Back to Journals » Clinical Epidemiology » Volume 9
Transmission of health care-associated infections from roommates and prior room occupants: a systematic review
Authors Cohen B, Cohen CC, Løyland B, Larson EL
Received 11 October 2016
Accepted for publication 7 February 2017
Published 23 May 2017 Volume 2017:9 Pages 297—310
DOI https://doi.org/10.2147/CLEP.S124382
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Henrik Sørensen
Bevin Cohen,1 Catherine Crawford Cohen,1 Borghild Løyland,2 Elaine L Larson1
1Columbia University School of Nursing, New York, NY, USA; 2Oslo and Akershus University College, Oslo, Norway
Abstract: Pathogens that cause health care-associated infections (HAIs) are known to survive on surfaces and equipment in health care environments despite routine cleaning. As a result, the infection status of prior room occupants and roommates may play a role in HAI transmission. We performed a systematic review of the literature evaluating the association between patients’ exposure to infected/colonized hospital roommates or prior room occupants and their risk of infection/colonization with the same organism. A PubMed search for English articles published in 1990–2014 yielded 330 studies, which were screened by three reviewers. Eighteen articles met our inclusion criteria. Multiple studies reported positive associations between infection and exposure to roommates with influenza and group A streptococcus, but no associations were found for Clostridium difficile, methicillin-resistant Staphylococcus aureus, Cryptosporidium parvum, or Pseudomonas cepacia; findings were mixed for vancomycin-resistant enterococci (VRE). Positive associations were found between infection/colonization and exposure to rooms previously occupied by patients with Pseudomonas aeruginosa and Acinetobacter baumannii, but no associations were found for resistant Gram-negative organisms; findings were mixed for C. difficile, methicillin-resistant S. aureus, and VRE. Although the majority of studies suggest a link between exposure to infected/colonized roommates and prior room occupants, methodological improvements such as increasing the statistical power and conducting universal screening for colonization would provide more definitive evidence needed to establish causality.
Keywords: health care-associated infections, hospital roommates, prior room occupants, multidrug-resistant organisms
Introduction
Despite decades of infection prevention research and quality improvement initiatives, health care-associated infections (HAIs) remain common adverse events in hospitals and long-term care facilities.1 Over 700,000 HAIs occur annually in the USA alone, leading to death in 6% of cases and costing the health care system 28–45 billion US dollars each year.2–4 Recently, there has been renewed interest in understanding the role of the physical environment in the spread of HAIs.5,6 Countless studies have reported that pathogenic organisms can survive on a variety of fomites in health care settings, including those at the patient bedside (eg, mattresses, linens, pillows, bedframes, bedrails), inside patient bathrooms (eg, toilets, floors, soap dispensers), and on medical instruments (eg, blood pressure cuffs, suctioning systems).7–14 Moreover, the effectiveness of cleaning regimens has been called into question as a number of studies have reported that pathogens remain on hospital surfaces even after they have been disinfected in accordance with recommended protocols.15–18 Pathogens that survive on fomites can subsequently be transferred from contaminated surfaces to patients through direct contact, indirect contact through the hands and gloves of health care workers, or by aerosolization of surface particles.8,11,19–21
Patients hospitalized with infections frequently contaminate their surrounding environments with pathogenic organisms; therefore, roommates and previous room occupants may serve as potential sources of exposure to other patients.8,22 Yet, our understanding of how such exposures contribute to a patient’s overall risk of infection remains limited, and the effects of these exposures may be dependent on a variety of factors unique to each organism species, such as their robustness to atmospheric conditions, susceptibility to cleaning agents, and virulence. Therefore, the aim of this study was to systematically review the literature describing organism transmission from concurrent roommates or previous room occupants in health care settings.
Methods
Inclusion criteria
This systematic literature review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.23 Studies were included if they met the following criteria: 1) compared infection and/or colonization rates between patients known to be exposed to infectious roommates and/or prior room occupants and patients not known to be exposed, 2) were conducted in an acute or long-term health care setting, 3) were original research studies, 4) were published in English, and 5) were published from January 1, 1990 through December 31, 2014.
