Back to Journals » Journal of Inflammation Research » Volume 16
Transient Increase in Patient Numbers with “Acute Macular Neuroretinopathy” Post SARS-CoV-2 Infection—Case Series During the First Surge of Infection in December 2022
Authors Xu B, Zhang J, Zhang Y, Cheng Y, Huang Q
Received 24 April 2023
Accepted for publication 15 June 2023
Published 3 July 2023 Volume 2023:16 Pages 2763—2771
DOI https://doi.org/10.2147/JIR.S413050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Bing Xu,* Jie Zhang,* Ying Zhang, Yang Cheng, Qiong Huang
Department of Ophthalmology, Union Hospital, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiong Huang; Yang Cheng, Department of Ophthalmology, Union Hospital, Huazhong University of Science and Technology, 1277 Jiefang Ave, Jianghan District, Wuhan, 430022, People’s Republic of China, Tel +86 27 85726533, Email [email protected]; [email protected]
Objective: Acute macular neuroretinopathy (AMN) has been associated with several conditions. The aim of this study is to report a surge of AMN cases diagnosed since the easing of epidemic control for COVID-19 in China, in early December 2022.
Case Report: Four cases presented with paracentral or central scotomas, or blurred vision soon after SARS-CoV-2 coronavirus infection. Fundus manifestations were recorded, including typical hyper-reflective segments of the outer plexiform layer (OPL) and outer nuclear layer (ONL), and associated disruption of ellipsoid, interdigitation zones, and retinal pigment epithelium (RPE) layers on optical coherence tomography (OCT). Oral prednisone was administered and gradually tapered. Slight scotoma persisted with hyper-reflective segments fading and irregularity of outer retina on OCT during the follow-up. Case 4 was lost to follow-up.
Conclusion: With the ongoing pandemic and extensive vaccination programs, it is expected that cases of AMN will surge. It is important for ophthalmologists to be aware of the possibility of COVID-19-induced AMN.
Keywords: AMN, COVID-19, OCT, retina, SARS-CoV-2
Introduction
Covid-19, a global pandemic caused by the SARS-CoV-2 coronavirus, has been spreading for the past three years. When writing this paper, Omicron was the most notable variant and was not under control unlike the initial Alpha or Delta variants. Since the easing of epidemic control for COVID-19 in China, in early December 2022, the pandemic has spread rapidly in the country. In recent reports, a wide variety of ophthalmic manifestations have been described and the degree of ocular symptoms varied widely among patients including conjunctivitis, scleritis, acute anterior uveitis, various retinal/choroid manifestations, and neuro-ophthalmologic manifestations, occasionally involving extraocular motility, cranial nerves, and orbital/ocular adnexa.1
Acute macular neuroretinopathy (AMN) is a rare retinal microvascular disorder characterized by the acute onset of brownish-red, wedge-shaped (or petaloid) perifoveal lesions that cause paracentral or central scotomas. Typical lesions are depicted with infra-red or red-free photography and optical coherence tomography (OCT). OCT studies have identified hyperreflectivity of the outer nuclear and plexiform layers and ischemia of the deep retinal capillary plexus may be a part of the disease’s origin. The prevalence of AMN has been reported to be less than 1 patient per million,2 though it has been increasingly prevalent since the wide application of multi-model imaging recently. AMN is commonly seen in young females and has been associated with various risk factors including flu-like illness, hormonal oral contraceptive pills, antecedent trauma, caffeine, epinephrine and pseudoephedrine injection, hypovolemia, and pregnancy-induced hypertension.3 Several studies have speculated the association of SARS-CoV-2 coronavirus and AMN, since there are more reports of a surge in the incidence of AMN onset during the COVID-19 pandemic, or after COVID-19 vaccinations.4–7
Here, we report a case series of AMN in young healthy patients, as there was a sudden rise during the first surge of the SARS-CoV-2 infection, immediately after the easing of epidemic control measures in early December 2022 in Wuhan, China. Patients were referred Union Hospital, Tongji Medical College shortly after the infection, and the first three cases, presented on the same day, which is uncommon for this rare disease.
