Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Transcutaneous Electrical Acupoint Stimulation for Preventing Postoperative Delirium: A Meta-Analysis
Authors Guo F, Yan Y, Sun L, Han R, Zheng L, Qin Y, Wang S, Sun X, Ji Z, Gao C
Received 14 January 2023
Accepted for publication 5 April 2023
Published 15 April 2023 Volume 2023:19 Pages 907—920
DOI https://doi.org/10.2147/NDT.S404805
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Fei Guo,1,* Yuting Yan,1,* Li Sun,1 Ruili Han,1 Lanlan Zheng,1 Yuan Qin,1 Shuang Wang,1 Xude Sun,1 Zhaohua Ji,2 Changjun Gao1
1Department of Anesthesiology, Second Affiliated Hospital of Air Force Medical University, Xi’an, 710038, People’s Republic of China; 2Department of Epidemiology, School of Public Health, Ministry of Education Key Laboratory of Hazard Assessment and Control in Special Operational Environment, Air Force Medical University, Xi’an, 710032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Changjun Gao, Department of Anesthesiology, Second Affiliated Hospital of Air Force Medical University, Xin Si Road & 569, Xi’an City, Shaanxi, 710038, People’s Republic of China, Tel/Fax +86 2984777439, Email [email protected] Zhaohua Ji, Department of Epidemiology, School of Public Health, Ministry of Education Key Laboratory of Hazard Assessment and Control in Special Operational Environment, Air Force Medical University, ChangLe Road & 169, Xi’an City, Shaanxi, 710032, People’s Republic of China, Tel/Fax +86 2884711316, Email [email protected]
Objective: This meta-analysis of randomized controlled trials (RCTs) aims to evaluate the efficacy and safety of transcutaneous electrical acupoint stimulation (TEAS) for postoperative delirium (POD) in surgical patients.
Methods: Based on database searches of the Wanfang, China National Knowledge Infrastructure (CNKI), VIP, Chinese Biology Medicine (CBM), PubMed, Cochrane Library, and Web of Science, relevant RCTs published before December 30, 2022, were extracted. Outcome indicators included the incidence of POD, changes in Confusion Assessment Method (CAM) scores, Visual Analogue Scale (VAS) scores, and the intraoperative consumption of anesthetics. Data were pooled and analyzed by Review Manager 5.3, and publication bias detection was conducted using Stata 17.0.
Results: A meta-analysis containing 715 experimental and 717 control participants from 12 RCTs was performed. The overall results showed that TEAS had obvious superiority with a lower incidence of POD on any day during the postoperative 1 week. In subgroup analyses, the CAM scores on the third postoperative day were significantly lower in the TEAS group than in the control group (MD = − 0.52, 95% CI: − 1.02 to − 0.03, P = 0.04), the VAS scores on the first postoperative day were significantly lower in the TEAS group than in the control group (MD = − 0.19, 95% CI: − 0.36 to − 0.02, P = 0.03), the consumption of propofol and remifentanil were both significantly lower in the TEAS group compared with the control group (MD = − 23.1, 95% CI: − 37.27 to − 8.94, P = 0.001; MD = − 105.69, 95% CI: − 174.20 to − 37.19, P = 0.002). No serious adverse events of TEAS were reported in any of the referenced studies.
Conclusion: TEAS has an obvious curative effect in preventing POD and pain in the earlier stage of surgical patients. It could be a promising assisted anesthesia technique in the future.
Keywords: transcutaneous electrical acupoint stimulation, TEAS, postoperative delirium, POD, meta-analysis
Introduction
Postoperative delirium (POD) is an acute neuropsychiatric syndrome that often occurs in surgical patients due to the vulnerability of cerebral functioning to pathophysiological stressors.1 It is characterized by fluctuating alterations in consciousness, attention, and cognition that are highly related to a deterioration in the prognosis,2 and is also associated with prolonged length of hospital stay, increased health-care costs, severe cognitive impairment, and increased incidence of accidental injuries and mortality.3,4 The occurrence of POD is closely related to a wide range of factors such as patient’s age, sex, anesthetic tolerance, and physical condition,5,6 however, the pathophysiology of delirium is still poorly understood.7 Over the past decades, surgeons and anesthesiologists have devoted great efforts to exploring effective pharmacological interventions of POD, nevertheless, they found antipsychotics, acetylcholinesterase inhibitors, steroids, and statins might cause heightened adverse effects and poorer long-term outcomes.8 Recently, primary prevention of POD with multicomponent nonpharmacologic approaches such as reorientation, early mobilization, sleep strategies, and hearing and vision adaptations are taken as promising strategies.9 Therefore, it is a priority to identify high-quality and adequately powered evidence of nonpharmacologic treatment that could be effective and safe to prevent POD.
