to make more accurate and comprehensive judgments.
In conclusion, ultrasound-guided radiofrequency ablation in the clinical treatment of patients with cystic-solid thyroid nodules can effectively reduce the volume of the solid portions of the thyroid nodules without significantly impacting thyroid function, with good therapeutic efficacy, a low incidence of postoperative adverse reactions, and good surgical safety.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the Affiliated Xiaoshan No.1 Hospital of Hangzhou Normal University Medical College. Written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from every individual whose data are included in this manuscript.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
No external funding received to conduct this study.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Mulita F. Thyroid Adenoma. Treasure Island (FL): StatPearls Publishing; 2023.
2. Mulita F, Plachouri MK, Liolis E, Vailas M, Panagopoulos K, Maroulis I. Patient outcomes following surgical management of thyroid nodules classified as Bethesda category III (AUS/FLUS). Endokrynol Pol. 2021;72(2):143–144. doi:10.5603/EP.a2021.0018
3. Mulita F, Iliopoulos F, Tsilivigkos C, et al. Cancer rate of Bethesda category II thyroid nodules. Med Glas. 2022;19(1):1413–1421.
4. Wong R, Farrell SG, Grossmann M. Thyroid nodules: diagnosis and management. Med J Aust. 2018;209(2):92–98. PMID: 29996756. doi:10.5694/mja17.01204
5. Kant R, Davis A, Verma V. Thyroid nodules: advances in evaluation and management. Am Fam Physician. 2020;102(5):298–304.
6. Kobaly K, Kim CS, Mandel SJ. Contemporary management of thyroid nodules. Annu Rev Med. 2022;73:517–528. doi:10.1146/annurev-med-042220-015032
7. Huang HY, Yu T, Dai W. Diagnostic value of thyroid ultrasound combined with thyroid function in benign and malignant thyroid nodules. China and Foreign Medical Treatment. 2018;37(31):176–178. Chinese. doi:10.16662/j.cnki.1674-0742.2018.31.176
8. Muhammad H, Santhanam P, Russell JO. Radiofrequency ablation and thyroid nodules: updated systematic review. Endocrine. 2021;72(3):619–632.
9. Zhang M, Luo Y, Zhang Y, Tang J. Efficacy and safety of ultrasound-guided radiofrequency ablation for treating low-risk papillary thyroid microcarcinoma: a prospective study. Thyroid. 2016;26(11):1581–1587. doi:10.1089/thy.2015.0471
10. Li J, Xue W, Xu P, et al. Efficacy on radiofrequency ablation according to the types of benign thyroid nodules. Sci Rep. 2021;11(1):22270.
11. Sung JY, Baek JH, Kim KS, et al. Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology. 2013;269(1):293–300. doi:10.1148/radiol.13122134
12. Franz AM, Seitel A, Bopp N, et al. First clinical use of the EchoTrack guidance approach for radiofrequency ablation of thyroid gland nodules. Int J Comput Assist Radiol Surg. 2017;12(6):931–940. doi:10.1007/s11548-017-1560-2
13. Yang M, Luo YK, Wang XX, Zhang Y, Zhang MB, Ji B. Efficacy and safety of radiofrequency ablation combined with ethanol in ablation of benign cystic solid nodules. Chin J Med Imaging. 2019;4:249–253. Chinese.
14. Meng WU, Zhou RH, Yuan R, Zhao P, Lan YP. Evaluation of curative effect of ultrasound-guided ethanol ablation and radiofrequency ablation in treatment of thyroid cystic solid nodules. J Clin Ultrasound Med. 2017;9:606–609. Chinese.
15. Che Y, Jin S, Shi C, et al. Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol. 2015;36(7):1321–1325. doi:10.3174/ajnr.A4276
16. Koh J, Moon HJ, Park JS, et al. Variability in interpretation of ultrasound elastography and gray-scale ultrasound in assessing thyroid nodules. Ultrasound Med Biol. 2016;42(1):51–59. doi:10.1016/j.ultrasmedbio.2015.08.005
17. Papini E, Monpeyssen H, Frasoldati A, Hegedüs L. 2020 European Thyroid Association Clinical Practice Guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9(4):172–185.
18. Zhang BS, Peng LN, Wang FB, Zhang JR, Li SY, Wang W. Study on the application value of intravascular imaging technology combined with TI-RADS classification in the differential diagnosis of benign and malignant thyroid nodules. Heilongjiang Med Pharm. 2019;1:28–30. Chinese.
19. Wu YM, Wang HJ, Wang D, Wang Y, Liu YX. Clinical observation of ultrasound-guided percutaneous ethanol sclerotherapy for thyroid cyst. J Clin Exp Med. 2012;3:182–183+185. Chinese.
20. Li M, Liu N, Bai YL, Jiang J, Zhou Q, Lei XY. Value of contrast-enhanced ultrasound and ultrasonic elastography in differential diagnosis of benign and malignant thyroid nodules. J Clin Ultrasound Med. 2011;8:516–520. Chinese.
21. Girolami I, Marletta S, Pantanowitz L, et al. Impact of image analysis and artificial intelligence in thyroid pathology, with particular reference to cytological aspects. Cytopathology. 2020;31(5):432–444.
22. Elliott Range DD, Dov D, Kovalsky SZ, Henao R, Carin L, Cohen J. Application of a machine learning algorithm to predict malignancy in thyroid cytopathology. Cancer Cytopathol. 2020;128(4):287–295. doi:10.1002/cncy.22238
23. Ludwig M, Ludwig B, Mikuła A, Biernat S, Rudnicki J, Kaliszewski K. The use of artificial intelligence in the diagnosis and classification of thyroid nodules: an update. Cancers. 2023;15(3):708.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.