Case 1
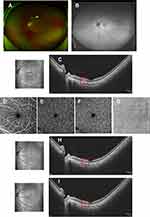
A 27-year-old woman complained of temporal paracentral scotoma in the left eye for two days. Three days prior to this she had fatigue, headache, chills, and fever. Antigen self-testing for SARS-CoV-2 was positive. Systemic symptoms were relieved after taking ibuprofen, but the paracentral scotoma persisted. She was healthy without any medical antecedent. On examination, visual acuity corrected to −4.75 diopters was 20/20 in both eyes. A slit-lamp examination was done, and intraocular pressures (IOPs) were normal. Fundus photography and autofluorescence (FAF) (Optos, Daytona Plus 200tx) did not reveal pathological changes (Figure 1A and B). OCT (VG200S; SVision Imaging, Henan, China) disclosed hyper-reflective segment of the outer plexiform layer (OPL) and outer nuclear layer (ONL). There was also associated disruption of ellipsoid, interdigitation zones, and retinal pigment epithelium (RPE) layers on the nasal macula, corresponding with the temporal scotoma (Figure 1C). Optical coherence tomography angiography (OCTA, VG200S; SVision Imaging, Henan, China) showed no flow defects either in the superficial/intermediate/deep retinal capillary plexuses or in the choroidal capillary plexus (Figure 1D–G). Fundus fluorescein angiography (FFA)/indocyanine green angiography (ICGA) examination, view field test, and further laboratory investigations were recommended but were denied due to persisting fatigue after infection. A possible diagnosis of unilateral AMN in the left eye was made. Informed consent was then obtained, 40 mg/day (0.8 mg/kg body weight) of oral prednisone was administered for one week and was continued with gradual tapering over a 2-month period. One week later, the patient returned without obvious alleviation of the scotoma, but hyper-reflectivity of the OPL and ONL mildly faded with definable ellipsoid, interdigitation zones, and RPE layers (Figure 1H). Six weeks later, OCT only showed slight irregularity of ellipsoid, interdigitation zones, and retinal pigment epithelium (RPE) layers, but the patient still complained of slight scotoma (Figure 1I).
Case 2
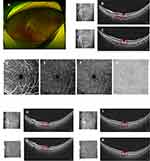
A 23-year-old man presented with acute onset of unilateral blurred vision in his left eye for 3 days. Five days before the onset, he suffered from fatigue, headache, chills, and fever and antigen self-testing for SARS-CoV-2 was positive. He recovered two days later but blurred vision persisted. His past medical history was unremarkable, with no medication or eye trauma. At presentation, visual acuity corrected to −2.25 diopters was 20/20 in both eyes, though the patient complained of blurred vision in the left eye. The anterior segment in both eyes under the slit-lamp examination was normal, as also IOPs. Fundus photography (Optos, Daytona Plus 200tx) did not indicate abnormalities (Figure 2A). OCT (VG200S; SVision Imaging, Henan, China) disclosed two hyper-reflective segments of the OPL and ONL and associated disruption of ellipsoid, interdigitation zones, and RPE layers, on the nasal and upper macular areas (Figure 2B and C). OCTA (VG200S; SVision Imaging, Henan, China) showed no flow defect either in the superficial/intermediate/deep retinal capillary plexuses or in the choroidal capillary plexus (Figure 2D–G). No further examinations were done, and a possible diagnosis of unilateral AMN on the left eye was made. The patient was administered 55 mg/day (0.8mg/kg body weight) of oral prednisone for 1 week, which was continued with gradual tapering over a 2-month period. Three weeks later, the patient returned with mild alleviation of blurred vision. Hyper-reflectivity of the OPL and ONL completely faded with definable ellipsoid, interdigitation zones, and RPE layers both on the nasal and upper macular areas (Figure 2H and I). Six weeks later, the patient still complained of a slightly blurred vision, and OCT showed a slight irregularity in the ellipsoid, interdigitation zones, and RPE layers (Figure 2J and K). Prednisone was continued with gradual tapering, and the patient was under observation.