Transcutaneous electrical acupoint stimulation (TEAS) combines the theory of traditional Chinese acupuncture with the modern technology of electrical stimulation and is widely used in clinics with characteristics of simplicity, stability, and safety. Subsequently, a variety of studies have focused on cerebral protection and found that TEAS could be used for preventing and curing cerebral ischemia-reperfusion injury, postoperative cognitive dysfunction (POCD), postoperative pain, and other surgical complications.10–12 Additionally, a meta-analysis suggested that TEAS could reduce the incidence of POCD among old patients who underwent general anesthesia in the early postoperative period.13 However, the incidence of POD was not analyzed in the study due to the limited number of articles. Notably, a previous study revealed that the inflammatory response might play an important role in the mutually overlapping processes of POCD.14 It is well known that the pathogenesis between POD and POCD is similar, moreover, POD seems to be a harbinger of developing POCD. Nevertheless, the limited clinical studies that explored the effect of TEAS on POD just provided class-2 evidence or weaker.
Considering that there was no meta-analysis on the association between TEAS and POD to offer class-1 evidence, we performed this meta-analysis to evaluate the efficacy and safety of TEAS on POD.
Materials and Methods
Study Design and Protocol
To evaluate the efficacy and safety of TEAS for preventing POD in surgical patients, the protocol of this meta-analysis was elaborated and performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15 Before the review started, it was registered on the PROSPERO database (registration number: CRD42022378709).
Data Sources and Search Strategy
We searched articles in English or Chinese through electronic databases including Wanfang, China National Knowledge Infrastructure (CNKI), VIP, Chinese Biology Medicine (CBM), PubMed, Cochrane Library, and Web of Science. The following search terms were used: “Transcutaneous electrical acupoint stimulation”, “TEAS”, “Transcutaneous electric nerve stimulation”, “Perioperative neurocognitive dysfunction”, “PND”, “Postoperative delirium”, and “POD.” Specific search strategies were performed as ((Transcutaneous electrical acupoint stimulation) OR (TEAS)) AND ((((Perioperative neurocognitive disorders) OR (PND)) OR (Postoperative delirium)) OR (POD)). All the publications until 30 December 2022 were searched without any restriction of countries or article type. A reference list of all selected articles was independently screened to identify additional studies left out in the initial search.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) RCTs that evaluated the effect of TEAS in preventing POD; 2) study participants were patients following selected operations regardless of age, sex, race, and anesthesia; 3) studies in which the incidence of POD was confirmed based on clinical diagnostic criteria; 4) the intervention in the experimental group was TEAS (In this group, patients received electrical stimulation that was provided by an electrical stimulator through electrode tabs on the target acupoints. The electrical stimulator was set with certain modes, frequencies, and intensities accordingly), while the control group served as the placebo. The exclusion criteria were as follows: 1) animal studies; 2) comments, case reports, cross-sectional studies, letters, editorials, review articles, meta-analysis, and retrospective studies; 3) available data was inadequate in the study; 4) method of intervention was inconsistent with TEAS; 5) the studies had no English abstracts; 6) the studies had fundamental errors.
Data Extraction
The process of data extraction was performed independently by two authors. Any discrepancies in the data will be solved by consultation with the third investigator. The following information was extracted from each study: 1) Characteristics of included trials: the first author’s name, publication year, American Society of Anesthesiologist (ASA) grade, sample size, type of surgery, test scales, test time; 2) Details of intervention: anesthesia protocol, experimental intervention, parameters, intervention time, acupoints, and stimulation sides; 3) The results including POD incidence, Confusion Assessment Method (CAM) scores, Visual Analogue Scale (VAS) scores, serum biomarkers, and the methods and time of detection. If the data were insufficient or missed, we tried to contact the authors to request the raw data.
Assessment of the Risk of Bias
Two authors evaluated the methodological qualities of the included trials using the Cochrane risk of bias (ROB) tool16 independently. The contents of the assessment tool included random sequence generation, allocation concealment, participants and performers blinding, outcome assessment blinding, outcome data completeness, selective reporting, and other sources of bias. The risk of bias was classified as “low-risk”, “high-risk”, or “unclear-risk.” If there were any different opinions, a third party was consulted to resolve them.
