Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Translation, Validation, and Psychometric Evaluation of the Diabetes Quality-of-Life Brief Clinical Inventory: The Urdu Version
Authors Haider S, Saleem F , Ahmad N, Iqbal Q , Bashaar M
Received 30 December 2021
Accepted for publication 28 March 2022
Published 29 April 2022 Volume 2022:15 Pages 955—966
DOI https://doi.org/10.2147/JMDH.S351330
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sajjad Haider,1 Fahad Saleem,1 Nafees Ahmad,1 Qaiser Iqbal,1 Mohammad Bashaar2
1Faculty of Pharmacy & Health Sciences, University of Balochistan, Quetta, Pakistan; 2SMART Afghan International Trainings and Consultancy, Kabul, Afghanistan
Correspondence: Mohammad Bashaar, SMART Afghan International Trainings and Consultancy, Shahri Naw, Hospital Street No. 1, Kabul, Afghanistan, Tel +93788233865, Email [email protected]
Purpose: The study is aimed to examine the psychometric properties of the Urdu version of the Diabetes Quality-of-Life Brief Clinical Inventory.
Methods: We adopted the forward–backward procedure to translate the Diabetes Quality-of-Life Brief Clinical Inventory (DQoL-BCI) into the Urdu language (lingua franca of Pakistan). The intraclass correlation (ICC) confirmed the consistency of retaining the items, and Cronbach’s alpha established the test–re-test reliability. The confirmatory factor analysis (principal axis factoring extraction and oblique rotation with Kaiser normalization) validated the DQoL-BCI in Urdu.
Results: A two-time point with an interval of 2 weeks was used, and the Urdu version of DQoL-BCI was piloted accordingly. The 15-item translated version (DQoL-BCI-U) exhibited a satisfactory Cronbach’s value of 0.866 (test) at week 1 and 0.850 at week 3 (re-test). Using the one-way random model with single measurements, the ICC for all 15 items exhibited coefficient values of > 0.80. The Kaiser–Meyer–Olkin measure of sampling adequacy and Bartlett’s Test of Sphericity revealed relationships of the data and suitability of CFA (0.899, p< 0.05). Seven factors explaining the total variance of 69% were extracted. With acceptable communalities, all 15 items of DQoL-BCI-U were retained.
Conclusion: The study concludes that the translated version of DQoL-BCI-U is a valid instrument in regions, where Urdu is a communal language of communication and can examine quality-of-life issues during the typical patient–provider encounter.
Keywords: translation, validation, psychometric evaluation, Urdu
Introduction
Traditionally, healthcare systems around the globe were concerned with decreasing morbidity and mortality. Healthcare systems concentrated on the “quantity of life,” and gave utility evaluation little consideration during disease management.1 However, the late 1960s observed significant variations in socio-demographic profiles and their influence on the healthcare systems of “late modern” societies. These variations developed another critical concern: “Quality-of-Life” (QoL). First introduced by Elkington in 1966,2 the author raised significant questions regarding the maintenance and improvement of QoL of an individual patient. Furthermore, the author also emphasized that there should be a specific criterion to utilize society’s resources to achieve excellent health and QoL for all members of that society.2 The contentions by Elkington were further supported by the development of new technologies that raised additional questions for clinical and non-clinical professionals. Hence, QoL was proposed as an additional yet substantial parameter for making decisions for health-related issues.1 Consequently, researchers from both medical and social sciences started to construct instruments designed to measure QoL. These efforts developed generic and disease-specific tools focusing on the status and methods of improving the patients’ physical, mental, and social well-being.3–5 Since then, QoL measures have been in continuous use during clinical trials and research initiatives to assess the impact of management, treatment, and medical interventions.6–9
There are multiple models of QoL, however, the models proposed by Wilson and Cleary,10 Ferrans et al,11 and the World Health Organization12 are frequently used in the literature. These models can also predict multidimensional aspects of QoL, and the diverse use of this term across health and disease conditions.13 Based on the postulates of these models, both generic and disease-specific instruments are developed and are repeatedly used in literature to assess the literature QoL and its related issues.13 Within this context, the differences between the generic and the disease-specific QoL measures are extensively discussed in the literature.14,15 Where generic measures focus on various health conditions, disease-specific measures tend to measure specific elements of a disease and are sensitive to changes and modifications during pharmacotherapy and disease-related management.16 Furthermore, disease-specific measures can give a detailed pattern of specific symptoms and impairment related to a particular disease.17 Therefore, disease-specific measures are much more effective at assessing the impact of disease-based interventions and identifying critical issues in making clinical care decisions.
