Back to Journals » Cancer Management and Research » Volume 15
Transformation of Lung Squamous Cell Carcinoma to Small Cell Lung Cancer After Immunotherapy Resistance: A Case Report
Authors Wang D , Ye W , Chen D, Shi Q, Ma D
Received 18 May 2023
Accepted for publication 24 July 2023
Published 10 August 2023 Volume 2023:15 Pages 803—808
DOI https://doi.org/10.2147/CMAR.S420485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Diming Wang,1,* Wei Ye,2,* Dongmei Chen,1 Qingming Shi,1 Dongchun Ma3
1Department of Oncology, Anhui Chest Hospital, Hefei, 230022, People’s Republic of China; 2Department of Pathology, Anhui Chest Hospital, Hefei, 230022, People’s Republic of China; 3Department of Thoracic Surgery, Anhui Chest Hospital, Hefei, 230022, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dongchun Ma, Department of Thoracic Surgery, Anhui Chest Hospital, Hefei, 230022, People’s Republic of China, Email [email protected] Qingming Shi, Department of Oncology, Anhui Chest Hospital, Hefei, 230022, People’s Republic of China, Email [email protected]
Abstract: The transformation of lung adenocarcinoma to small cell lung cancer (SCLC) following treatment with epidermal growth factor (EGFR) receptor tyrosine kinase inhibitors (TKIs) is a relatively common phenomenon. However, transformation of non-small cell lung cancer (NSCLC) to SCLC following treatment with immunotherapy is very rare. Here, we report a case of a 56-year-old patient diagnosed with driver gene mutation-negative lung squamous cell carcinoma (SCC). He received four cycles of immunotherapy with sugemalimab and chemotherapy with albumin paclitaxel in combination with carboplatin, and a partial response was achieved. Subsequently, the patient received 5 cycles of immunotherapy with sugemalimab. However, he developed rapid progression of mediastinal lymph nodes, and biopsy results showed transformation to SCLC. His tumor did not respond to the next line of carboplatin combined with etoposide, and he died six months after the discovery of SCLC transformation. In conclusion, SCLC transformation is also an important resistance mechanism for lung SCC patients treated with immunotherapy and predicts a very poor outcome. Repeat biopsy is needed for advanced lung SCC that has progressed with immunotherapy.
Keywords: lung squamous cell carcinoma, immunotherapy, small cell transformation
Introduction
Currently, immunotherapy has changed the treatment strategy for non-small cell lung cancer (NSCLC) patients.1 In particular, antibodies specific for the programmed death (PD-1) receptor and programmed death ligand 1 (PD-L1) are preferred as first- or second-line treatment for advanced NSCLC.2,3 In addition, several studies have found that neoadjuvant immunotherapy for locally advanced lung squamous cell carcinoma (SCC) leads to superior pathological response rates.4,5 In the real world, a proportion of patients with advanced lung SCC treated with immunotherapy show durable clinical benefits.6 Nevertheless, some patients also present with relatively rapid disease progression.7 However, the resistance mechanisms for immune checkpoint inhibitors (ICIs) are still unclear.8 Several studies have shown that small cell transformation is commonly recognized as one of the resistance mechanisms to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in EGFR-mutant NSCLC, which accounts for 3–14% of resistant cases.9–11 In addition, small cell transformation in advanced NSCLC patients treated with EGFR-TKIs is associated with poor survival outcomes.12 Recently, small cell transformation is extremely rare for advanced NSCLC patients who have failed immunotherapy.13 It was recently reported that when first-line treatment with EGFR-TKIs fails for patients with advanced NSCLC, repeat biopsy for detecting EGFR T790M is preferred.14 However, repeat biopsy is not regularly recommended for advanced NSCLC patients treated with ICIs after disease progression.15 Here, we report a case of small cell transformation in locally advanced lung SCC treated with immunotherapy.