Statistical Analysis
The dichotomous outcomes were reported as pooled relative risks (RRs) and corresponding 95% confidence intervals (CIs), and continuous outcomes were reported as mean differences (MDs) with 95% CIs. Furthermore, heterogeneity between studies was analyzed using Cochran’s Q test and I2 statistic. If the result of the Q test was P > 0.1 and I2 < 50%, the fixed effects model (FEM) was used to calculate the pooled RRs. Otherwise, the random effects model (REM) was used. In addition, potential publication bias was assessed using the funnel plot and Egger’s regression test (significance threshold set at P < 0.05). All meta-analyses were conducted using Review Manager Version 5.3 statistical software (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark), and the publication bias was detected by Stata 17.0 software (Stata Corp, College Station, Texas, USA).
Results
Literature Search
The literature screening process and flow diagram can be found in Figure 1. Firstly, 118 retrieved studies were searched through the electronic database (including 13 in CNKI, 17 in Wanfang, 11 in VIP, 18 in CBM, 16 in Web of Science, 39 in PubMed, and 4 in Cochrane Library). Then, a total of 39 duplicate studies were removed, and 54 irrelevant studies were also excluded in the preliminary screening of the title and abstract. Finally, after 13 studies were excluded by a further reading of the full text because without an English abstract, there were no journal articles, and intervention, and main outcome indicators were inconsistent and had fundamental errors, 12 studies17–28 were included in this meta-analysis.
 |
Figure 1 Flow diagram of the trials screening procedures. |
Study Characteristics
Of the 12 RCTs (Table 1), 11 studies17–25,27,28 were conducted in the last 2 years, and only 1 study26 was conducted in 2018. A total of 1432 participants were analyzed in these RCTs, with 715 in the TEAS group and 717 in the control group. The surgery types of three studies were abdominal surgery,17,25,28 five studies were spinal surgery,18,20,23,26,27 three studies were orthopedic surgery,21,22,24 and one study was cardiac surgery.19 In the included studies, POD was assessed by the Richmond Agitation-Sedation Scale (RASS), CAM, and their variants differed at postoperative first day to seventh day, respectively.
 |
Table 1 Characteristics of Included Trails |
The details of the intervention are shown in Table 2. Eleven trials’ anesthesia protocol was general anesthesia,17–20,22–28 except 1 trial was spinal anesthesia.21 Furthermore, the selected acupoints with higher frequency were Neiguan (PC6, 8 times), Hegu (LI4, 6 times), Baihui (GV20, 4 times), and the acupoints were stimulated bilaterally when multiple combined. In addition, the commonly applied parameter of TEAS was 2/100 Hz (disperse-dense waves) which was performed 30 min before induction of anesthesia until the end of surgery.
 |
Table 2 Details of Interventions |
Quality Assessment
The risk of bias for each research and all studies are described in Figure 2a and b, respectively. Most biases were concentrated on selection bias, performance bias, and detection bias. Except for three studies that were evaluated as having low bias,23,25,27 the other studies were identified as uncertain based on the risk of bias.17–22,24,26,28 Besides, three studies19,23,27 had a high risk of reporting bias due to the presence of some outcome indicators without analysis. Therefore, the quality of the included RCTs was moderate.
The Incidence of POD
Considering that the incidence of POD was assessed from the postoperative first day to the seventh day, and the reported data were expressed as the incidence of POD on the postoperative first or second day or within 3 days, 5 days, and 7 days, respectively. Thus, we performed a comparison for the incidence of POD at different assessed times.
The incidence of POD on the first and second postoperative days was compared in three studies.17–19 As it was proven that there was no evident heterogeneity in these data (I2 = 0), the analysis of pooled data adopting a FEM revealed that the participants in the TEAS group had a lower incidence of POD than the control participants in the first postoperative day (RR: 0.61, 95% CI: 0.37–1.01, P = 0.05; Figure 3a) and second postoperative day (RR: 0.49, 95% CI: 0.25–0.98, P = 0.04; Figure 3b), respectively.
 |
Figure 3 Forest plot of the incidence of POD in both groups on different days. Note: (a) On postoperative first day; (b) On postoperative second day. |
Based on the reported incidence of POD within different days postoperatively, comparisons were also conducted between these studies. As is shown in Figure 4, low heterogeneity was detected between studies in each analysis (I2 = 0, 47%, and 0); thus, all the comparisons used a FEM. The results showed that the difference in the incidence of POD was statistically significant in the TEAS group compared to the control group within postoperative 3 days17,19,20,24–26 (RR: 0.44, 95% CI: 0.29–0.66, P < 0.0001; Figure 4a), 5 days23,27 (RR: 0.52, 95% CI: 0.33–0.83, P = 0.006; Figure 4b), and 7 days18,22,28 (RR: 0.39, 95% CI: 0.18–0.83, P = 0.01; Figure 4c).