In line with what is being discussed, evaluating QoL in chronic diseases such as diabetes is imperative. The management requires a long-term enduring change that impedes patients’ lifestyles and day-to-day activities. Patients with diabetes are exposed to physical and psychological complications that directly influence the QoL. Even though patients with diabetes are managed according to the established guidelines, improvement in QoL is not frequently reported.18 This is understandable as QoL is a multi-factor phenomenon, and progress in overall health status is not dependent on a single factor.19 Our observations are supported by various studies whereby disease-related, personal, and psychosocial factors negatively affect QoL.20–22 Therefore, to comprehensively assess the QoL in diabetes patients, an instrument capable of evaluating significant experiences of the patients is required. In literature, multiple tools are available but with certain limitations. Some tools are extensively built, others are either Type I or Type II focused. Some focus on QoL during clinical trials, while others lack generalizability because of their specific nature.23 Several QoL measures are available that assess the QoL in T2DM. The Audit of Diabetes-Dependent QoL measure, Diabetes Health Profile, Problem Areas in Diabetes, Diabetes Impact Measurement Scales, Diabetes Quality-of-Life Clinical Trial Questionnaire, The Asian Diabetes Quality-of-Life, and Diabetes-Dependent Quality-of-Life are frequently used and reported in the literature.24,25 Nevertheless, an instrument focusing on the live experiences of diabetes patients during the point of care was missing in the literature. These limitations led to the Diabetes Quality-of-Life Brief Clinical Inventory (DQoL-BCI); an instrument derived from the Diabetes Quality-of-Life Questionnaire (DQoL). The DQoL-BCI evaluated three domains (satisfaction, impact, and worries) that were not addressed by other QoL instruments.23 Moreover, the DQoL-BCI targeted both Types I and II diabetes, was less time-consuming, and predicted self-care behaviors and specific treatment-related concerns. Most importantly, it identified QoL issues left unaddressed during the consultation process. Consequently, DQoL-BCI showed clear advantages over other tools while addressing issues related to QoL in patients with diabetes. Therefore, the developers concluded that, while addressing QoL in diabetes patients, DQoL-BCI can replace other generic instruments.23
In this study, we aimed to generate a validated Urdu version of DQoL-BCI and examined its psychometric properties to be used for patients with diabetes. We believe that a psychometrically valid diabetes-specific questionnaire would be most suitable for assessing the QoL during diabetes management for territories where Urdu is used as a communication medium.26–28 In addition, it will be of critical importance to diabetes as that disease is associated with long-term complications and disabilities.
Materials and Methods
Study Design, Settings, and Pilot
We used a cross-sectional study design to test the DQoL-BCI psychometrically. The study was conducted at the Sandeman Provisional Hospital Quetta (SPHQ). Established in 1939, SPHQ is a tertiary care teaching hospital located in the city’s center. With well-developed and equipped wards, SPHQ has the complete facility and advanced technologies to manage patients with T2DM.29
Thirty patients with T2DM attending medicine wards of SPHQ for their routine consultation were approached for the pilot phase. In addition to the face and content validity, the pilot study was conducted to establish the internal consistency of DQoL-BCI-U. Respondents’ views on the translated instrument were considered and were discussed in a second expert panel discussion (post-pilot). One hundred and sixty-five patients were later enrolled for the field study.
Study Participants and Criteria
Patients with T2DM, consenting to participate with an understanding of long-term collaboration (for test–re-test analysis) and familiar (speaking, reading, and writing) with the national language of Pakistan (Urdu) were approached for data collection. We excluded illiterates, patients with mental impairments, patients requiring assistance in responding to the principal investigator, and immigrants.
Permission to Translate and Validate the DQoL-BCI
We sent a formal request to the developer of DQoL-BCI (Prof. Dr. Tom Burroughs, SLU College for Public Health and Social Justice, 3545 Lafayette Avenue, St. Louis, MO 63104, USA), who provided the English version of the DQoL-BCI and permission through email.
