Back to Journals » Vascular Health and Risk Management » Volume 17
Transvenous Lead Extraction without Procedure-Related Deaths in 1000 Consecutive Patients: A Single-Center Experience
Authors Stefańczyk P, Nowosielecka D , Tułecki, Tomków K, Polewczyk A , Jacheć W , Kleinrok A, Borzęcki W, Kutarski A
Received 30 April 2021
Accepted for publication 17 June 2021
Published 5 August 2021 Volume 2021:17 Pages 445—459
DOI https://doi.org/10.2147/VHRM.S318205
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Daniel Duprez
Paweł Stefańczyk,1 Dorota Nowosielecka,1 Łukasz Tułecki,2 Konrad Tomków,2 Anna Polewczyk,3,4 Wojciech Jacheć,5 Andrzej Kleinrok,1,6 Wojciech Borzęcki,1 Andrzej Kutarski7
1Department of Cardiology, The Pope John Paul II Province Hospital of Zamość, Zamość, Poland; 2Department of Cardiac Surgery, The Pope John Paul II Province Hospital of Zamość, Zamość, Poland; 3Department of Physiology, Pathophysiology, and Clinical Immunology, Collegium Medicum of Jan Kochanowski University, Kielce, Poland; 4Department of Cardiac Surgery, Świętokrzyskie Cardiology Center, Kielce, Poland; 5Silesian Medical University, 2nd Department of Cardiology, Zabrze, Poland; 6Medical College, Department of Physiotherapy, University of Information Technology and Management, Rzeszów, Poland; 7Department of Cardiology, Medical University, Lublin, Poland
Correspondence: Anna Polewczyk
Department of Physiology, Pathophysiology, and Clinical Immunology, Collegium Medicum of Jan Kochanowski University, 19A, Aleja IX Wieków Kielc Str 25-317, Kielce, Poland
Tel +48600024074
Email [email protected]
Background: Transvenous lead extraction (TLE) is now a first-line technique for the treatment of complications related to cardiac implantable electronic devices. The aim of the study was to demonstrate that it is possible to safely perform difficult TLE procedures with a maximum reduction of peri-procedural major complications.
Methods: A total of 1000 consecutive patients undergoing TLE in a single high-volume center from 2016 to 2019 were studied. All procedures were performed in a hybrid room or operating room by a specialized TLE team. TLE was performed under general anesthesia and monitored by transesophageal echocardiography, and the operating room was suitably equipped for immediate surgical intervention. The effectiveness and safety of the procedures were assessed, with particular emphasis on major complications.
Results: In all, 1952 leads with the mean implant duration of 111.7 ± 77.6 months had been extracted. Complete procedural success of patients was achieved in 95.9% and clinical success in 99.1%. Major complications, predominantly cardiac tamponade (63.3%), occurred in 22 patients (2.2%). Rapid diagnosis and immediate intervention were the key to a 100% survival in patients with this complication.
Conclusion: Performing procedures in a hybrid operating room under general anesthesia in the presence of a cardiac surgeon and with the use of transesophageal echocardiography significantly improves the safety of transvenous lead extraction.