Search strategy
The literature search was conducted in February 2015 to ensure that all manuscripts published within the inclusion period had been indexed. All databases indexed within PubMed were searched using the following combination of keywords and Medical Subject Heading (MeSH) search terms linked with Boolean operators: ([MeSH {Patients’ Rooms}] AND [MeSH {Infection Control Practitioners} OR MeSH {Infection Control} OR MeSH {Cross Infection} OR MeSH {Infection} OR MeSH {Wound Infection} OR MeSH {Surgical Wound Infection} OR Keyword {Infection}]) OR (Keyword [Prior Room Occupant*]) OR (Keyword [Roommate] AND Keyword [Transmission] OR Keyword [Infection*] OR Keyword [Outbreak*]).
Article selection, review, and quality scoring
Three reviewers (BC, CCC, and BL) independently assessed each article at all stages of the review and quality scoring processes. Discrepancies among reviewers were discussed as a group until a consensus was reached. First, the reviewers screened the titles and abstracts of all articles and eliminated those that were not relevant to the aims of the review. The remaining articles underwent full-text review to determine whether they met the inclusion criteria. A hand search of the references of all articles meeting the inclusion criteria was also performed. Articles meeting the inclusion criteria were scored according to a modified 20-item version of the Checklist for Measuring Study Quality developed by Downs and Black (Table 1).24 Some measures were not applicable to all articles; these items were removed from the score denominator and not assessed for studies in which they were not relevant. Final scores were converted to percentages.
  | Table 1 Assessment of study quality Note: aData collection tool from Downs and Black.24 |
Results
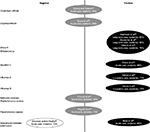
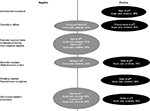
The database search returned 330 articles. No additional articles were identified from the hand search and no duplicates were found. Twenty articles were excluded during the title screening phase, and 223 articles were excluded during the abstract screening phase. The remaining 87 articles underwent full-text review, and 18 of these were determined to meet the inclusion criteria. Figure 1 describes the reasons for exclusion during the full-text review. Ten articles investigated the effects of exposure to infected or colonized roommates,25–34 six investigated the effects of exposure to infected or colonized prior room occupants,35–40 and two investigated both exposures.41,42
Study designs and definitions of exposures and outcomes
The articles in this review represent a range of observational and interventional designs, including retrospective and prospective cohort studies (n=11),26,28–32,35,38–40,42 case–control studies (n=4),25,27,33,34 and quasi-experimental studies (n=3).36,37,41 The studies varied considerably in their definitions of exposure and outcome measures. Among studies that examined exposure to roommates with nonviral pathogens, four (44%) defined the exposure as having a roommate with a clinical infection25–27,42 and five (56%) defined the exposure as having a roommate who was either infected or colonized.31–34,41 Among studies that examined exposure to previous room occupants, there was variation both in the determination of whether a previous occupant was infectious and in the timeframe during which they occupied the room. Four studies (50%) defined the exposure as a previous occupant who was infected or colonized,35,38,39,41 two studies (25%) – both of Clostridium difficile – defined the exposure as a previous occupant with a history of infection,40,41 and two studies (25%) did not specify.36,37
With regard to timing of the exposure, most of the studies implied that only the occupant immediately prior to the study subject was included, although only three articles stated this explicitly.35,37,40 One study also analyzed exposure to any infectious patient who had occupied the same room within the previous 2-week period.37 Finally, there was notable variation in the definition of study outcomes. Half of the articles used an outcome measure of infection,25–30,32,40,42 while the other half used an outcome measure of infection or colonization.31,33–39,41 Methods of case detection ranged from universal screening to sampling based on clinical indication.
Findings of studies examining exposure to infected or colonized roommates
The 12 articles investigating the effects of exposure to infected or colonized roommates are described in Table 2 and their findings are summarized in Figure 2. Five studies evaluated bacterial pathogens that are transmitted by contact.31,33,34,41,42 No significant associations between roommate exposure and infection with methicillin-resistant Staphylococcus aureus (MRSA), C. difficile, or Pseudomonas cepacia were identified.31,33,42 Results for vancomycin-resistant enterococci (VRE) were inconsistent, with Bass et al41 reporting a statistically significant positive association (hazard ratio [HR]: 18.8, 95% confidence interval: [5.4–66.2]) and Shorman and Al-Tawfiq34 reporting a statistically significant negative association (odds ratio [OR]: 0.04 [0.004–0.4]).