Translation of the DQoL-BCI
The translation of DQoL-BCI followed the forward–backward procedure that is proposed by the guidelines issued by the International Society of Pharmacoeconomics and Outcomes Research and World Health Organization.30,31
Forward Translation of DQoL-BCI (English to Urdu)
The research team approached four independent linguistic translators (native of Urdu language with academic proficiency in the English language). All translators were blinded because we wanted the Urdu version of DQoL-BCI to be theoretically to the DQol-BCI. Furthermore, our focus was to avoid discrepancies and partiality in the translation process and assure that the translated version received by the translators was without any communal discussion.
Reverse Translation of DQoL-BCI (Urdu to English)
Four independent translators (native English language with academic proficiency in the Urdu language) performed the reverse translation. As discussed above, the translators were also blinded. Once the research team received the two translated versions, we conducted an expert panel discussion (pre-pilot) to discuss issues associated with the questionnaire translation process.
Expert Panel Discussion (Pre-Pilot)
A bilingual (English and Urdu) expert panel convened by the research supervisor discussed the two translated versions of the questionnaire. The panel members comprised the translators, the research team, two diabetologists, and three experienced scientists of Health System Research. After reaching a mutual consensus, the Urdu version of DQoL-BCI, termed “The Diabetes Quality-of-Life Brief Clinical Inventory-Urdu (DQoL-BCI-U),” was subjected to a pilot study.
Expert Panel Discussion (Post-Pilot)
The DQoL-BCI-U, along with the reservations and recommendations of the patients, was discussed in the post-pilot expert panel discussion. The DQoL-BCI-U required minor changes, and the updated version was again presented to respondents of the pilot study for their consideration. Once the panel-respondents understanding was established, the finalized version of DQoL-BCI-U was made available for the field study (Supplementary Material).
Sampling for Pilot Phase (Test–Re-Test at Two Different Points)
According to Cohen,32 the population correlation coefficient as the effect size measurement should be used to benchmark sample size calculation. As described in the literature (p<0.05 with statistical power at 80%), 30 respondents were contacted for test–re-test analysis.33
Sampling for Factor Analysis (Field Test)
We used the Confirmatory factor analysis (CFA) to establish the construct validity.34 Determining an acceptable sample selection for CFA is essential because an inadequate sample may lead to problems with an estimation and item interpretation.33,34 Therefore, as suggested by Wolf et al,35 a 10 subjects to 1 variable ratio were used to determine the sample for CFA. One hundred and fifty participants were needed and, by adding a drop-out of 10%, the final sample size was 165, which was supposed to generate a good factor resolution.34
Ethical Approval
We conduct this study in compliance with the principles of the Declaration of Helsinki. The Institutional Review Board at the Faculty of Pharmacy and Health Sciences, University of Balochistan approved the study (UoB/Reg/67). Permission from the Medical Superintendent of SPHQ was also taken into consideration. Written consent from the participants was taken, and they were informed about their rights of participation in the study.
Statistical Analysis
The data were coded and entered in SPSS v 21.0 with an alpha value of 0.05 (two-tailed).36 Frequencies and percentages were used to explain the demographic variables. The test–re-test reliability was assessed and interpreted through Cronbach’s alpha reliability analysis.34 Intraclass Correlation Coefficient (ICC) via the One-Way Random effects model with single measures was used to establish the stability of the items.34–37 Confirmatory Factor Analysis confirmed the validity of the DQoL-BCI-U through principal axis factoring extraction and Oblique rotation with Kaiser Normalization.34
Results
Demographic Characteristics of the Study Respondents (Pilot Phase)
The demographic information for the pilot phase is presented in Table 1. The majority (19, 63.4%) of the patients were 30–40 years old. Males dominated the cohort, and almost 50% were public employees. Nearly 40% had T2DM for less than 5 years, and 63.4% took oral hypoglycemic agents.