Keywords: lead extraction, complications of lead extraction, venue of TLE, mechanical dilatation, safety precautions
Introduction
The number of patients with pacemakers and implantable cardioverter defibrillators (ICDs) has grown significantly in recent decades. Technological and pharmacological advances have significantly prolonged the lives of patients with cardiac implantable electronic devices (CIED). Unfortunately, the increase in the number of implanted devices goes in line with the increased number of complications.1,2 Transvenous lead extraction (TLE) is the gold standard for treating complications of CIED therapy.1–6 Initial experience with successful extraction of the leads was described in the 1980s and ‘90s.7,8 The effectiveness of TLE is high (more than 90% in general) but rates of major complications vary between studies from 0.4 to 3.4%, whereas mortality risk is 0.00–1.86%.3–8 Generally, many factors may be associated with different outcomes of procedures, including: age of patients, New York Heart Association Class III/IV or pre-operative use of antithrombotic therapy.9
There are a wide variety of techniques and tools which can be used for transvenous lead extraction: conventional mechanical systems with non-powered sheaths (Byrd polypropylene sheaths, Cook) for dilating adherent fibrotic tissue and extracting the leads,7 and the new techniques including rotational mechanical sheaths with threaded tips such as Evolution (Cook)10 and TightRail (Spectranetics, CA),11 rotating mechanical dilator sheaths as well as sheaths powered by ablative energy sources, ie, excimer laser sheaths (SLS II Laser Sheath).8 Mechanical dilation is associated with a lower incidence of vascular complications during transvenous lead extraction as compared to the use of powered sheaths.12
Major complications of TLE are quite rare, but their consequences can be very serious if proper management is delayed.3–6 These include hemopericardium requiring drainage or cardiac surgery, hemothorax requiring thoracic surgery or pleural drainage, pulmonary embolism requiring cardiac surgery, stroke, acute heart failure and severe tricuspid valve damage.3–6
Major complications are linked to lead implant duration, number of leads and other, mainly patient-related risk factors (female gender, renal failure, patient age).13,14 Risk is inseparable from the removal procedure and may be reduced only if TLE is performed by an experienced operator in high volume center.15 But the main goal is to prevent deaths due to major complications. This may be achieved through detection of major complications at the earliest possible opportunity, before circulatory collapse occurs, and through proper immediate management. Success depends on patient monitoring (arterial line, transesophageal echocardiography, exhaust gases) and organization of the lead removal procedure to ensure sternotomy within 5–10 minutes after complication detection (venue, presence of cardiac surgical and anesthesia teams).3–6 According to the latest recommendations additional precautions should be adopted during TLE to avoid serious consequences, but there are no reports on their effectiveness in clinical practice.3–6
Methods
Study Population
The first 1000 consecutive patients undergoing TLE in a single high-volume center from 2016 to 2019 were enrolled in the study. All participants provided written informed consent prior to study enrollment, and before the TLE procedure as medically indicated. All data were held anonymous. The study was approved by the Bioethics Committee at the Regional Chamber of Physicians in Lublin no. 288/2018/KB/VII, and complied with the Declaration of Helsinki.
Organization and Course of the TLE Procedure
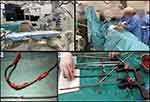
All procedures were performed in a hybrid operating room or operating room equipped with C-arm fluoroscopic X-ray machine and simultaneous surgical standby. The team consisted of a first operator having experience with CIED therapy and TLE, a second cardiologist, a cardiac surgeon, an anesthesiologist and an experienced echocardiographer. Patients were prepped and draped in standard fashion as for emergency sternotomy or thoracotomy. The rooms were equipped with an “open chest” cart including all necessary equipment for immediate surgical intervention, and the surgical instruments were opened and placed on the procedure table. The cardiopulmonary bypass machine was primed, and a perfusionist was immediately available. All TLE procedures were performed under general anesthesia, and blood pressure readings were obtained from invasive catheters (Arterial Line – AL) (Figure 1).
Transesophageal echocardiography was routinely used during most procedures (91%). Femoral venous access was obtained for temporary pacing, transfemoral lead extraction and as an additional site in emergency situations.
TLE procedures were performed according to our center’s routine. A primary access site was the implant vein whenever possible. In patients with the proximal (usually broken) end in the cardiovascular system or with the lead fractured during the extraction procedure, the restored access site via the femoral, jugular or subclavian vein was selected. First, adherent fibrous tissue around the leads was excised in the surgical pouch, and then the sleeves were removed. In most procedures, standard stylets were used to stiffen the leads. Locking stylets (Liberator Locking Stylet, Cook Medical Inc., USA) were used only for extraction of the oldest leads when estimated risk of lead fracture was high. Simple traction or traction on a locking stylet with insulation-bound suture was very rarely applied (usually in patients with infection, when prolonged temporary pacing was not planned). Lead extraction was performed using mainly non-powered mechanical telescoping polypropylene sheaths (Byrd Dilator Sheaths, Cook Medical Inc., USA) of all diameters and lengths and using various stylets. When the polypropylene telescoping sheaths appeared ineffective, powered mechanical sheath systems (Evolution Mechanical Dilator Sheath, Cook Medical Inc., USA; TightRail Rotating Dilator Sheath, Spectranetics, USA) were used. A combined approach, using two or more different (jugular, subclavian, femoral) access sites, was selected when conventional methods were insufficient. An Evolution Mechanical Dilator was used in 25 patients, and TightRail Rotating Dilator Sheath in 4 patients. Laser and electrosurgical dissection sheaths were not used.
