Back to Journals » International Journal of Nanomedicine » Volume 15
Trastuzumab Targeted Neratinib Loaded Poly-Amidoamine Dendrimer Nanocapsules for Breast Cancer Therapy
Authors Aleanizy FS , Alqahtani FY , Seto S, Al Khalil N , Aleshaiwi L, Alghamdi M, Alquadeib B , Alkahtani H , Aldarwesh A , Alqahtani QH, Abdelhady HG , Alsarra I
Received 4 April 2020
Accepted for publication 8 July 2020
Published 30 July 2020 Volume 2020:15 Pages 5433—5443
DOI https://doi.org/10.2147/IJN.S256898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Fadilah Sfouq Aleanizy,1,* Fulwah Yahya Alqahtani,1,* Sara Seto,1 Nora Al Khalil,1 Lama Aleshaiwi,1 Manar Alghamdi,1 Bushra Alquadeib,1 Hamad Alkahtani,2 Amal Aldarwesh,3 Qamraa Hamad Alqahtani,4 Hosam Gharib Abdelhady,5 Ibrahim Alsarra1
1Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 2Department of Pharmaceutical Chemistry, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 3Department of Optometry, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; 4Department of Pharmacology and Toxicology, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia; 5Department of Pharmaceutical Sciences, Eugene Applebaum College of Pharmacy and Health Sciences, Wayne State University, Detroit, MI, USA
*These authors contributed equally to this work
Correspondence: Fadilah Sfouq Aleanizy Email [email protected]
Background: Human epidermal growth factor receptor2 (Her2) positive breast cancer represents 25% of breast cancer cases. Targeted therapy with Her2 monoclonal antibody, trastuzumab (TZ), represents the first-line treatment for this type of breast cancer. In addition, neratinib, an irreversible inhibitor of the HER-2 receptor tyrosine kinase, has recently been approved as adjuvant therapy to TZ. This study aims to formulate (TZ)-grafted dendrimers loaded with neratinib, allowing a dual treatment alongside reducing the associated resistance as well as targeted therapy.
Methods: TZ was conjugated on the surface of dendrimer using hetero-cross linker, MAL-PEG-NHS, and the zeta potential, and in vitro release of neratinib from dendrimers was characterized. Formulated dendrimers were also fluorescently conjugated with fluorescein isothiocyanate to visualize and quantify their SKBR-3 cellular uptake.
Results: The G4 PAMAM dendrimer showed successful encapsulation of neratinib and a sustained release profile. Comparative in vitro studies revealed that these TZ-targeted dendrimers loaded with neratinib were more selective and have higher antiproliferation activity against SKBR-3 cells compared to neratinib alone and neratinib loaded dendrimer.
Conclusion: In the current study, neratinib loaded in plain and trastuzumab-grafted dendrimer were successfully prepared. Enhanced cellular uptake of trastuzumab conjugated dendrimers was shown, together with a higher cytotoxic effect than plain neratinib dendrimers. These findings suggest the potential of TZ-conjugated dendrimers as targeting carrier for cytotoxic drugs, including neratinib.