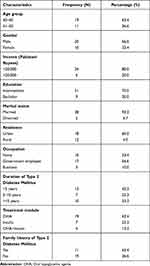 |
Table 1 Patients’ Characteristics and Descriptive Statistics (N=30, Pilot Test) |
Reliability Analysis (Two-Time Point)
The DQoL-BCI-U was pilot tested on 30 respondents at two time points with an interval of 2 weeks (Table 2). Based on what is reported in the literature,34,38,39 the following criterion was referred; 0.9≤α (excellent), 0.8≤α<0.9 (good), and 0.7≤α<0.8 (acceptable). At week 1, the 15-item DQoL-BCI-U exhibited an acceptable Cronbach’s value of 0.866 (test). Furthermore, the DQoL-BCI-U reported a good Cronbach’s value of 0.850 at week 3 (re-test) that illustrated satisfactory internal consistency at two time points.
 |
Table 2 Reliability Values at Two-Time Points (N=30, Pilot Study) |
Construct Stability Assessment
Using the One-Way Random Model (Model 1) with single measurements, the Intraclass Correlation Coefficient (ICC) was calculated to establish the stability of the items. The ICC values of <0.50 (low), 0.50–0.75 (moderate), and >0.75 (good), as suggested by Portney and Watkins,37 were used as reference. As shown in Table 3, the ICC for all items tested for intra-rater (test–re-test) reliability was good and exhibited coefficient values of >0.80.
 |
Table 3 Reliability of Test–Re-Test (N=30; Intraclass Correlation Coefficient) |
Description of Field Test (n=165)
The demographic characteristics of the study respondents for field testing are described in Table 4. The cohort was dominated by 41–50 years (92, 70.8%), and most of the respondents had rural locality (70, 53.7%). Thirty-six (27.6%) were illiterate, while 115 (88.4%) were married. The internal consistency using Cronbach’s alpha statistics (n=165) for pooled 15 items showed well acceptable reliability at α=0.801 (Table 5).
 |
Table 4 Respondents’ Characteristics and Descriptive Statistics (n=165, Field Test) |
 |
Table 5 Reliability Values for All Items (N=165, Field Test) |
Confirmatory Factor Analysis: Construct Validity
The Kaiser–Meyer–Olkin (KMO) sampling adequacy for the factor analysis was 0.899; hence was rated meritorious.40 Also, with χ2=1071.21 and p<0.05, Bartlett's Test of Sphericity revealed relationships of the data and suitability of CFA.41
The extracted communalities and loadings factors for the DQoL-BCI-U are presented in Table 6. We used both matrices (pattern and structure) to avoid probabilities of value suppression because of the factorial relationships.42 Seven factors explaining the total variance of 69% were extracted. For the retention of items, we used Field’s proposal of communalities >0.30.33 As shown in Table 6, loading values for all 15 items were acceptable (>0.40). All items of the translated DQoL-BCI-U were retained, proving the validity of the translated questionnaire.
 |
Table 6 Survey Items, Rotated Factor Loading, and Communalities (n=165) |
Discussion
Diabetes is a global public health concern and is approaching epidemic proportions. The disease is a burden to the healthcare systems that adversely affect the socio-economic development of nations.43 Four hundred and fifty-one million adults had diabetes in 2017. The International Diabetes Federation estimated a projected increase of 693 million by 2045 (IDF), provided no intervention is offered or adopted.44 While the past decades have seen significant progress in promoting population health and extending life expectancy; diabetes still has the second biggest negative effect on reducing global health adjusted life expectancy worldwide.45
Within this context, diabetes is highly prevalent in developing countries, and Pakistan is no exception. The IDF in 2019 estimated that >19 million adults in Pakistan had diabetes. Alarmingly, 8.5 million of these 19 million were undiagnosed, hence are at risk of developing micro- and macrovascular complications.46 In addition to the negative effect of diabetes on QoL, the healthcare system of Pakistan is faced with a lack of human resources, optimal facilities, income disparities, and differences of living status that further deteriorate the QoL.22 Therefore, assessing QoL in diabetes patients becomes imperative. For that reason, we psychometrically validated the DQoL-BCI-U, which will be utilized for the assessment of QoL in the healthcare settings of Pakistan.
The current study showed that the Urdu version of the questionnaire had good psychometric properties and was also coherent to the study participants. The DQoL-BCI-U demonstrated a Cronbach alpha index of 0.81 that revealed high reliability and internal consistency. Furthermore, the test–re-test reliability was also excellent, like the parent study23 and other versions of the questionnaire.47–50 Likewise, the ICC for all 15 items tested for intra-rater reliability was excellent, with significant coefficient values proposed in the literature.33 Hence, our assessment confirmed the repeatability of item measurements between two time intervals. This also demonstrated the correlation between multiple items of DQoL-BCI-U that intended to measure the same items.