Echocardiographic Monitoring
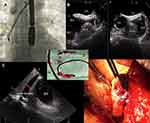
At the stage of selection for the procedure, each patient underwent a comprehensive transthoracic echocardiogram. Transesophageal echocardiography was routinely performed during most procedures. The echocardiographic probe was inserted after the patient was intubated. TEE monitoring was performed in the following stages: 1) pre-procedural assessment 2) intra-procedural phase – procedure monitoring, and 3) post-procedural phase to evaluate the impact of TLE on tricuspid valve function, retention of lead fragments, vegetation remnants and delayed fluid (blood) accumulation in the pericardial space16–19 (Figure 2).
Echocardiographic Evaluation of the Tricuspid Valve Function
Tricuspid valve function was assessed by comparing the degree of regurgitation before and after the procedure. A deterioration in the degree of regurgitation by at least 2 degrees was considered a significant dysfunction after TLE. Grade III or IV deterioration of valve function was considered a significant dysfunction of the valve, qualifying for surgery.
Anesthesia Protocol
All 1000 extractions were performed under general anesthesia given by attending cardiac anesthesiologist who monitored vital signs. Before each TLE procedure, patients were comprehensively evaluated, with particular emphasis on the respiratory system and heart function. In addition to transthoracic or transesophageal echocardiography all patients underwent chest x-ray. Routine monitoring (pulse oximetry, invasive arterial blood pressure measurements, 5-lead electrocardiogram, serial arterial blood gas analyses, urine output) was implemented in all patients during the procedure. Upon completion of the procedure, patients were moved to the recovery room, where monitoring (invasive blood pressure measurements and electrocardiography) continued for at least 2 hours.
Indications, Procedure Effectiveness and Complications
Indications for TLE, procedure effectiveness, and complications were assessed according to the 2009 and 2017 HRS consensus and 2017 EHRA guidelines.3–5 The efficacy of TLE was determined based on the percentage of procedural success and clinical success. Procedural success was defined as the removal of all targeted leads and lead material from the vascular space with the absence of any permanently disabling complication or procedure-related death.3,4 Clinical success was defined as the removal of all targeted leads or retention of a small portion (<4 cm) of the lead that did not negatively impact the outcome goals of the procedure, with the absence of any permanently disabling complication or procedure-related death.3,4
The complications of TLE were also defined as major complications being those that were life threatening, resulted in significant or permanent disability or death, or required surgical intervention and minor complications being those that required medical or minor procedural interventions.5
Dataset and Statistical Methods
The dataset was split into the following groups: patient demographic and clinical data (Table 1), pacing system data, lead management and TLE organization (Table 2), TLE procedure information (Table 3), effectiveness of lead extraction in terms of radiographic, clinical and complete procedural success, major and minor complications (Table 4).
 |
Table 1 Patient Demographic and Clinical Data, Pacing System Data, Indications for TLE |
 |
Table 2 Lead Management and Organization of TLE Procedure |
 |
Table 3 TLE Procedure Information |
 |
Table 4 Effectiveness of Lead Extraction Expressed as Radiographic, Clinical and Procedural Success, Major and Minor Complications in 1000 Procedures |
Categorical variables were expressed as counts and percentages, whereas continuous variables as either the mean and standard deviation (SD) or median and the interquartile range (25–75%).
Results
Baseline Characteristics
In all, 1952 leads were extracted from the first 1000 patients (61.9% males) with a mean age of 67.32 ± 14.34 years. The most common pacing system was PM DDD (46%); systems with ICD leads and left ventricular leads (LV) were less popular (32.5% and 12.5%, respectively). The mean dwell time of the oldest lead in one patient was 112.9±77.40 months, the mean sum of dwell times of all leads in one patient was 17.31±14.13 years. Non-infectious indications for TLE were present in 77.8% of patients; local pocket infection and endocarditis were less frequent (6.3% and 15.9%, respectively) (Table 1).
The most common indication for TLE was the need to replace the lead (51.5%); other reasons included prevention of lead abandonment (up-grading, down-grading, superfluous lead extraction, system removal with deferred reimplantation and removal of the redundant entire system; 26.3%), whereas removal of the entire system due to infection was rarest of all (22%).
Analysis of lead management options showed that all leads were removed in 77% of patients, non-functional, superfluous leads were extracted in 9.1%.