Case 3
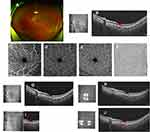
The third patient was a 30-year-old lactating woman with a 21-month-old baby, who presented with the sudden onset of central scotoma in her left eye for 5 days. Five days before she had suffered fatigue, headache, chills, and fever, antigen self-testing for SARS-CoV-2 was positive. Scotoma persisted while systemic symptoms were relieved. The patient had refractive myopia correction in both eyes 10 years ago, with no remarkable systemic medical history. At presentation, visual acuity corrected to −0.5 diopters was 20/20 in both eyes. Slit-lamp examination and IOPs were unremarkable. Fundus photography (Optos, Daytona Plus 200tx) showed no obvious abnormal findings (Figure 3A), and OCT (VG200S; SVision Imaging, Henan, China) showed a para-foveal hyper-reflective segment of the OPL and ONL with associated disruption of ellipsoid, interdigitation zones, and RPE layers (Figure 3B). OCTA (VG200S; SVision Imaging, Henan, China) showed no flow defect either in the superficial/intermediate/deep retinal capillary plexuses or in the choroidal capillary plexus (Figure 3C–F). A possible diagnosis of unilateral AMN in the left eye was made. The patient was prescribed 40 mg/day (0.8 mg/kg body weight) of oral prednisone for 1 week, which was continued with gradual tapering over a 2-month period. The patient was asked to pause breastfeeding during medication. Three days later, OCT indicated that OPL and ONL hyper-reflectivity had mildly faded (Figure 3G). Ten days later, OPL and ONL hyper-reflectivity had decreased further with definable ellipsoid, interdigitation zones, and RPE layers on the OCT (Figure 3H); however, the patient still complained of slight scotoma. During follow-up, the patient still complained of a subtle scotoma and OCT only showed a tiny segmental discontinuity of ellipsoid, interdigitation zones, and RPE layers at the 4-week visit and 7-week visit (Figure 3I and J). The patient was still under observation.
Case 4
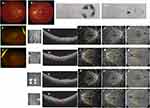
The fourth patient was a 35-year-old woman, who presented with a sudden onset of central scotoma in both eyes for 6 days. Before the onset, she had suffered fatigue, headache, chills, and fever, and antigen self-testing for SARS-CoV-2 was positive. She had no remarkable systemic medical history. From previous records, the visual acuity corrected to −4.5 diopters was 0.5 in both eyes. Slit-lamp examination and IOPs were unremarkable. Fundus photography disclosed white-yellow lesions in the posterior retina (Figure 4A and B, photocopy from local records); however, the OCT scan was not available. A 30–2 visual field test on the right eye showed defects on nasal and temporal fields but was normal in the left eye (Figure 4C and D, photocopy from local records). Visual evoked potential (VEP) was normal in both eyes. At presentation, the patient still had serious systemic symptoms and visual acuity corrected to −4.5 diopters was 0.4 in the right eye and 0.2 in the left eye. The anterior segments in both eyes were unremarkable. Fundus photography (Optos, Daytona Plus 200tx) showed reduced white-yellow lesions (Figure 4E and F) and OCT scan (VG200S; SVision Imaging, Henan, China) disclosed hyper-reflective segments of OPL and ONL in the fovea of both eyes, also with associated disruption of ellipsoid, interdigitation zones, and RPE layers (Figure 4G and H). OCTA (VG200S; SVision Imaging, Henan, China) showed perfusion defects in the superficial/deep retinal capillary plexuses, also in the choroidal capillary plexus on wide field OCTA in both eyes (Figure 4I–N). The patient was diagnosed with bilateral AMN and paracentral acute middle maculopathy (PAMM) according to the initial fundus photography, though with mild resolution at presentation. Oral prednisone 40 mg/day (0.8 mg/kg body weight) was administered for one week. A week later, the systemic symptoms relieved completely, and the corrected visual acuity improved to 0.8 in the right eye and 0.4 in the left eye. OCT showed that hyper-reflectivity of the OPL and ONL slightly faded (Figure 4O and P) and retinal capillary defects resolved on OCTA (Figure 4Q–V). The patient was suggested to continue with oral prednisone with gradual tapering over a 2-month period. However, the patient was lost to follow-up.