We applied the CFA to identify the underlying relationships between the measured items of DQoL-BCI-U. Before adopting the CFA, the sampling adequacy and completeness of the dataset for each variable under observation is to be established as proposed in the literature.31 In the current study, the KMO value for the current model was 0.899, indicating the sample adequacy and data completeness for CFA.40,51 Likewise, the positive zero-order correlations reported in our study verified that no corrective actions were needed.52 To conclude, the acceptable values and positive correlations confirmed the application of CFA for the current data.
The construct validity was established by applying the principal factor analysis (PFA). For the PFA, the oblique rotation method with Kaiser normalization was selected based on the values extracted by the Shapiro–Wilk test as the data set violated the assumption of normality.34 Because the delta was set at zero, direct oblimin was selected based on the assumption that factors are correlated. Results of the study reported a significant Bartlett's Test of Sphericity (p<0.05) which further confirmed the suitability of CFA.
Extracted communalities were used as a reference while considering the item retention. As recommended by Field33,34 and Pallant, loading values of >0.30 have considerable influence and are to be retained during the CFA. As presented in Table 6, the extracted communalities for all items of DQoL-BCI-U ranged from 0.4–0.7, confirming that all items of the original DQoL-BCI can be adopted in the translated version. This also verified that the factors extracted during the CFA were applicable to establish the validity of DQoL-BCI-U. Likewise, items identified during the CFA were like the original DQoL-BCI and other validated versions.23,47,50,53 Concluding, high scale reliability and good construct validity rated the Urdu version of DQoL-BCI as a reliable tool in research and individual diagnostics.
Conclusion
Assessing QoL is important while recommending or adopting preventative measures during Diabetes Mellitus. An improvement in QoL ensures the well-being of diabetic patients with a reduced frequency of complications. With satisfactory reliability (test and re-test), satisfactory correlations and acceptable communalities, all 15 items of DQoL-BCI-U were retained during the validation. Based on our analysis, we conclude that DQoL-BCI-U is a valid instrument to assess QoL in regions where Urdu is a prime language of communication. We believe that using DQoL-BCI-U will help healthcare professionals identify potential areas for improvement for diabetic patients that will affect the overall quality of care. Consequently, a large study to generalize the results and establish the QoL profile of T2DM in the country is recommended.
Strengths and Limitations
We agree with the developers of DQoL-BCI that this specific instrument should be viewed as an initial screening device to identify specific patient issues. In addition, probing other problems that patients may be facing must be kept in mind. Nevertheless, the informal communication during data collection revealed that respondents of the current study felt the DQoL-BCI-U managed to extract their specific experiences and concerns while living with diabetes. Consequently, the DQoL-BCI-U provides an opportunity to capture patients’ experiences that is not possible by adopting other instruments.
Acknowledgment
We acknowledge the patients for their help and support during the data collection process. The developers of DQoL-BCI are also acknowledged for their permission and continuous support during the study period.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pennacchini M, Bertolaso M, Elvira M, De Marinis M. A brief history of the Quality of Life: its use in medicine and in philosophy. Clin Ther. 2011;162(3):e99–e103.
2. Elkington JR. Medicine and the Quality of Life. Ann Intern Med. 1966;64:711–714. doi:10.7326/0003-4819-64-3-711
3. Gill TM, Feinstein AR. A critical appraisal of the quality of quality-of-life measurements. JAMA. 1994;272(8):619–626. doi:10.1001/jama.1994.03520080061045
4. Greenfield S, Nelson EC. Future Issues in the use of health status assessment measures in clinical settings. Med Care. 1992;30M:S23–MS41.
5. Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118(8):622–629. doi:10.7326/0003-4819-118-8-199304150-00009
6. Bakas T, McLennon SM, Carpenter JS, et al. Systematic review of health-related quality of life models. Health Qual Life Outcomes. 2012;10(1):1–12. doi:10.1186/1477-7525-10-134
7. Chachamovich JR, Chachamovich E, Ezer H, Fleck MP, Knauth D, Passos EP. Investigating quality of life and health-related quality of life in infertility: a systematic review. J Psychosom Obstet Gynaecol. 2010;31(2):101–110. doi:10.3109/0167482X.2010.481337
8. Hanaoka H, Okamura H. Study on effects of life review activities on the quality of life of the elderly: a randomized controlled trial. Psychother Psychosom. 2004;73(5):302–311. doi:10.1159/000078847
9. Megari K. Quality of life in chronic disease patients. Health Psychol Res. 2013;1(3):e27. doi:10.4081/hpr.2013.932
10. Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: a conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. doi:10.1001/jama.1995.03520250075037
11. Ferrans CE, Zerwic JJ, Wilbur JE, Larson JL. Conceptual model of health‐related quality of life. J Nurs Scholar. 2005;37(4):336–342. doi:10.1111/j.1547-5069.2005.00058.x
12. World Health Organization. International classification of impairments, disabilities, and handicaps: a manual of classification relating to the consequences of disease. Available from: https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf?sequence=1&isAllowed=y.
13. Sousa WP, Kwok O. Putting Wilson and Cleary to the test: analysis of a HRQOL conceptual model using structural equation modeling. Qual life Res. 2006;15:725–737. doi:10.1007/s11136-005-3975-4
14. Coons SJ, Rao S, Keininger DL, Hays RD. A comparative review of generic quality-of-life instruments. Pharmacoeconomics. 2000;17(1):13–35. doi:10.2165/00019053-200017010-00002
15. Haraldstad K, Wahl A, Andenæs R, et al. A systematic review of quality of life research in medicine and health sciences. Qual life Res. 2019;28(10):2641–2650. doi:10.1007/s11136-019-02214-9
16. Seow LSE, Tan THG, Abdin E, Chong SA, Subramaniam M. Comparing disease-specific and generic quality of life measures in patients with schizophrenia. Psychiatry Res. 2019;273:387–393. doi:10.1016/j.psychres.2019.01.034
17. Remor E, Young N, Von Mackensen S, Lopatina E. Disease‐specific quality‐of‐life measurement tools for haemophilia patients. Haemophilia. 2004;10:30–34. doi:10.1111/j.1365-2516.2004.01004.x
18. Palamenghi L, Carlucci MM, Graffigna G. Measuring the quality of life in diabetic patients: a scoping review. J Diabetes Res. 2020;2020:5419298. doi:10.1155/2020/5419298
19. Saleem F, Hassali MA, Shafie AA, et al. Does treatment adherence correlates with health related quality of life? Findings from a cross sectional study. BMC Public Health. 2012;12(1):1–7. doi:10.1186/1471-2458-12-318
20. Timar R, Velea I, Timar B, et al. Factors influencing the quality of life perception in patients with type 2 diabetes mellitus. Patient Prefer Adherence. 2016;10:2471. doi:10.2147/PPA.S124858
21. Jing X, Chen J, Dong Y, et al. Related factors of quality of life of type 2 diabetes patients: a systematic review and meta-analysis. Health Qual Life Outcomes. 2018;16(1):1–14. doi:10.1186/s12955-018-1021-9
22. Iqbal Q, Ul Haq N, Bashir S, Bashaar M. Profile and predictors of health related quality of life among type II diabetes mellitus patients in Quetta city, Pakistan. Health Qual Life Outcomes. 2017;15(1):1–9. doi:10.1186/s12955-017-0717-6
23. Burroughs TE, Desikan R, Waterman BM, Gilin D, McGill J. Development and validation of the diabetes quality of life brief clinical inventory. Diabetes Spectr. 2004;17(1):41–49. doi:10.2337/diaspect.17.1.41
24. Nair R, Kachan P. Outcome tools for diabetes-specific quality of life: study performed in a private family practice clinic. Can Fam Physician. 2017;63(6):e310–e315.
25. Oluchi SE, Manaf RA, Ismail S, Kadir Shahar H, Mahmud A, Udeani TK. Health related Quality of Life measurements for diabetes: a systematic review. Int J Environ Res Public Health. 2021;18(17):9245. doi:10.3390/ijerph18179245
26. Britannica. Indian languages. Available from: https://www.britannica.com/topic/Indian-languages.
27. The Nation. National language. Available from: https://nation.com.pk/13-Sep-2015/national-language.
28. Ethnologue. What are the top 200 most spoken languages? Available from: https://www.ethnologue.com/guides/ethnologue200.