Keywords: neratinib, targeted dendrimer, trastuzumab, Her2-positive, breast cancer
Introduction
Breast cancer is responsible for roughly 30% of all recent cancer cases annually, with an incidence of about 4.5 thousand new cases of breast cancer diagnosed daily worldwide.1,2 Moreover, around 25% of breast cancers have an overexpressed human epidermal growth factor receptor2 (Her2),3 which is a transmembrane protein belonging to the HER family of 4 growth factor receptors (HER1 to HER4) that are very important in cellular growth and are often up-regulated in several cancers including breast cancer. HER-2 has become an important therapeutic target in breast cancer, and this could be explained by the fact that: (i) HER-2 level is a very important component of the pathogenesis and prognosis of breast cancer; (ii) human cancer cells showed higher expression and proven amplification of the HER-2 gene compared to normal adult tissues, suggesting that anti-HER-2 therapy would target most cancer cells in a given patient; and (iii) HER-2 overexpression is present both in the primary tumor and in metastatic sites, indicating that anti-HER-2 therapy may be effective in all disease stages.4–6 HER2-positive breast cancer is generally more aggressive and spreads more rapidly than HER2-negative breast cancer. Additionally, HER2-positive breast cancer is more difficult to treat with the hormonal therapy used for other types of breast cancer.7,8 The most important target in anticancer development is the discovery of targeted therapy in which the treatment is designed to kill cancer cells while minimizing damage to the healthy cells by subsiding the action of abnormal proteins that induce cell growth and out of control division. For example, trastuzumab (Herceptin) is a humanized monoclonal antibody directed against the human epidermal growth factor receptor-2 (HER2), which significantly reduced the risk of relapse and increased the survival in women with early-stage and metastatic cancer. Back then, it was the first oncogene-targeted therapy approved by the US Food and Drug Administration for treating breast cancer in 1998.9 Although trastuzumab is a successful targeted therapy for the treatment of HER2 overexpressing (HER2-positive) breast cancer, unfortunately, this treatment is associated with an increased risk of cardiotoxicity.10 In addition, a significant number of patients with HER2-overexpressing breast cancer would initially or eventually become resistant to anti-HER2-based therapy with trastuzumab.5,11,12 Therefore, several studies have been conducted to understand the mechanism of trastuzumab resistance and develop combination strategies to overcome this resistance.5,11,12 Several cases have shown that the combination of trastuzumab and neratinib was effective in overcoming drug resistance, improved the response rate and even led to a synergistic effect. Neratinib is a kinase inhibitor,13 indicated for the extended adjuvant treatment of adult patients with early-stage HER2-overexpressed/amplified breast cancer. Neratinib is only available in tablet dosage form and it is required to be used in a high dose for over a year, which is associated with more adverse effects.14,15 Cancer nanotherapeutics are rapidly progressing and are being implemented to solve several limitations of conventional drug delivery systems such as nonspecific biodistribution and targeting, lack of water solubility, poor oral bioavailability, drug resistance, and low therapeutic indices.16 Dendrimers are branched, synthetic polymers with layered architectures that show promise in several biomedical applications.17–19 Advances in our understanding of the role of molecular weight and architecture on the in vivo behavior of dendrimers, together with recent progress in the design of biodegradable chemistries, have enabled the application of these branched polymers as targeted carriers of chemotherapeutics. This work aims to prepare a nanoformula of PAMAM dendrimers loaded with neratinib labeled with FITC and conjugated with trastuzumab as dual treatment of breast cancer. The use of a nanoformula would allow using a small dose of neratinib, avoiding the side effects associated with high oral doses. Conjugating the loaded dendrimers with trastuzumab would allow a dual treatment alongside reducing the associated resistance as well as targeted therapy.
Materials and Methods
Loading of Neratinib into G4 PAMAM Dendrimers
Excess amounts of neratinib (UFC Biotechnology, USA) were thoroughly vortexed with predetermined concentrations of pegylated poly amidoamine dendrimers [10% PAMAM] (Generation 4) (PEG-PAMAM) (Sigma-Aldrich, USA) in a 5:1 molar ratio. The mixture was left overnight with stirring at room temperature for equilibration. The free drug was removed by Dialysis (MWCO 1000 Da) against double-distilled water for 10 min. The loaded Stealth Dendritic Nanospheres were then estimated spectrophotometrically in ethanol at λmax 382 nm.
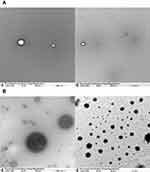
Morphological Studies, TEM
Blank and loaded dendrimer was subjected to morphological evaluation to determine the size and shape of the blank and loaded dendrimer using a transmission electron microscope (TEM) JEOL JEM-1011.
Synthesis and Characterization of TZ-Grafted PAMAM Dendrimers
Synthesis of FITC-Labeled Dendrimers
FITC-labeled dendrimers were made as previously described;19 in brief, dendrimers were dissolved in PBS (pH 7.4), and then mixed with FITC (Merc, Germany), in 1:6 molar ratio. The mixture was stirred overnight and dialyzed against PBS for 24 h. The molar ratio of FITC conjugation to dendrimers was determined from the absorbance at 495 nm using a UV-VIS spectrophotometer. Samples were scanned in the wavelengths-range of 200–800nm for qualitative analysis.