Case Presentation
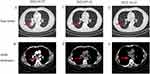
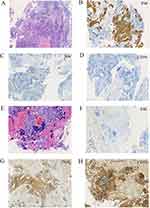
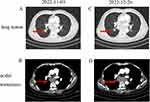
A 56-year-old male was admitted to the Oncology Department of the Anhui Chest Hospital in March 2022 due to cough and expectoration for 1 month. Chest computed tomography (CT) revealed an irregular mass (3.5×3.6 cm) in the lower lobe of his right lung with intramediastinal lymph node metastasis (Figure 1A and B). The patient had a 30-year history of smoking. Serum tumor marker levels at diagnosis were carcinoembryonic antigen (CEA) 1.7 ng/mL and neuron-specific enolase (NSE) 10.5 ng/mL. On 8 April 2022, bronchoscopy was performed, and the pathological findings were compatible with lung SCC (Figure 2A). Immunohistochemical (IHC) staining was positive for P40 (Figure 2B) but negative for CD56 and synaptophysin (Figure 2C and D). The patient was diagnosed with driver gene mutation-negative lung SCC. The tumor was classified as clinical T2N2M0, which is stage IIIA according to the TNM classification of the UICC, 8th edition. Based on multidisciplinary team discussions, we performed neoadjuvant therapy to reduce staging and reassess the disease. From 10 April 2022 to 10 July 2022, the patient received four cycles of immunochemotherapy: sugemalimab 1200 mg ig d1, albumin paclitaxel 400 mg ig d1, and carboplatin area under the curve (AUC) 5 ig d1/q3w. After the patient received 4 cycles of treatment on July 10, 2022, chest CT showed significant remission compared with baseline CT (27 March 2022) and achieved a partial response (PR) according to the Response Evaluation Criteria in Solid Tumors 1.1 (Figure 1C). In addition, chest CT showed that the lymph nodes achieved complete response (CR) compared with baseline CT (Figure 1D). After a multidisciplinary team discussion, we suggested that surgical removal was possible. However, the patient refused surgical treatment and radiotherapy. From July 2022 to October 2022, the patient received 5 cycles of sugemalimab treatment. However, chest CT (3 November 2022) showed that the mass was slightly larger than the previous CT scan (12th, October 2022), and the lymph nodes were very significantly increased (Figures 1E, F and 3A, B), which was evaluated as progressive disease (PD). In addition, a CT scan revealed thoracic spine bone destruction, which suggested a neoplastic lesion in the thoracic spine. Notably, serum tumor marker levels at progression were CEA 2.9 ng/mL and NSE 60.5 ng/mL, significantly higher than the baseline levels. We were surprised by the rapid progression of this patient after seven months of immunotherapy. Therefore, we performed endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymph nodes on November 05, 2022. The pathological diagnosis was small cell carcinoma (Figure 2E). Immunohistochemical (IHC) staining was positive for CD56 and synaptophysin but negative for P40 (Figure 2F–H). Subsequently, this patient was treated with 2 cycles of etoposide combined with cisplatin, and the CT scan showed sustained disease progression (Figure 3C and D). He died 6 months after the detection of small cell transformation.
Discussion
Immunotherapy constitutes irreplaceable treatment for locally advanced lung SCC.16 Sugemalimab is a fully human, full-length, anti-PD-L1 monoclonal antibody developed by CStone Pharmaceuticals.17 It is an immunoglobulin G4 monoclonal antibody engineered to block interaction between PD-L1 and PD-1 on T cells and CD80 on immune cells and exhibits antitumor activity by eliminating the immunosuppressive effect of PD-L1 on cytotoxic T cells.18 The GEMSTONE-301 study19 showed that sugemalimab can significantly prolong progression-free survival in patients with stage III NSCLC whose disease has not progressed after sequential or concurrent chemoradiotherapy. In addition, the GEMSTONE-302 study20 showed a clinically significant meaningful outcome for patients with advanced lung SCC treated with first-line therapy with sugemalimab plus chemotherapy. The present case is a locally advanced lung SCC treated with sugemalimab combined with chemotherapy. When the patient’s disease progressed rapidly, repeat biopsy pathology was performed to assess transformed small cell carcinoma.