Discussions
Here, we report a case series of typical AMN in young healthy patients, a sudden rise during the first surge of the SARS-CoV-2 infection immediately after the easing of the epidemic control measures in early December 2022 in Wuhan, China. More cases with AMN followed during the next several weeks for further consultation. We also speculate the association between rare retinal lesions and the high incidence of viral infection during the wave immediately after the easing of the epidemic control measures. The typical cases reported were systemically healthy with no medication history. Before the onset of ocular symptoms, patients, including their family members, experienced systemic symptoms and antigen self-testing showed SARS-CoV-2 infection. Several days after recovery from the systemic symptoms, the onset of ocular symptoms occurred, mainly paracentral or central scotomas. The first three cases presented on the same day, which is uncommon for this rare disease.
AMN, described by Bos and Deutman in 1975, is a rare disease, characterized by the sudden onset of one or more paracentral scotomas, bilaterally or unilaterally.8 AMN is commonly seen in young females and associated risk factors include flu-like illness, hormonal oral contraceptive pills, antecedent trauma, caffeine, injection of epinephrine and pseudoephedrine, hypovolemia, and pregnancy-induced hypertension.3 The most common pathogenesis is ischemia of the deep capillary plexus. It is most easily identified by wedge-shaped parafoveal lesions on infra-red or red-free imaging. Characteristic OCT findings showed OPL and ONL hyperreflectivity in the fovea, also with associated disruption of ellipsoid, interdigitation zones, and RPE layers. In some reports, OCTA detected flow voids in the choriocapillaris or deep retinal capillary plexus, corresponding to the wedge-shaped lesions on near-infrared imaging.9 However, they were not always remarkable.
In the cases described, initial OCT findings revealed characteristic hyperreflectivity of the OPL and ONL in the macular area, accompanied by associated disruption of ellipsoid, interdigitation zones, and RPE layers. However, OCTA detected unremarkable changes in the retinal capillary plexuses or in the choroidal capillary plexus in most cases reported here, except that there was a perfusion defect in retinal and choroidal capillary plexuses in Case 4, with suggested PAMM initially. FAF was normal in Case 1, and VEP was normal in Case 4. However, visual field tests varied among the cases based on the degree of the visual function impairment. There were visual field defects in the right eye in Case 4 detected during 30-2 Humphrey test. The 10-2 visual field test and Amsler test may be more specific in detecting scotomas, but they were not available at the time.
Although the exact mechanism of AMN after SARS-CoV-2 infection is unknown, it has been hypothesized to be an acute vascular event in the outer retina or choroid because of the inflammatory reaction, leading to ischemia of the deep retinal capillary plexus and choroid capillary. SARS-CoV-2 is known to enter host cells through the interaction of its spike protein with the entry receptor angiotensin-converting enzyme (ACE)-2, in the presence of transmembrane serine protease (TMPRSS). Interestingly, ACE-II receptor has been found in the retinal ganglion cell layer, inner plexiform layer, inner nuclear layer, and photoreceptor outer segments. TMPRSS2 is also found in multiple retinal neuronal cells, vascular and perivascular cells, and in retinal Müller glial cells.10 These may suggest possible viral entry in the retinal cells, though there is no conclusive evidence of virus existence in the retina for the cases reported. Indeed, SARS-CoV-2 RNA has been found in the retina of some patients who died from COVID-19.11
For extrapulmonary manifestations of COVID-19, besides direct viral tissue damage, others including endothelial damage and dysregulation of immune responses, and maladaptation of ACE2-related pathways are the plausible mechanisms of injury.12 Hyperinflammatory response and thromboembolic events leading to endothelial damage in the retinal deep capillary plexus or choroidal capillary may be the key mechanisms contributing to AMN induced by SARS-CoV-2. Hyper-reflectivity of the outer retina on OCT may be an indirect sign of damage to adjacent the photoreceptors and the RPE, or with corresponding flow voids in the choroidal capillary or the deep retinal capillary plexus on OCTA scans.