Conjugation of Heterocross-Linker, Maleimide-Poly(Ethylene) Glycol-N-Hydroxysuccinimide (NHS-PEG-MAL) to FITC-Labeled Dendrimers
This was done as previously described;19 in brief, NHS ester of PEG-MAL was reacted with FITC-conjugated dendrimer in a molar ratio of 3:1 in PBS (pH 8), then stirred for 30 min at room temperature. The synthesis of dendrimer-PEG-MAL was confirmed by 1H NMR spectroscopy (data not shown). The obtained dendrimer-PEG-MAL conjugate was then dissolved in D2O and scanned using Avance 500NMR (Bruker, UK).
TZ Thiolation
As a separate reaction, TZ was dissolved in PBS and allowed to be reacted with 2-iminothiolane hydrochloride in a 1:10 molar ratio for 2 h. Thiolated TZ was purified by dialysis (MW cutoff 12,000–14,000) in PBS to remove the 2-iminothiolane hydrochloride. The number of thiol groups on the TZ was determined using Ellmann’s test.20 Then, SDS-PAGE was used for analysis.
Bioconjugation of Thiolated TZ with Dendrimers
Thiolated TZ was coupled with heterocross-linked, conjugated, FITC-labeled dendrimers (10:1 ratio) to give a bioconjugate. The NHS-PEG-MAL cross-linked dendrimer solution was mixed with the thiolated TZ solution at room temperature overnight.TZ-dendrimer conjugate was dialyzed with PBS, pH 7.4.
Characterization of TZ-Dendrimer Conjugate
The surface potential of various nanoconjugates was measured using a Malvern Zetasizer Nano-ZS (Malvern Instruments Ltd, Malvern, UK). Samples were diluted in deionized water and analyzed at 25°C. TZ-dendrimer conjugates were also characterized by SDS-PAGE analysis. All gels were electrophoresed using Mini-Protean II electrophoresis units (Invitrogen, USA) at a constant voltage of 200 V in Tris/glycine/SDS buffer. The hydrodynamic diameter of PAMAM dendrimers was determined using a Malvern Zetasizer Nano-ZS (Malvern Instruments Ltd).
In vitro Drug Release Studies: Using a Dialysis Method in PBS (pH 7.4)
Dendrimer formulations equivalent to 2 mg of neratinib were placed in dialysis tubing (MWCO 2000) in the release medium (100 mL of PBS) at 37°C and stirred at 100 rpm. At different time intervals, 1 mL was withdrawn from the release medium and replaced with the same volume of fresh medium. The samples were diluted, filtered through a 0.22 μm nylon filter, and analyzed with Nanodrop.
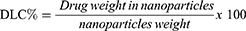
Cell Culturing and Cytotoxicity Assay
Human breast cancer SK-BR-3 cell line (HER2-positive) was obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were grown in McCoy’s 5A medium (Invitrogen, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 2 mM/L L-glutamine. Cells were maintained at 37°C in 5% CO2.
A cytotoxicity assay was performed using alamar blue assay. First 500 µL of cell suspension were seeded in 24-well plates at a density of 3 × 105 cells/mL and incubated for overnight at 37°C in 5% CO2. Then, cells were incubated with neratinib, dendrimer-neratinib, or TZ-dendrimer-neratinib at equivalent drug concentrations ranging from 45 to 250 ng/mL for 48 h. After the incubation period, media were then replaced with fresh medium and 50 μL of alamar blue reagent (Bio-Rad, UK) was added in each well, and plates were subsequently incubated for 2–3 h. To quantify cell survival, the fluorescence intensity of the reagent was measured using a microplate reader (iM3) (BioTek, USA) at 560 nm excitation wavelength and 590 nm emission wavelength. Then, the fluorescence of treated cells was compared with the fluorescence of control untreated cells.
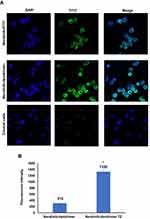
Cellular Uptake Studies
Cell-covered coverslips in 6-well plates were incubated with dendrimer-FITC or TZ-dendrimer-FITC conjugates, at an equivalent FITC concentration of 100 μg/mL for 5 h; after that the cells were washed with PBS three times and stained with DAPI for 10 min then fixed with 4% paraformaldehyde in PBS. Cellular uptake of FITC-labeled nanoparticles were visualized using confocal microscopy (Carl Zeiss, Jena, Germany). Images were analyzed using ZEN LSM (Carl Zeiss) software.