Notably, the transformation of NSCLC to small cell lung cancer (SCLC) after immunotherapy is rare, and the mechanism is unclear.21 Bar et al22 and Sehgal et al23 reported the detection of the same genomic features in initial non-small cell lung cancer and secondary small cell carcinoma. They suggested that NSCLC cells may be histologically transformed into small cell carcinoma cells. Imakita et al13 described two lung SCC patients with tumors that transformed to small cell carcinoma after immunotherapy. However, the treatment options and prognosis after conversion to small cell carcinoma were not reported.13 Dong et al24 presented a case of stage IIIA lung SCC by bronchoscopic biopsy. Subsequently, this patient was treated with neoadjuvant immunotherapy and surgical pathology of SCC, carcinosarcoma, and SCLC. Unfortunately, this case was not labeled as small cell carcinoma in the initial pathological diagnosis. In our case, the patient was initially diagnosed with lung SCC and was excluded by immunohistochemistry for small cell, neuroendocrine, and adenocarcinoma components. In addition, we also understand that bronchoscopy biopsy does not reveal the histology of the whole tumor. The presence of mixed histology of small and non-small cells at diagnosis is possible. However, there are no guidelines on how to distinguish transformed small cell carcinoma from primary SCLC.
Currently, etoposide in combination with platinum is still the preferred treatment option for transformed SCLC patients who have failed EGFR-TKIs.25 However, the treatment options for NSCLC patients who have failed immunotherapy and then undergo transformation to small cell carcinoma are unclear. Sehgal et al23 reported a case of advanced lung SCC transformed to SCLC after receiving nivolumab in the second line, then received etoposide in combination with carboplatin, yet progressed again after 10 months. This is similar to the previously reported overall survival of 10.9 months (95% CI, 8.0 months–13.7 months) for transformation to SCLC after EGFR-TKIs in patients with EGFR-mutated lung adenocarcinoma.25 In this case, etoposide in combination with cisplatin was chosen after transformation to small cell carcinoma, but the disease continued to progress after 2 cycles.
In conclusion, it is rare for advanced lung SCC with immunotherapy to transform into SCLC in the real world, and this may be related to the lack of knowledge about repeat tissue biopsy after disease progression. Therefore, it is very important to conduct another biopsy after the progression of lung SCC patients treated with immunotherapy in the future. In addition, the treatment of lung SCC transformed into SCLC with immunotherapy needs to be further investigated.
Ethical Approval
This study was approved by the Human Research Ethics Committee of Anhui Chest Hospital.
Disclosure
Diming Wang and Wei Ye are co-first authors for this study. The authors have no conflicts of interest to declare for this work.
References
1. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40(6):586–597. doi:10.1200/JCO.21.01497
2. Veluswamy R, Hirsch FR, Taioli E, et al. Real-world longitudinal practice patterns in the use of PD-1 and PD-L1 inhibitors as first-line therapy in patients with non-small cell lung cancer in the United States. Cancer Med. 2022;11(22):4265–4272. doi:10.1002/cam4.4785
3. Julian C, Machado RJM, Girish S, et al. Real-world data prognostic model of overall survival in patients with advanced NSCLC receiving anti-PD-1/PD-L1 immune checkpoint inhibitors as second-line monotherapy. Cancer Rep. 2022;5(10):e1578. doi:10.1002/cnr2.1578
4. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi:10.1056/NEJMoa2202170
5. Chaft JE, Oezkan F, Kris MG, et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm Phase II trial. Nat Med. 2022;28(10):2155–2161. doi:10.1038/s41591-022-01962-5
6. Waterhouse D, Lam J, Betts KA, et al. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer. 2021;156:41–49. doi:10.1016/j.lungcan.2021.04.007