For now, there is no guideline for treating AMN related to SARS-CoV-2 infection or vaccination. Some reported AMN as a self-limiting disease, which usually resolves without specific treatment.13,14 Some cases were reportedly just under observation, and the disease course may last weeks to months with diverse outcomes, ranging from persistent scotomas to complete visual recovery.15–17 Tapering corticosteroid therapy, either for a short time (less than 1 month) or for a longer time (over 4 months) was applied in some cases following SARS-CoV-2 vaccination for any possible rapid recovery of vision, but also with diverse outcomes.18–20 For the cases reported here, an initial prednisone (0.8 mg/kg body weight) dose was administered and was continued with gradual tapering over a 2-month period. During the follow-up, OCT revealed resolution of previous hyper-reflective lesion but there was subsequent ellipsoid and interdigitation zone loss. For cases 1, 2, and 3, visual acuity remained unchanged with only slightly scotoma persisting during the follow-up. In case 4, there was remarkable improvement of visual acuity during the first one week after prednisone administration, also a corresponding resolution of hyper-reflective lesion on OCT. We assume that early corticosteroid administration may play a positive role in shortening the disease course and visual function improvement, as there was no improvement of visual function in cases where medication was delayed due to incorrect diagnosis elsewhere caused by the subtlety in retinal lesions and rarity of the disease. However, the cases are still under investigation and we are awaiting more long-term results.
Moreover, an interesting correlation between COVID-19 vaccination and AMN has been lately observed. Mambretti et al reported AMN in two young females two days after receiving Vaxzevria Coronavirus disease (COVID-19) vaccination who were on long-term oral contraceptives.21 Franchi et al reported two cases of young females presenting with AMN within 48 h of administration of the COVID-19 Oxford AstraZeneca vaccine.22 Additionally, Valenzuela et al reported a case of AMN following administration of the Pfizer-BioNTech COVID-19 vaccine.16 Several similar findings have also been reported in other case reports23,24 and may be suggestive of a causal relationship between COVID-19 vaccination and AMN.
Conclusion
Here we report a case series of AMN, a sudden increase in the onset of the disease, since the easing of epidemic control measures for COVID-19 in China in early December 2022. There have been similar reports of cases with AMN during the COVID-19 pandemic or after SARS-CoV-2 vaccination globally. The rise of this rare disease linked to SARS-CoV-2 infection or vaccination seems to confirm a possible correlation. However, further studies are needed to confirm the association of AMN with COVID-19 and its vaccines, also to understand the exact mechanism and treatment of the disease.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Union Hospital, Huazhong University of Science and Technology. A written informed consent was obtained from all participants.
Consent for Publication
Consent for publication was obtained from every individual whose data are included in this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu K, Patel J, Swiston C, Patel BC. Ophthalmic manifestations of coronavirus (COVID-19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. PMID: 32310553.
2. Orphanet. Acute macular neuroretinopathy. Available from: https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=488239.
3. Bhavsar KV, Lin S, Rahimy E, et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61(5):538–565. PMID: 26973287. doi:10.1016/j.survophthal.2016.03.003
4. Ahmed W, Suri A, Ahmed A. COVID-19 and acute macular neuroretinopathy - an underlying association? Ann Med Surg. 2022;78:103847. PMID: 35734676; PMCID: PMC9207085. doi:10.1016/j.amsu.2022.103847
5. Jalink MB, Bronkhorst IHG. A sudden rise of patients with acute macular neuroretinopathy during the COVID-19 pandemic. Case Rep Ophthalmol. 2022;13(1):96–103. PMID: 35350236; PMCID: PMC8921888. doi:10.1159/000522080