HPLC Assay for Neratinib
A simple new HPLC method was utilized for determination of neratinib. It is a simple process appropriate for routine work. The concentration of neratinib was measured applying a Waters HPLC system, utilizing Waters 2707 autosampler delivery system and using a symmetry C18 column (4.6 x 1.0 cm) packed with 5-µm spherical particles with a gradient mobile phase 10 mM ammonium acetate buffer pH 3–0.1% formic acid:methanol – 0.1% formic acid; 80:20, v/v. The mobile phase was prepared daily during the study, filtered through a 0.22-µm millipore filter, and degassed under vacuum. The total run time was 5.0 min and the autosampler temperature was ambient temperature. The flow rate was 1 mL/min and the injection volume was 20 μL and detection was performed at 220 nm. Data were analyzed with an Empower Pro Chromatography Manager Data Collection System.
Standard and Sample Solutions
A stock solution containing 10 mg of drug in mobile phase was stored in 4.0-mL glass vials at −30°C. A daily standard calibration curve (n=3) ranging from 10, 20, 40, 60, 80, and 100 µg/mL was prepared. The standards were transferred to glass autosampler vials with pre-slit septum (Waters, USA), where 20 µL was injected into the HPLC system for analysis.
Stability Studies
Short- and long-term stability was assessed in stock solution after storage at room temperature, 4 and −30°C. Stability in the stock solution was expressed as the percentage recovery of the stored solution (0, 1, 3, and 6 months) relative to a fresh solution. All stability testing was determined in triplicate. Additionally, the effect of 3 freeze/thaw cycles on analyte concentrations in samples was evaluated by assaying samples after they had been frozen (−30°C) and thawed on 3 separate days and comparing the results with those of freshly prepared samples.
Statistical Analysis
Results are expressed as the mean and standard deviation of three experiments each performed in triplicate (n = 9). Statistical significance was analyzed using the Student’s t-test. A probability (p) of equal or less than 0.05 was considered statistically significant.
Results and Discussion
Formulation, Loading and Characterization of Neratinib in PAMAM Dendrimers
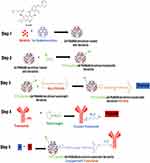
Neratinib was loaded into G4 PAMAM dendrimers then conjugated to trastuzumab by a 5-step reaction (Figure 1). When loading neratinib into G4 PAMAM dendrimer, data from previous reports have been considered;21 the study indicated that the loading of dendrimers after bioconjugation to trastuzumab yield a lower loading percentage.19,21 Thus, loading dendrimers was achieved via strong vertexing in a molar ratio of 5:1 of neratinib to dendrimer. TEM imaging was used for morphological characterization of the encapsulated dendrimers (Figure 2). The percentage of encapsulation efficiency was calculated by comparing neratinib released form the formula to the original concentration used. Using the equation EE% = [(Total drug-free drug)/Total drug ∙100] the calculated EE% was around 19.24%. Then, loaded dendrimers were subjected to fluorescence labeling with FITC and crosslinked with maleimide-poly(ethylene) glycol-N-hydroxysuccinimide (NHS-PEG-MAL) as previously described19 to allow successful linking to thiolated trastuzumab. Thiolation of trastuzumab was accomplished using Traut’s reagent (2-iminothiolane hydrochloride) where the number of thiol groups on the TZ was determined using Ellmann’s test.20 Approximately 3.6 thiol groups were generated per one molecule of TZ. The activity of TZ was checked after thiolation using Bradford assay and compared to TZ before thiolation (Figure 3). Finally, the pegylated dendrimers were reacted with the thiolated TZ, and the conjugation was confirmed by SDS-PAGE analysis as seen in Figure 3. Figure 4 shows the in vitro release of neratinib from dendrimers and from TZ-conjugated dendrimers. Both formulas showed sustained release pattern with about 10% less amount released from TZ-conjugated loaded dendrimers. This could be attributed to the loss or the release of some drug during the conjugation process.