Fifty percent of procedures were performed in a hybrid room, 40% in an operating room (cardiac surgery room in cooperation with a cardiac surgeon), and over 90% of procedures were performed under TEE guidance (Table 2).
Dwell time of the oldest extracted lead (111.7 months) and the sum of dwell times of the extracted leads (15.6 years) were slightly different than before the procedure, because functional leads had been left in place for prolonged use in 227 patients. The mean duration of the procedure (skin–skin time) was 63.03±27.17 min, whereas average sheath–sheath time (measured as the time from insertion of the dilator to its removal) was 15.44±24.38 min. An average duration of single-lead extraction was 9.0 min. Intraprocedural temporary pacing in pacemaker-dependent patients was used in 23.4% cases. More than one lead was extracted in 52.0% and 3 or more leads in 11.5% of patients.
Leads were most commonly accessible from their venous entry site (98.3%). A variety of technical difficulties occurred during 212 out of 1000 procedures (21.2%). Rescue tools were necessary in about 20% of cases: metal sheaths were used in 11.3% of procedures, lasso catheters in 4.1% of patients, and the lasso and guide wire loop in 1.5% of cases (Table 3).
Complete procedural success was achieved in 95.9% of procedures, clinical success was obtained in 99.1% of patients. Absent clinical success was related to abandonment of non-extractable lead remnants in infectious cases (in 0.2%) and marked tricuspid valve damage (in 0.7% of procedures) (Table 4).
Major complications were detected in 22 patients (2.2%). They were: hemopericardium requiring cardiac surgery (1.3%), hemothorax requiring cardiac surgery (0.1%), acute heart failure requiring intensive therapy (0.1%) and tricuspid valve damage requiring tricuspid valve repair urgently (one patient) or in the future (6 patients; 7.0%) (Table 4).
A careful analysis of 22 cases with serious complications showed that hemodynamically significant cardiac tamponade was predominant (63.3%). The causes of tamponade were: damage to the atrial wall in 11 cases (78.5%), perforation of the right ventricular wall in one (7.1%), damage to the coronary sinus and the superior vena cava wall in one case. Extracorporeal circulation was necessary in 3 out of 13 interventions (18.7%) (Table 4).
Another important determinant of TLE safety is tricuspid valve function. In this study, tricuspid valve injury occurred in 33 (3.3%) patients, with significant valve dysfunction qualifying for cardiac surgery being diagnosed in 0.7% of cases (Table 4).
It is also noteworthy that 14 (1.4%) patients who were previously diagnosed with lead-dependent tricuspid dysfunction showed a significant reduction in tricuspid regurgitation after TLE.
The group of 22 patients with major complications consisted mainly of women (68%). Renal failure occurred in 14% of patients, average Charlson index was 2.8. Of the factors related to the implanted systems, only 9% of patients had an ICD, none had an LV lead. An abandoned lead was detected in 24% of patients. The mean number of leads in the heart before TLE was 2.05. Dwell time of the oldest lead before TLE was 200.3 months, the sum of lead dwell times in the heart was 33.2 years. Excessive lead loops in the heart were found in 18% of patients. The average number of CIED-related procedures before TLE was 2.89 (>1 procedure in 77% of patients). Dwell time of the extracted lead was relatively long (oldest: 468, average 111.7 months), but three major complications (acute heart failure-AHF, tricuspid valve dysfunction-TVD, hemothorax requiring surgery – HTS) occurred in patients with relatively younger leads, ie, with implant duration of less than 111.7 months (50, 97, 69, 108 months). In order to evaluate TLE-related risk in these patients, we used a previously devised SAFeTY TLE score15 and an online calculator (available at http://alamay2.linuxpl.info/kalkulator/), confirming that the mean score for these four patients was 12.0, reflecting a high (8–10%) risk of major complications (Table 5).
 |
Table 5 Major Complications of TLE in Patients with Lead Implant Duration of Less Than 10 Years |
In the group of 22 patients with major complications, lead dwell time ranged from 50 to 385 months (average 200, 3 months), but the mean value may be misleading because major complications occurred in 6 patients with the implant duration of less than 10 years. What’s more, even the SAFeTY TLE scores did not predict procedural complications in these patients (Table 5).