Three studies conducted in long-term care settings examined group A streptococcus, which is transmitted by contact and droplet routes.25,27,32 All three found significant positive associations between roommate exposure and infection, with ORs ranging from 2.0 (1.1–5.1) to 15.3 (2.5–110.9; point estimate not reported by Auerbach et al25).
Three studies examined exposure to roommates infected with viral pathogens.28–30 Two studies of influenza conducted within the same long-term care facility found significantly elevated risks of infection among those with infected roommates (relative risk: 3.1 [1.6–5.8] for influenza A and relative risk: 2.6 [1.2–5.6] for influenza B).28,29 One study evaluated transmission of hepatitis C, a viral bloodborne pathogen, in a liver transplant ward of an acute care hospital and found significantly increased odds of infection after sharing a room with an infected patient (OR: 12.0 [1.4–103.0]).30 One parasitic pathogen spread by fecal–oral contact, Cryptosporidium parvum, was evaluated in an acute care human immunodeficiency virus ward and no association was found.26
Findings of studies examining exposure to rooms previously occupied by infected or colonized patients
The eight articles investigating the effects of exposure to rooms previously occupied by infected or colonized patients are described in Table 3 and their findings are summarized in Figure 3. All of the articles studied bacterial pathogens spread through contact transmission in acute care hospitals, with all but two41,42 taking place in intensive care units. Nseir et al39 found that exposure to rooms previously occupied by patients with Acinetobacter baumannii and Pseudomonas aeruginosa resulted in significantly higher odds of infection or colonization (OR: 4.2 [2.0–8.8] and OR: 2.3 [1.2–4.3], respectively), while the two studies that examined extended-spectrum beta-lactamase-producing gram-negative organisms found no association.35,39 Effects of exposure to rooms previously occupied by patients with C. difficile, MRSA, and VRE were examined by at least two studies each. For each of these organisms, significant positive associations were reported by one article (C. difficile, HR: 2.4 [1.2–4.5];40 MRSA, OR: 1.4 [p=0.04];36 VRE, HR: 3.8 [2.0–7.4]37), with the remainder of articles finding no significant associations.38,41,42
Quality of included articles
Quality scores ranged from 50% to 95%, with the majority of articles scoring at or above 80% (median=83%, mean=82%). Table 1 provides a summary of scores for each item. All of the articles had clearly stated aims, adequate descriptions of study populations, appropriate control groups, and acceptable reporting of results. However, many of the studies did not appropriately control for confounding (50%, n=9), address differential follow-up between exposed and unexposed patients (33%, n=6), or use acceptable statistical methods (17%, n=3). In addition, some articles did not include sufficient or precise definitions of the exposures (17%, n=3) or outcomes (6%, n=1) under investigation. Notably, none of the articles reported a sample size calculation indicating adequate power to detect differences between patients exposed versus unexposed to infected/colonized roommates or prior room occupants.
Discussion
More than half of the articles identified in this systematic literature review reported at least one statistically significant positive association between the infection/colonization status of a roommate or previous room occupant and the development of HAIs.25,27–30,32,36,37,39–41 Only a single article identified a statistically significant negative association.34 The remainder found no associations that reached statistical significance, though this may be due to the fact that they were insufficiently powered; none of the articles reviewed included a statement indicating that statistical power was adequate for the analyses presented. Another factor that may have contributed to findings of no association is that many studies included patients who were either infected or colonized as potential sources of exposure. Patients with symptomatic infections may shed greater amounts of infectious body fluids to surrounding fomites, compared with patients who are asymptomatically colonized.43 Therefore, if a causal association does indeed exist, including both infected and colonized patients as potential sources of exposure may have driven findings toward the null, since exposure to colonized roommates and prior room occupants could present less risk to patients. Heterogeneity of the exposure may have also arisen from variation in the infection or colonization site of a roommate or prior room occupant. In a study of patients with MRSA, environmental contamination was more prevalent on fomites surrounding patients with positive wound or urine cultures, compared with patients who had positive blood or sputum cultures.8
The studies we reviewed revealed consistent findings for some pathogens (influenza, group A streptococcus) and inconsistent findings for others (VRE, MRSA, C. difficile). For endemic health care pathogens such as VRE, MRSA, and C. difficile, it may be difficult to isolate the effects of roommates and previous room occupants, since the exposure and outcome are common and may originate from multiple sources.44 On the contrary, pathogens such as influenza and group A streptococcus are more commonly associated with outbreak scenarios, making it easier to single out the effects of particular exposures.45 Other factors that may have contributed to inconsistent findings across studies are variations in how exposures and outcomes were defined and operationalized (eg, differences in case definitions, case finding methods, and timing of exposure).