The surface charge of the dendrimers was estimated at various stages (Table 1). Plain dendrimers showed a positive zeta potential (14.6 mV) due to the cationic surface groups (64-amine) on their surface. After conjugation with NHS-PEG-MAL, the zeta potential decreased to 5.6 mV, while after TZ was grafted onto the dendrimer surface it was further reduced to −3.98 mV. Since TZ has an isoelectric point (pI) of 5.91, with an overall anionic nature at physiological pH, the reduction in zeta potential value supports successful conjugation of TZ with the dendrimers.
 |
Table 1 Zeta Potential Value of Different Conjugates and Plain Dendrimers |
In vitro Cytotoxicity and Cellular Uptake
SKBR-3 (HER2-positive) breast cancer cell line was used to examine the anticancer action of neratinib formulations. This cell line was used to provide a good understanding of the contribution of TZ in targeting HER2-positive breast cancers.18 The cytotoxic effect of neratinib, neratinib-loaded-dendrimers and neratinib-loaded-dendrimers-TZ grafted against SKBR-3 cell line are shown in Figure 5. It is clearly shown in Figure 5 that at a 45 ng/mL concentration of neratinib, the SKBR-3 cell viability after 48 h was 40%, 36%, and 33% (average % ± SD) for neratinib, neratinib-loaded-dendrimers and neratinib-loaded-dendrimers-TZ grafted, respectively. The results revealed that neratinib-loaded dendrimers were more cytotoxic than neratinib alone. Trastuzumab-grafted neratinib dendrimers were more cytotoxic than plain neratinib dendrimers. This could be attributed to the affinity of trastuzumab to the HER2 receptors expressed in SKBR-3 cells, leading to binding and subsequent nanoparticle internalization via receptor-mediated endocytosis.21
Cellular uptake studies were conducted to examine the internalization of the fluorescently labeled neratinib-loaded-dendrimers prepared. As illustrated in Figure 6, the uptake of formulated dendrimers was observed after 5 h; however, neratinib-loaded-dendrimers-FITC-TZ grafted showed higher fluorescence signal than neratinib-loaded-dendrimers-FITC. This was confirmed by the results of fluorescence intensity quantitative analysis, in which neratinib-loaded-dendrimers-FITC showed statistically significant higher fluorescence intensity compared to that of neratinib-loaded-dendrimers-FITC alone (p ˂ 0.05) (Table 2). These findings were in agreement with previous studies,18,21,22 in which cellular uptake and antiproliferative effect of TZ-conjugated dendrimers loaded with docetaxel were significantly higher than for plain dendrimers.21
 |
Table 2 Quantitative Uptake of Neratinib-Dendrimer-FITC-Trastuzumab by SKBR-3 Cells with or Without Trastuzumab After 5 h of Incubation. *indicates Comparison with Neratinib-Dendrimer (*p< 0.05) |
HPLC Analysis of Neratinib
A simple, accurate, and precise RP-HPLC assay for neratinib was developed for the determination of the concertation of neratinib. The linearity was over the range 10–100 µg/mL with a regression coefficient value of 0.9997 (Figure 7A). The retention time was 3.8 min (Figure 7B) with the condition mentioned above.
Stability
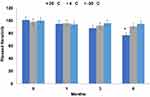
Neratinib was stable in the treated samples held in the autosampler at 25°C for 24 h with mean calculated values within 4.2±0.21% of the nominal concentration (Figure 8). However, the samples lost 6.52±0.24% of their nominal concentration within one month if stored protected from light in the autosampler. Therefore, it is a stable drug and can be kept at room temperature. However, after one month the amount lost decreases up to 12 within 3 and significantly reduced by 23% after 6 months (p > 0.05). So, it is recommended to protect the drug standard solution at 4°C or −30°C. The freeze–thaw temperature cycles did not significantly (p > 0.05) affect the stability of neratinib in all three cycles, even after 72 h, with the mean calculated values within 4.24% of the nominal concentration.
Conclusion
The result of the current study revealed the successful preparation of neratinib loaded dendrimers and trastuzumab-grafted neratinib dendrimer. TEM images showed the spherical surface of dendrimers in the nanoscale range and successful encapsulation of the drug. An in vitro release study demonstrated a sustained pattern of release from both formulae. A significant increase in cellular uptake of trastuzumab-grafted dendrimer was obtained when compared with plain dendrimer. Cytotoxicity studies revealed that neratinib-loaded dendrimers were more cytotoxic to cancerous cells than neratinib alone; however, trastuzumab-grafted neratinib dendrimers had slightly higher cytotoxic effect compared to untargeted plan neratinib dendrimers. The targeted nanosystems used in our study could provide the benefit of the use of a lower dose of any cytotoxic drugs, e.g. neratinib, overcoming the unbearable side effects of chemotherapeutic agents.