The complexity of procedures in patients with major complications was high, any technical difficulty occurred in 68% of patients. Lead-to-lead fibrotic-binding sites were found in 59% of cases, lead fracture during extraction occurred in 36% of patients, more than one technical problem occurred in 41%. The most common minor complications were: worsening tricuspid valve function in 4% of cases, bleeding requiring blood transfusion in 1.8% of patients, hematoma requiring evacuation in 1.2% and pericardial effusion without surgical intervention in 1% of cases.
None of the patients died in the periprocedural period due to major complications. Mortality at 7 days was 0.3%, at 30 days 1.3%, at 6 months 4.1% and mortality over the whole follow-up time (from January 2016 to January 2020) was 14.7% (Table 4).
Discussion
As the need for transvenous lead extraction is increasing, it becomes more and more important to ensure its efficiency and safety. It was shown previously that the occurrence of major TLE complications was determined by risk factors related to patient characteristics (female gender, renal failure) and CIED characteristics (implant duration, number of leads, abandoned leads).13–15 Experience of the first operator and the team, as well as the use of less invasive tools, can prevent major complications but only to a limited extent.3–6,13 Procedure-related death remains the most serious complication of lead extraction.3–6,13 Safety precautions outlined in the guidelines (procedure site, TEE monitoring, general anesthesia, arterial line, gas analysis, presence of a cardiac surgeon during surgery, availability of tools and pumps for sternotomy) were designed to effectively treat serious complications as early as possible before the onset of general hypoxemia.3–6,13 There are many reports describing practical utility of the proposed organizational model, but our analysis has demonstrated probably for the first time the effectiveness of a comprehensive safety system in each of the 1000 procedures.
This study shows that simple, cheap and conventional tools (non-powered polypropylene mechanical sheaths) used as first-line support help us achieve excellent results (complete radiographic success in 95.9%, clinical success in 99.1% and procedural success in 95.9%).
This report also demonstrates that such a stepwise approach can result in a safe, successful and complete lead removal in up to over 95% of patients, regardless of the specific extraction tool used. It should also be emphasized that these results were obtained in the country’s TLE tertiary reference center that has the efficiency and experience to perform the most difficult procedures. Dwell time of the oldest extracted lead was 112 months (more than 10 years) which had a significant impact on the occurrence of serious complications (2.2%) despite the main operator’s extensive experience (>3500 TLEs performed personally), but even so, a zero mortality rate was observed. Major complications can occur even during the extraction of leads with implant duration of less than 10 years (4/22, 18%), which clearly shows that safety strategy should be implemented during all procedures without exception. Additionally, the present study reveals that the use of minimally aggressive tools (polypropylene sheaths) as the only possible way and, if necessary, only mechanical rotating threaded tips, may reduce the risk of superior vena cava rupture (there was only one case, when using the old model of Evolution).
Out of 25 reports comprising 43,940 patients, summarized in Table 6, 13,15,22–43 13 studies involved more than 1000 TLE procedures.13,15,22,23,25,32,34–36,42
 |
Table 6 List of Publications Describing Effectiveness and Safety of TLE Procedures |
The average implant duration in those reports was 74.5 months. In the present study, the implant duration was markedly longer (112 months), and there were two smaller studies reporting such a long lead dwell time.29,40 Infection was the main indication for lead extraction in 41.7% of reports. In the current study, this percentage was lower (22%), and there were only 3 studies with a higher rate of non-infectious indications.26,30,34 Laser sheaths were used as a first-line or predominant (50%) tool in 11 reports comprising 22,890 patients.13,24,25,27,28,30,32–34,36,38 Mechanical dilation with non-powered sheaths was used as a first-line or predominant (50%) option in 11 reports comprising 17,953 patients,15,22,23,26,29,31,35,39,40,42 whereas mechanical rotational sheaths with threaded tips were used as a first-line or predominant (50%) tool in 2 reports35,38 (a relatively new tool; 2,605 patients). In one report, femoral approach and Needle’s Eye Snare was used as a first-line option in 492 patients.43 Comparison of procedural/clinical success, percentage of major complications and percentage of procedure-related death showed no significant differences between “laser” and “non-laser” centers. The average incidence of major complications in the studies was 1.67%, but this parameter should be evaluated in parallel with implant duration, which is the main risk factor for their occurrence. Only two studies demonstrated a higher rate of MC than in the current investigation,32,36 but there were only 2 studies with slightly longer implant duration32,43 and one of them reported a higher rate of MC.32 Procedure-related death is the most important indicator of the effectiveness of safety measures. Four studies reported a zero procedure-related mortality rate,24,27,37,42 but the mean implant duration in those reports was markedly shorter than in the current study (72–92 vs 112 months). The mean rate of procedure-related deaths was 0.23%, but rates of 0.5%, 0.65% and 1.10% were also reported.15,29,30 There were 12 studies in which mortality rate was above the average (0.23%)22,23,25,28–30,32–35,38 and in all but one of the studies implant duration was shorter than in the current study. The above data indirectly show that the organizational model of lead extraction plays a key role.