While the inconsistency of findings for some of the organisms could be due to artifact, there may nevertheless be real differences in the effects of roommate and prior room occupant exposure based on the biologic characteristics of the infecting species. Microorganisms vary in their abilities to produce spores and survive changes to atmospheric temperature and moisture conditions.46 In addition, some organisms favor specific sites of colonization or infection that may produce greater shedding of infectious material and higher potential for environmental contamination.46 For example, a study of multidrug-resistant pathogens found that environmental contamination was more common surrounding patients with gram-positive versus gram-negative infections.22 Furthermore, organism species differ in their resiliency to withstand cleaning agents and methods.47,48
The preponderance of evidence presented in this review suggests that there is a link between exposure to infected or colonized roommates and previous room occupants and the risk of HAIs. These findings present a number of practice and policy implications. First, the fact that patient rooms may serve as a reservoir for pathogens deposited by roommates and previous occupants highlights the importance of proper hand hygiene, not just for staff but for competent patients and their visitors as well.49 To underscore this point, a molecular typing study demonstrated that 12% of patients who became newly colonized with MRSA while in the intensive care unit acquired a strain that most probably came from contamination in their immediate environment.13 Second, these results emphasize the need for improved cleaning and disinfection of patient rooms, both during patients’ hospital stays and upon their discharge. For patients with known infection or colonization, targeted daily and terminal cleaning procedures that are tailored to specific organisms may reduce environmental contamination and infection rates.50 Enhancement of routine cleaning measures should not be limited to patients with known infection or colonization, however, since patients may contaminate their environments during incubation periods before the infections are detected or when colonization is not detected through active surveillance.
There were some limitations to this systematic review. It is possible that some studies which would have met the inclusion criteria were not identified. Only databases indexed in PubMed were included, so any unpublished reports and other gray literature would not have been detected by our search. Similarly, studies that found significant positive associations may have been more likely to appear in the literature due to publication bias. Our restriction to articles published in English may have also excluded some relevant papers. While a major strength of this study is its coverage of two and a half decades of literature, changes in the epidemiology of HAIs, infection control policies and procedures, and study methodology over time may have introduced some variability to the studies we reviewed. Lastly, we were unable to conduct a meta-analysis or provide a funnel plot because the studies assessed a wide variety of outcomes.
Notwithstanding these limitations, it is notable that the studies which reported significant findings were conducted across a range of institutions in several different countries across multiple decades. Presumably, the diverse study facilities employed a variety of cleaning products, methods, and infection control policies. Despite possible variations in practice, exposure to roommates and prior room occupants may have played a role in infection outcomes. Several gaps in the literature remain, however, specifically with regard to organisms that are endemic in health care settings and, therefore, difficult to associate with specific sources of exposure. The use of molecular typing would provide more definitive evidence concerning the role of roommates and prior room occupants in the epidemiology of HAIs.
Acknowledgment
The abstract of this paper was presented at IDWeek 2015 as a poster presentation with interim findings. The poster’s abstract was published in “IDWeek 2015 Abstracts” in Open Forum Infectious Diseases: http://ofid.oxfordjournals.org/content/2/suppl_1/1706.full. doi: 10.1093/ofid/ofv133.1256
Disclosure
The authors report no conflicts of interest in this work.