Acknowledgment
The authors are very grateful to the Deanship of Scientific Research and Research Centre, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Disclosure
All authors declare no conflict of interest.
References
1. Stewart B, Wild CP. World Cancer Report 2014. lyon, france: International agency for research on cancer. World Health Organization. 2014; 630.
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
3. Dean-Colomb W, Esteva FJ. Her2-positive breast cancer: herceptin and beyond. Eur J Cancer. 2008;44(18):2806–2812. doi:10.1016/j.ejca.2008.09.013
4. Esteva FJ, Cheli CD, Fritsche H, et al. Clinical utility of serum HER2/neu in monitoring and prediction of progression-free survival in metastatic breast cancer patients treated with trastuzumab-based therapies. Breast Cancer Res. 2005;7(4):R436. doi:10.1186/bcr1020
5. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977–984. doi:10.1093/annonc/mdl475
6. Yarden Y. The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37:3–8. doi:10.1016/S0959-8049(01)00230-1
7. Senkus E, Cardoso F, Pagani O. Time for more optimism in metastatic breast cancer? Cancer Treat Rev. 2014;40(2):220–228. doi:10.1016/j.ctrv.2013.09.015
8. Figueroa-Magalhães MC, Jelovac D, Connolly RM, Wolff AC. Treatment of HER2-positive breast cancer. Breast. 2014;23(2):128–136. doi:10.1016/j.breast.2013.11.011
9. Arteaga CL. Trastuzumab, an appropriate first-line single-agent therapy for HER2-overexpressing metastatic breast cancer. Breast Cancer Res. 2003;5(2):96. doi:10.1186/bcr574
10. Guglin M, Cutro R, Mishkin JD. Trastuzumab-induced cardiomyopathy. J Card Fail. 2008;14(5):437–444. doi:10.1016/j.cardfail.2008.02.002
11. Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. 2006;232(2):123–138. doi:10.1016/j.canlet.2005.01.041
12. Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15(24):7479–7491. doi:10.1158/1078-0432.CCR-09-0636
13. Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28(8):1301–1307. doi:10.1200/JCO.2009.25.8707
14. Swaby R, Blackwell K, Jiang Z, et al. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: a Phase I/II study. J Clin Oncol. 2009;27(15S):1004.
15. Canonici A, Gijsen M, Mullooly M, et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget. 2013;4(10):1592. doi:10.18632/oncotarget.1148
16. Cho K, Wang X, Nie S, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14(5):1310–1316. doi:10.1158/1078-0432.CCR-07-1441
17. Kumar D, Sharma P. Nanoparticulate system for cancer therapy: an updated review. Int J Nanomater Nanotechnol Nanomed. 2018;4(2):022–034. doi:10.17352/2455-3492.000027
18. Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7(2):572–579. doi:10.1021/bm0506142
19. Kulhari H, Pooja D, Shrivastava S, et al. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci Rep. 2016;6:23179. doi:10.1038/srep23179
20. Moser M, Behnke T, Hamers-Allin C, Klein-Hartwig K, Falkenhagen J, Resch-Genger U. Quantification of PEG-maleimide ligands and coupling efficiencies on nanoparticles with Ellman’s reagent. Anal Chem. 2015;87(18):9376–9383. doi:10.1021/acs.analchem.5b02173
21. Marcinkowska M, Sobierajska E, Stanczyk M, Janaszewska A, Chworos A, Klajnert-Maculewicz B. Conjugate of PAMAM dendrimer, doxorubicin and monoclonal antibody—trastuzumab: the new approach of a well-known strategy. Polymers. 2018;10(2):187. doi:10.3390/polym10020187
22. Marcinkowska M, Stanczyk M, Janaszewska A, Sobierajska E, Chworos A, Klajnert-Maculewicz B. Multicomponent conjugates of anticancer drugs and monoclonal antibody with PAMAM dendrimers to increase efficacy of HER-2 positive breast cancer therapy. Pharm Res. 2019;36(11):154. doi:10.1007/s11095-019-2683-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.