29. Shahzad F, Saleem F, Iqbal Q, et al. A cross-sectional assessment of health literacy among hypertensive community of Quetta City, Pakistan. Biomed J. 2018;14(4):1–9.
30. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR task force for translation and cultural adaptation. Value Health. 2005;8(2):94–104. doi:10.1111/j.1524-4733.2005.04054.x
31. World Health Organization. Process of translation and adaptation of instruments. Available from: http://www.who.int/substance_abuse/research_tools/translation/en/.
32. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge Academic; 1988.
33. Field A. Discovering Statistics Using IBM SPSS Statistics. Sage; 2013.
34. Pallant J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS. Routledge; 2020.
35. Wolf EJ, Harrington KM, Clark SL, Miller MW. Sample size requirements for structural equation models: an evaluation of power, bias, and solution propriety. Educ Psychol Meas. 2013;73(6):913–934. doi:10.1177/0013164413495237
36. IBM Corp. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; 2012.
37. Portney LG, Watkins MP. Foundations of Clinical Research.
38. Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16(3):297–334. doi:10.1007/BF02310555
39. Nunnally JC. Psychometric Theory. McGraw-Hill; 1978.
40. Kaiser HF. An index of factorial simplicity. Psychometrika. 1974;39(1):31–36. doi:10.1007/BF02291575
41. Tobias S, Carlson JE. Brief report: bartlett’s test of sphericity and chance findings in factor analysis. Multivariate Behav Res. 1969;4(3):375–377. doi:10.1207/s15327906mbr0403_8
42. Graham JM, Guthrie AC, Thompson B. Consequences of not interpreting structure coefficients in published CFA research: a reminder. Struct Equ Model. 2003;10(1):142–153. doi:10.1207/S15328007SEM1001_7
43. World Health Organization. Diabetes. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes.
44. Cho N, Shaw J, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
45. Chen H, Chen G, Zheng X, Guo Y. Contribution of specific diseases and injuries to changes in health adjusted life expectancy in 187 countries from 1990 to 2013: retrospective observational study. BMJ. 2019;4:364.
46. International Diabetes Federation. Latest figures show over 19 million people now living with diabetes in Pakistan as the numbers continue to rise. Available from: file:///C:/Users/HP/Downloads/WDD2019%20Regional-PR-Pakistan_Final.pdf.
47. Eekleiti M, Souliotis K, Sarafis P, Kyriazis I, Tsironi M. Measuring the reliability and validity of the Greek edition of the diabetes Quality of Life brief clinical inventory. Diabetes Res Clin Pract. 2018;140:61–71. doi:10.1016/j.diabres.2018.01.019
48. Huang I-C, Liu J-H, Wu AW, Wu M-Y, Leite W, Hwang -C-C. Evaluating the reliability, validity and minimally important difference of the Taiwanese version of the diabetes quality of life (DQOL) measurement. Health Qual Life Outcomes. 2008;6(1):1–12. doi:10.1186/1477-7525-6-87
49. Yildirim A, Akinci F, Gozu H, Sargin H, Orbay E, Sargin M. Translation, cultural adaptation, cross-validation of the Turkish diabetes quality-of-life (DQOL) measure. Qual life Res. 2007;16(5):873–879. doi:10.1007/s11136-007-9172-x
50. Dudzińska M, Tarach JS, Burroughs TE, et al. Validation of the polish version of diabetes Quality of Life–Brief Clinical Inventory (DQL-BCI) among patients with type 2 diabetes. AMS. 2014;10(5):891. doi:10.5114/aoms.2014.46210
51. IBM Support. Kaiser-Meyer-Olkin measure for identity correlation matrix. Available from: https://www.ibm.com/support/pages/kaiser-meyer-olkin-measure-identity-correlation-matrix.
52. Yong AG, Pearce S. A beginner’s guide to factor analysis: focusing on exploratory factor analysis. Tutor Quant Methods Psychol. 2013;9(2):79–94. doi:10.20982/tqmp.09.2.p079
53. Bakalidou D, Antoniadis P, Levantis A, et al. Psychometric properties of the diabetes Quality of Life-brief clinical inventory in Greek diabetic patients. Biol Exerc. 2018;14(1):61–73. doi:10.4127/jbe.2018.0131
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