References
Haley RW, Shachtman RH. The emergence of infection surveillance and control programs in US hospitals: an assessment, 1976. Am J Epidemiol. 1980;111(5):574–591. | ||
Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166. | ||
Scott RD II; Centers for Disease Control and Prevention. The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention. Available from: http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf. Accessed October 1, 2015. | ||
Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. | ||
Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38(5 Suppl 1):S25–S33. | ||
Weber DJ, Rutala WA. Understanding and preventing transmission of healthcare-associated pathogens due to the contaminated hospital environment. Infect Control Hosp Epidemiol. 2013;34(5):449–452. | ||
Duckro AN, Blom DW, Lyle EA, Weinstein RA, Hayden MK. Transfer of vancomycin-resistant enterococci via health care worker hands. Arch Intern Med. 2005;165(3):302–307. | ||
Boyce JM, Potter-Bynoe G, Chenevert C, King T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: possible infection control implications. Infect Control Hosp Epidemiol. 1997;18(9):622–627. | ||
Sitzlar B, Deshpande A, Fertelli D, Kundrapu S, Sethi AK, Donskey CJ. An environmental disinfection odyssey: evaluation of sequential interventions to improve disinfection of Clostridium difficile isolation rooms. Infect Control Hosp Epidemiol. 2013;34(5):459–465. | ||
Wilson AP, Smyth D, Moore G, et al. The impact of enhanced cleaning within the intensive care unit on contamination of the near-patient environment with hospital pathogens: a randomized crossover study in critical care units in two hospitals. Crit Care Med. 2011;39(4):651–658. | ||
Ray AJ, Hoyen CK, Taub TF, Eckstein EC, Donskey CJ. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA. 2002;287(11):1400–1401. | ||
Creamer E, Humphreys H. The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect. 2008;69(1):8–23. | ||
Hardy KJ, Oppenheim BA, Gossain S, Gao F, Hawkey PM. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect Control Hosp Epidemiol. 2006;27(2):127–132. | ||
Giannini MA, Nance D, McCullers JA. Are toilet seats a vector for transmission of methicillin-resistant Staphylococcus aureus? Am J Infect Control. 2009;37(6):505–506. | ||
Guerrero DM, Carling PC, Jury LA, Ponnada S, Nerandzic MM, Donskey CJ. Beyond the hawthorne effect: reduction of clostridium difficile environmental contamination through active intervention to improve cleaning practices. Infect Control Hosp Epidemiol. 2013;34(5):524–526. | ||
Carling PC, Briggs J, Hylander D, Perkins J. An evaluation of patient area cleaning in 3 hospitals using a novel targeting methodology. Am J Infect Control. 2006;34(8):513–519. | ||
Carling PC. Evaluating the thoroughness of environmental cleaning in hospitals. J Hosp Infect. 2008;68(3):273–274. | ||
Attaway HH 3rd, Fairey S, Steed LL, Salgado CD, Michels HT, Schmidt MG. Intrinsic bacterial burden associated with intensive care unit hospital beds: effects of disinfection on population recovery and mitigation of potential infection risk. Am J Infect Control. 2012;40(10):907–912. | ||
Hess AS, Shardell M, Johnson JK, et al. A randomized controlled trial of enhanced cleaning to reduce contamination of healthcare worker gowns and gloves with multidrug-resistant bacteria. Infect Control Hosp Epidemiol. 2013;34(5):487–493. | ||
Bhalla A, Pultz NJ, Gries DM, et al. Acquisition of nosocomial pathogens on hands after contact with environmental surfaces near hospitalized patients. Infect Control Hosp Epidemiol. 2004;25(2):164–167. | ||
Dancer SJ. Hospital cleaning in the 21st century. Eur J Clin Microbiol Infect Dis. 2011;30(12):1473–1481. | ||
Lemmen SW, Häfner H, Zolldann D, Stanzel S, Lütticken R. Distribution of multi-resistant gram-negative versus gram-positive bacteria in the hospital inanimate environment. J Hosp Infect. 2004;56(3):191–197. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. | ||
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. | ||
Auerbach SB, Schwartz B, Williams D, et al. Outbreak of invasive group A streptococcal infections in a nursing home. Arch Intern Med. 1992;152(5):1017–1022. | ||