An important part of the TLE procedure is the venue and a standby team of cardiac surgeons. A hybrid room, a cardiac surgeon ready for immediate intervention and a perfusion pump ready for immediate use significantly improve procedure safety and reduce mortality.24,27,37,42 Our study is in line with these reports. Out of the 1000 patients undergoing TLE, 16 individuals required sternotomy and urgent cardiac surgery (14 with acute cardiac tamponade).
The cardiac surgeon’s presence was essential for survival of all patients, mortality was 0%. The role of continuous TEE monitoring cannot be overestimated, either. The American guidelines recommend echocardiographic examinations at the following stages: before the procedure for initial assessment, during the procedure for monitoring, and after the procedure for final assessment in accordance with the precisely determined criteria.4 Analogically, the European statements emphasize improved efficacy and safety of the TLE procedure, if it is supported by continuous echocardiographic monitoring.5 In the present study, more than 90% of TLEs were performed under transesophageal echocardiographic guidance. The role of continuous TEE monitoring during TLE procedures was discussed in previous publications.16–21
Due to economic and organizational problems, many TLE centers use a graded approach in the application of safety requirements: from TLE in the cath-lab, in deep analgesia-sedation, through TLE in the operating room/hybrid room with general anesthesia in the presence of a cardiac surgeon along with additional monitoring of the procedure (TEE, arterial line). Several predictors of major complications or even indicators have been proposed for pre-operative selection of the appropriate degree of protection.13,38,44 However, as shown in the present study, serious complications can occur even when leads with implant duration of less than 10 years (4/22, 18%) are being extracted. This is an argument for adopting a comprehensive safety strategy during all procedures without exception.
Conclusions
- Performing transvenous lead extraction in a hybrid operating room, under general anesthesia, in the presence of a cardiac surgeon and under transesophageal echocardiographic guidance. contributes to the improvement of the safety of TLE. This organizational model should be recommended for all TLE procedures.
- Serious complications may arise during the extraction of leads with implant duration of less than 10 years; therefore, all TLE procedures should be carried out with the same precautions.
Disclosure
The authors declare no conflict of interest in this work.
References
1. Tarakji KG, Wilkoff BL. Management of cardiac implantable electronic device infections: the challenges of understanding the scope of the problem and its associated mortality. Expert Rev Cardiovasc Ther. 2013;11(5):607–616. doi:10.1586/erc.12.190
2. Rao A, Garner D, Starck C, et al. Knowledge gaps, lack of confidence, and system barriers to guideline implementation among European physicians managing patients with CIED lead or infection complications: a European Heart Rhythm Association/European Society of Cardiology educational needs assessment survey. Europace. 2020;22:1743–1753.
3. Kusumoto FM, Schoenfeld MH, Wilkoff B, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551.
4. Wilkoff BL, Love CJ, Byrd CL, et al.; Heart Rhythm Society; American Heart Association. Transvenous lead extraction: heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6:1085–1104.
5. Bongiorni MG, Burri H, Deharo JC, et al.; ESC Scientific Document Group. 2018 EHRA expert consensus statement on lead extraction: recommendations on definitions, endpoints, research trial design, and data collection requirements for clinical scientific studies and registries: endorsed by APHRS/HRS/LAHRS. Europace. 2018;20:1217.
6. Deharo JC, Bongiorni MG, Rozkovec A, et al. European Heart Rhythm Association. Pathways for training and accreditation for transvenous lead extraction: a European Heart Rhythm. Europace. 2012;14:124–134.
7. Byrd CL, Schwartz SJ, Hedin NB, Goode LB, Fearnot NE, Smith HJ. Intravascular lead extraction using locking stylets and sheaths. Pacing Clin Electrophysiol. 1990;13:1871–1875.