7. Zhang L, Bai L, Liu X, et al. Factors related to rapid progression of non-small cell lung cancer in Chinese patients treated using single-agent immune checkpoint inhibitor treatment. Thorac Cancer. 2020;11(5):1170–1179. doi:10.1111/1759-7714.13370
8. Passaro A, Brahmer J, Antonia S, et al. Managing resistance to immune checkpoint inhibitors in lung cancer: treatment and novel strategies. J Clin Oncol. 2022;40(6):598–610. doi:10.1200/JCO.21.01845
9. Wang Z, Zhang L, Xu W, et al. The multi-omics analysis of key genes regulating EGFR-TKI resistance, immune infiltration, SCLC transformation in EGFR-mutant NSCLC. J Inflamm Res. 2022;15:649–667. doi:10.2147/JIR.S341001
10. Yang M-H, Yu J, Cai C-L, et al. Small cell lung cancer transformation and tumor heterogeneity after sequential targeted therapy and immunotherapy in EGFR-mutant non-small cell lung cancer: a case report. Front Oncol. 2022;12:1029282. doi:10.3389/fonc.2022.1029282
11. Lee P-H, Huang Y-H, Lin H. Histological transformation after acquired resistance to the third-generation EGFR-TKI in patients with advanced EGFR-mutant lung adenocarcinoma. Medicina. 2022;58(7):908. doi:10.3390/medicina58070908
12. Yu L, Bazhenova L, Gold K, et al. Clinicopathologic and molecular characteristics of EGFR-mutant lung adenocarcinomas that transform to small cell lung cancer after TKI therapy. Transl Lung Cancer Res. 2022;11(3):452–461. doi:10.21037/tlcr-21-665
13. Imakita T, Fujita K, Kanai O, et al. Small cell transformation of non-small cell lung cancer under immunotherapy: case series and literature review. Thorac Cancer. 2021;12(22):3062–3067. doi:10.1111/1759-7714.14180
14. Kudo K, Nishii K, Makimoto G, et al. First and repeat rebiopsy for detecting EGFR T790M mutation in non-small-cell lung cancer: CS-lung-003 prospective observational registry study. J Cancer Res Clin Oncol. 2022;148(8):1869–1877. doi:10.1007/s00432-021-03893-z
15. Wang F, Wang S, Zhou Q. The resistance mechanisms of lung cancer immunotherapy. Front Oncol. 2020;10:568059. doi:10.3389/fonc.2020.568059
16. Feng Y, Xiong X, Wang Y, et al. Genomic analysis reveals the prognostic and immunotherapeutic response characteristics of ferroptosis in lung squamous cell carcinoma. Lung. 2022;200(3):381–392. doi:10.1007/s00408-022-00537-y
17. Dhillon S, Duggan S. Sugemalimab: first approval. Drugs. 2022;82(5):593–599. doi:10.1007/s40265-022-01693-4
18. Gong J, Cao J, Zhang Q, et al. Safety, antitumor activity and biomarkers of sugemalimab in Chinese patients with advanced solid tumors or lymphomas: results from the first-in-human Phase 1 trial. Cancer Immunol Immunother. 2022;71(8):1897–1908. doi:10.1007/s00262-021-03102-3
19. Zhou Q, Chen M, Jiang O, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, Phase 3 trial. Lancet Oncol. 2022;23(2):209–219. doi:10.1016/S1470-2045(21)00630-6
20. Zhou C, Wang Z, Sun Y, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23(2):220–233. doi:10.1016/S1470-2045(21)00650-1
21. Shen Q, Qu J, Sheng L, et al. Case report: transformation from non-small cell lung cancer to small cell lung cancer during anti-PD-1 Therapy: a report of two cases. Front Oncol. 2021;11:619371. doi:10.3389/fonc.2021.619371
22. Bar J, Ofek E, Barshack I, et al. Transformation to small cell lung cancer as a mechanism of resistance to immunotherapy in non-small cell lung cancer. Lung Cancer. 2019;138:109–115. doi:10.1016/j.lungcan.2019.09.025
23. Sehgal K, Varkaris A, Viray H, et al. Small cell transformation of non-small cell lung cancer on immune checkpoint inhibitors: uncommon or under-recognized? J Immunother Cancer. 2020;8(1):e000697. doi:10.1136/jitc-2020-000697
24. Dong Y, Li Q, Li D, et al. Whole-process treatment of combined small cell lung cancer initially diagnosed as “lung squamous cell carcinoma”: a case report and review of the literature. Front Immunol. 2022;13:831698. doi:10.3389/fimmu.2022.831698
25. Wang W, Xu C, Chen H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: a multicenter retrospective study. Lung Cancer. 2021;155:20–27. doi:10.1016/j.lungcan.2021.03.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.