6. Dinh RH, Tsui E, Wieder MS, et al. Acute macular neuroretinopathy and coronavirus disease 2019. Ophthalmol Retina. 2023;7(2):198–200. PMID: 36216223; PMCID: PMC9546455. doi:10.1016/j.oret.2022.09.005
7. Azar G, Bonnin S, Vasseur V, et al. Did the COVID-19 pandemic increase the incidence of acute macular neuroretinopathy? J Clin Med. 2021;10(21):5038. PMID: 34768555; PMCID: PMC8585041. doi:10.3390/jcm10215038
8. Bos PJ, Deutman AF. Acute macular neuroretinopathy. Am J Ophthalmol. 1975;80(4):573–584. PMID: 1180301. doi:10.1016/0002-9394(75)90387-6
9. Casalino G, Arrigo A, Romano F, Munk MR, Bandello F, Parodi MB. Acute macular neuroretinopathy: pathogenetic insights from optical coherence tomography angiography. Br J Ophthalmol. 2019;103(3):410–414. PMID: 29844084. doi:10.1136/bjophthalmol-2018-312197
10. Zhou L, Xu Z, Guerra J, et al. Expression of the SARS-CoV-2 receptor ACE2 in human retina and diabetes-implications for retinopathy. Invest Ophthalmol Vis Sci. 2021;62(7):6. PMID: 34086044; PMCID: PMC8185397. doi:10.1167/iovs.62.7.6
11. Reinhold A, Tzankov A, Matter MS, Mihic-Probst D, Scholl HPN, Meyer P. Ocular pathology and occasionally detectable intraocular severe acute respiratory syndrome Coronavirus-2 RNA in five fatal Coronavirus disease-19 cases. Ophthalmic Res. 2021;64(5):785–792. PMID: 33472206. doi:10.1159/000514573
12. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26(7):1017–1032. PMID: 32651579. doi:10.1038/s41591-020-0968-3
13. Amin P, Cox TA. Acute macular neuroretinopathy. Arch Ophthalmol. 1998;116(1):112–113. PMID: 9445223. doi:10.1001/archopht.116.1.112
14. Neuhann IM, Inhoffen W, Koerner S, Bartz-Schmidt KU, Gelisken F. Visualization and follow-up of acute macular neuroretinopathy with the Spectralis HRA+OCT device. Graefes Arch Clin Exp Ophthalmol. 2010;248(7):1041–1044. PMID: 20198487. doi:10.1007/s00417-010-1324-y
15. Aidar MN, Gomes TM, de Almeida MZH, de Andrade EP, Serracarbassa PD. Low visual acuity due to acute macular neuroretinopathy associated with COVID-19: a case report. Am J Case Rep. 2021;22:e931169. PMID: 33930011; PMCID: PMC8097744. doi:10.12659/AJCR.931169
16. Valenzuela DA, Groth S, Taubenslag KJ, Gangaputra S. Acute macular neuroretinopathy following Pfizer-BioNTech COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101200. PMID: 34485760; PMCID: PMC8409052. doi:10.1016/j.ajoc.2021.101200
17. Miller MH, Spalton DJ, Fitzke FW, Bird AC. Acute macular neuroretinopathy. Ophthalmology. 1989;96(2):265–269. PMID: 2704546. doi:10.1016/s0161-6420(89)32906-x
18. Drüke D, Pleyer U, Hoerauf H, Feltgen N, Bemme S. Acute macular neuroretinopathy (AMN) following COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101207. PMID: 34580648; PMCID: PMC8457905. doi:10.1016/j.ajoc.2021.101207
19. Fekri S, Khorshidifar M, Dehghani MS, Nouri H, Abtahi SH. Acute macular neuroretinopathy and COVID-19 vaccination: case report and literature review. J Fr Ophtalmol. 2023;46(1):72–82. PMID: 36496293; PMCID: PMC9684098. doi:10.1016/j.jfo.2022.09.008
20. Rennie AT, DeWeerd AJ, Martinez MG, Kay CN. Acute macular neuroretinopathy following COVID-19 mRNA Vaccination. Cureus. 2022;14(7):e27502. PMID: 36060339; PMCID: PMC9426360. doi:10.7759/cureus.27502
21. Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G. Acute macular neuroretinopathy following Coronavirus disease 2019 vaccination. Ocul Immunol Inflamm. 2021;29(4):730–733. PMID: 34187278. doi:10.1080/09273948.2021.1946567
22. Franchi A, Rauchegger T, Palme C, et al. Two cases of acute macular neuroretinopathy associated with the adenovirus-based COVID-19 vaccine vaxzevria (AstraZeneca). Ocul Immunol Inflamm. 2022;30(5):1234–1239. PMID: 35050829. doi:10.1080/09273948.2022.2027463
23. Book BAJ, Schmidt B, Foerster AMH. Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. 2021;139(7):e212471. PMID: 34287612. doi:10.1001/jamaophthalmol.2021.2471
24. Bøhler AD, Strøm ME, Sandvig KU, Moe MC, Jørstad ØK. Acute macular neuroretinopathy following COVID-19 vaccination. Eye. 2022;36(3):644–645. PMID: 34158652; PMCID: PMC8217204. doi:10.1038/s41433-021-01610-1.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.