Bruce BB, Blass MA, Blumberg HM, Lennox JL, del Rio C, Horsburgh CR Jr. Risk of Cryptosporidium parvum transmission between hospital roommates. Clin Infect Dis. 2000;31(4):947–950. | ||
Deutscher M, Schillie S, Gould C, et al. Investigation of a group A streptococcal outbreak among residents of a long-term acute care hospital. Clin Infect Dis. 2011;52(8):988–994. | ||
Drinka PJ, Krause P, Nest L, Goodman BM, Gravenstein S. Risk of acquiring influenza A in a nursing home from a culture-positive roommate. Infect Control Hosp Epidemiol. 2003;24(11):872–874. | ||
Drinka PJ, Krause PF, Nest LJ, Goodman BM, Gravenstein S. Risk of acquiring influenza B in a nursing home from a culture-positive roommate. J Am Geriatr Soc. 2005;53(8):1437. | ||
Forns X, Martínez-Bauer E, Feliu A, et al. Nosocomial transmission of HCV in the liver unit of a tertiary care center. Hepatology. 2005;41(1):115–122. | ||
Furuno JP, Shurland SM, Zhan M, et al. Comparison of the methicillin-resistant Staphylococcus aureus acquisition among rehabilitation and nursing home residents. Infect Control Hosp Epidemiol. 2011;32(3):244–249. | ||
Greene CM, Van Beneden CA, Javadi M, et al. Cluster of deaths from group A streptococcus in a long-term care facility – Georgia, 2001. Am J Infect Control. 2005;33(2):108–113. | ||
Pegues DA, Schidlow DV, Tablan OC, Carson LA, Clark NC, Jarvis WR. Possible nosocomial transmission of Pseudomonas cepacia in patients with cystic fibrosis. Arch Pediatr Adolesc Med. 1994;148(8):508–812. | ||
Shorman M, Al-Tawfiq JA. Risk factors associated with vancomycin-resistant Enterococcus in intensive care unit settings in Saudi Arabia. Interdiscip Perspect Infect Dis. 2013;2013:369674. | ||
Ajao AO, Johnson KJ, Harris AD, et al. Risk of acquiring extended-spectrum β-lactamase-producing Klebsiella species and Escherichia coli from prior room occupants in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34(5):453–458. | ||
Datta R, Platt R, Yokoe DS, Huang SS. Environmental cleaning intervention and risk of acquiring multidrug-resistant organisms from prior room occupants. Arch Intern Med. 2011;171(6):491–494. | ||
Drees M, Snydman DR, Schmid CH, et al. Prior environmental contamination increases the risk of acquisition of vancomycin-resistant enterococci. Clin Infect Dis. 2008;46(5):678–685. | ||
Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166(18):1945–1951. | ||
Nseir S, Blazejewski C, Lubret R, Wallet F, Courcol R, Durocher A. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect. 2011;17(8):1201–1208. | ||
Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32(3):201–206. | ||
Bass P, Karki S, Rhodes D, et al. Impact of chlorhexidine-impregnated washcloths on reducing incidence of vancomycin-resistant enterococci colonization in hematology-oncology patients. Am J Infect Control. 2013;41(4):345–348. | ||
Chang VT, Nelson K. The role of physical proximity in nosocomial diarrhea. Clin Infect Dis. 2000;31(3):717–722. | ||
Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention. Management of multidrug-resistant organisms in healthcare settings; 2006. Available from http://www.cdc.gov/hicpac/pdf/MDRO/MDROGuideline2006.pdf. Accessed January 10, 2016. | ||
Siegel JD, Rhinehart E, Jackson M, Chiarello L; Healthcare Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. Available from: http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf. Accessed January 10, 2016. | ||
Wong SS, Yuen KY. Streptococcus pyogenes and re-emergence of scarlet fever as a public health problem. Emerg Microbes Infect. 2012;1(7):e2. | ||
Dancer SJ. Mopping up hospital infection. J Hosp Infect. 1999;43(2):85–100. | ||
Otter JA, French GL. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J Clin Microbiol. 2008;47(1):205–207. | ||
Weinstein RA, Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39(8):1182–1189. | ||
Morgan DJ, Rogawski E, Thom KA, et al. Transfer of multidrug-resistant bacteria to healthcare workers’ gloves and gowns after patient contact increases with environmental contamination. Crit Care Med. 2012;40(4):1045–1051. | ||
Manian FA, Griesnauer S, Bryant A. Implementation of hospital-wide enhanced terminal cleaning of targeted patient rooms and its impact on endemic Clostridium difficile infection rates. Am J Infect Control. 2013;41(6):537–541. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.