8. Wilkoff BL, Byrd CL, Love CJ, et al. Pacemaker lead extraction with the laser sheath: results of the pacing lead extraction with the excimer sheath (PLEXES) trial. J Am Coll Cardiol. 1999;33:1671–1676.
9. Di Cori A, Auricchio A, Regoli F, et al., ESC-EHRA ELECTRa Investigators. Clinical impact of antithrombotic therapy in transvenous lead extraction complications: a sub-analysis from the ESC-EORP EHRA ELECTRa (European Lead Extraction ConTRolled) Registry. Europace. 2019;21:1096–1105.
10. Migliore F, Testolina M, Sagone A, et al. Multicenter experience with the Evolution RL mechanical sheath for lead extraction using a stepwise approach: safety, effectiveness, and outcome. Pacing Clin Electrophysiol. 2019;42:989–997.
11. Zucchelli G, Di Cori A, Segreti L, et al., ELECTRa Investigators. Major cardiac and vascular complications after transvenous lead extraction: acute outcome and predictive factors from the ESC-EHRA ELECTRa (European Lead Extraction ConTRolled) registry. Europace. 2019;21:771–780.
12. Mazzone P, Melillo F, Radinovic A, et al. Use of the new rotating dilator sheath TightRail for lead extraction: a bicentric experience. J Arrhythm. 2020;36:343–350.
13. Brunner MP, Cronin EM, Duarte VE, et al. Clinical predictors of adverse patient outcomes in an experience of more than 5000 chronic endovascular pacemaker and defibrillator lead extractions. Heart Rhythm. 2014;11:799–805.
14. Jacheć W, Polewczyk A, Polewczyk M, Tomasik A, Kutarski A. Transvenous lead extraction safety score for risk stratification and proper patient selection for removal procedures using mechanical tools. J Clin Med. 2020;9:356–361.
15. Bongiorni MG, Kennergren C, Butter C, et al., ELECTRa Investigators. The European Lead Extraction ConTRolled (ELECTRa) study: a European Heart Rhythm Association (EHRA) registry of transvenous lead extraction outcomes. Eur Heart J. 2017;38:2995–3005.
16. Jacheć W, Polewczyk A, Polewczyk M, Tomasik A, Janion M, Kutarski A. Risk factors predicting complications of transvenous lead extraction. Biomed Res Int. 2018;2018:8796704.
17. Nowosielecka D, Polewczyk A, Jacheć W, Ł T, Kleinrok A, Kutarski A. Echocardiographic findings in patients with cardiac implantable electronic devices-analysis of factors predisposing to lead-associated changes. Clin Physiol Funct Imaging. 2021;41:25–41.
18. Nowosielecka D, Polewczyk A, Jacheć W, Kleinrok A, Tułecki Ł, Kutarski A. Transesophageal echocardiography for the monitoring of transvenous lead extraction. Kardiol Pol. 2020;78:1206–1214.
19. Nowosielecka D, Jacheć W, Polewczyk A, et al. Transesophageal echocardiography as a monitoring tool during transvenous lead extraction-Does it improve procedure effectiveness? J Clin Med. 2020;9:1382.
20. Nowosielecka D, Polewczyk A, Jacheć W, et al. A new approach to the continuous monitoring of transvenous lead extraction using transesophageal echocardiography-Analysis of 936 procedures. Echocardiography. 2020;37:601–611.
21. Nowosielecka D, Jacheć W, Polewczyk A, Kleinrok A, Tułecki Ł, Kutarski A. The prognostic value of transesophageal echocardiography after transvenous lead extraction - landscape after battle. Cardiovasc Diagn Ther. 2021;11(2):394.
22. Byrd CL, Wilkoff BL, Love CJ, et al. Intravascular extraction of problematic or infected permanent pacemaker leads: 1994-1996 U S extraction database, MED Institute pacing. Clin Electrophysiol. 1999;22:1348–1357.
23. Bongiorni MG, Soldati E, Zucchelli G, et al. Transvenous removal of pacing and implantable cardiac defibrillating leads using single sheath mechanical dilatation and multiple venous approaches: high success rate and safety in more than 2000 leads. Eur Heart J. 2008;29:2886–2893.
24. Kennergren C, Bjurman C, Wiklund R, Gäbel J. A single-centre experience of over one thousand lead extractions. Europace. 2009;11:612–617.
25. Wazni O, Epstein LM, Carrillo RG, et al. Lead extraction in the contemporary setting: the LExICon study: an observational retrospective study of consecutive laser lead extractions. J Am Coll Cardiol. 2010;55:579–586.
26. Gomes S, Cranney G, Bennett M, Li A, Giles R. Twenty-year experience of transvenous lead extraction at a single centre. Europace. 2014;16:1350–1355.
27. Maytin M, Jones SO, Epstein LM. Long-term mortality after transvenous lead extraction. Circ Arrhythm Electrophysiol. 2012;5:252–257.
28. Fu HX, Huang XM, Zhong LI, et al. Outcomes and complications of lead removal: can we establish a risk stratification schema for a collaborative and effective approach? Pacing Clin Electrophysiol. 2015;38:1439–1447.
29. El-Chami MF, Merchant FM, Levy M, et al. Outcomes of Sprint Fidelis And Riata Lead Extraction: data from 2 high-volume centers. Heart Rhythm. 2015;12:1216–1220.
30. Merchant FM, Levy MR, Kelli HM, et al. Predictors of long-term survival following transvenous extraction of defibrillator leads. Pacing Clin Electrophysiol. 2015;38:1297–1303.
31. Gomes S, Cranney G, Bennett M, Giles R. Lead extraction for treatment of cardiac device infection: a 20-year single centre experience. Heart Lung Circ. 2017;26:240–245.
32. Bashir J, Fedoruk LM, Ofiesh J, Karim SS, Tyers GF. Classification and surgical repair of injuries sustained during transvenous lead extraction. Circ Arrhythm Electrophysiol. 2016;9:e003741.
33. Barakat AF, Wazni OM, Tarakji K, et al. Transvenous lead extraction at the time of cardiac implantable electronic device upgrade: complexity, safety, and outcomes. Heart Rhythm. 2017;14:1807–1811.
34. Hussein AA, Tarakji KG, Martin DO, et al. Cardiac implantable electronic device infections: added complexity and suboptimal outcomes with previously abandoned leads. JACC Clin Electrophysiol. 2017;3:1–9.
35. Kutarski A, Czajkowski M, Pietura R, et al. Effectiveness, safety, and long-term outcomes of non-powered mechanical sheaths for transvenous lead extraction. Europace. 2018;20:1324–1333.
36. Sood N, Martin DT, Lampert R, Curtis JP, Parzynski C, Incidence CJ. Predictors of perioperative complications with transvenous lead extractions: real-world experience with national cardiovascular data registry. Circ Arrhythm Electrophysiol. 2018;11:e004768.
37. Sharma S, Ekeruo IA, Nand NP, et al. Efficacy of transvenous lead extraction utilizing the evolution mechanical lead extraction system: a single-center experience. JACC Clin Electrophysiol. 2018;4:212–220.
38. Gould J, Sidhu BS, Porter B, et al. Prolonged lead dwell time and lead burden predict bailout transfemoral lead extraction. Pacing Clin Electrophysiol. 2019;42:1355–1364.
39. Jacheć W, Polewczyk A, Segreti L, Bongiorni MG, Kutarski A. To abandon or not to abandon: late consequences of pacing and ICD lead abandonment. Pacing Clin Electrophysiol. 2019;42:1006–1017.
40. Segreti L, Giannotti Santoro M, Di Cori A, et al. Safety and efficacy of transvenous mechanical lead extraction in patients with abandoned leads. Europace. 2020;22:1401–1408.
41. Starck CT, Gonzalez E, Al-Razzo O, et al. Results of the Patient-Related Outcomes of Mechanical lead Extraction Techniques (PROMET) study: a multicentre retrospective study on advanced mechanical lead extraction techniques. Europace. 2020;22:1103–1110.
42. Giannotti Santoro M, Segreti L, Zucchelli G, et al. Transvenous lead extraction: efficacy and safety of the procedure in octogenarian patients. Pacing Clin Electrophysiol. 2020;43:382–387.
43. Zhou X, Ze F, Li D, et al. Transfemoral extraction of pacemaker and implantable cardioverter defibrillator leads using Needle’s Eye Snare: a single-center experience of more than 900 leads. Heart Vessels. 2020;35:825–834.
44. Bontempi L, Vassanelli F, Cerini M, et al. Predicting the difficulty of a lead extraction procedure: the LED index. J Cardiovasc Med. 2014;15:668–673.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.