 |
Figure 4 Forest plot of the incidence of POD in both groups within different days. Note: (a) Within postoperative 3 days; (b) Within postoperative 5 days; (c) Within postoperative 7 days. |
CAM Score
CAM scores on the first and third postoperative days were compared in three studies.21,22,24 There was low heterogeneity among the results for CAM scores on the first (I2 = 0%) and third (I2 = 21%) postoperative days. So the FEM was chosen for the measures. The results showed that the CAM scores on the third postoperative day were significantly lower in the TEAS group than in the control group (MD = −0.52, 95% CI: −1.02 to −0.03, P = 0.04; Figure 5b). On the first postoperative day, CAM scores were not significantly different between the two groups (MD = −0.42, 95% CI: −0.878–0.05, P = 0.08; Figure 5a).
 |
Figure 5 Forest plot of CAM scores in both groups. Note: (a) On the postoperative first day; (b) On the postoperative third day. |
VAS Score
Postoperative pain was evaluated in four studies using VAS. Four studies20,21,24,27 assessed VAS scores on first postoperative day and two studies21,24 assessed on second postoperative day. No significant heterogeneity was found among the studies for VAS scores on the first postoperative day (I2 = 0%). However, there was significant heterogeneity among the studies on second postoperative day (I2 = 67%). The results showed that the VAS scores on the first postoperative day were significantly lower in the TEAS group than in the control group (MD = −0.19, 95% CI: −0.36 to −0.02, P = 0.03; Figure 6a) based on the FEM, while no significant difference of VAS scores was found between the two groups on the second postoperative day (MD = −0.09, 95% CI: −0.48 to –0.30, P = 0.65; Figure 6b) based on a REM.
 |
Figure 6 Forest plot of VAS scores in both groups. Note: (a) On the postoperative first day; (b) On the postoperative second day. |
Intraoperative Consumption of Anesthetics
Intraoperative consumption of propofol and remifentanil was collected in five studies.20,22,26–28 Due to a significant heterogeneity among the studies (I2 = 79%; 84%), the meta-analysis was performed using a REM. The results showed that the consumption of propofol and remifentanil were both significantly lower in the TEAS group compared with the control group (MD = −23.1, 95% CI: −37.27 to −8.94, P = 0.001; Figure 7a; MD = −105.69, 95% CI: −174.20 to −37.19, P = 0.002; Figure 7b).
 |
Figure 7 Forest plot of intraoperative consumption of anesthetics in both groups. Note: (a) propofol; (b) remifentanil. |
Serum Biomarkers
As shown in Table 3, six studies17,19,20,23,26,27 measured serum levels of biomarkers including NSE, IL-6, MMP-9, IL-1β, SOD, BDNF, S-100β, GFAP, ACTH, Cor, and CRP at perioperative times. Most of them were assessed by enzyme-linked immunosorbent assay, however, only the IL-6 in two studies19,20 was detected at the same time (postoperative day 1). In addition, there was a significant heterogeneity between the studies (I2 = 99%); thus, a REM was chosen for analysis. The results showed that there was no significant difference in the serum levels of IL-6 on the first day after surgery between the TEAS group and control group (MD = −10.96, 95% CI: −26.74–4.81, P = 0.17; Figure 8).
 |
Table 3 Details of Serum Biomarkers |
 |
Figure 8 Forest plot of serum levels of IL-6 on the postoperative first day. |
Publication Bias
Publication bias tests were performed for outcome indicators with six or more studies. A funnel plot was used to assess publication bias based on the incidence of POD within 3 days after the surgery (Figure 9). It showed that some of the points were asymmetrically distributed around, which indicated potential publication bias might exist. Therefore, Stata 17.0 was also used to test the publication bias by Egger’s method. The results showed no obvious publication bias among the studies (P = 0.5019 > 0.05).
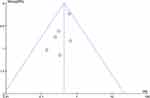 |
Figure 9 Funnel plot of the incidence of POD within postoperative 3 days. |
Discussion
This study was the first to explore the relationship between TEAS and POD by performing a meta-analysis, and the results of our analyses confirmed that TEAS could decrease the incidence of POD postoperatively. In the present study, most of the included studies used the CAM or its variants to test the POD among surgical patients at postoperative 1 to 7 days. Such assessments were aligned with the newly proposed concept that POD was the acute event of perioperative neurocognitive disorder (PND) within 1 week after surgery.29 So the results of this analysis could also be robust evidence for TEAS on preventing PND in the future.
Based on this meta-analysis, the outcomes showed that TEAS had a significant effect on preventing POD within postoperative 7 days. Simultaneously, the present study also found a significant decline in the CAM scores in the TEAS group compared to the control group, which further supported that TEAS could prevent POD. In the included studies, four researchers conducted one session (30 min before anesthesia induction until the end of surgery), and four performed more sessions of TEAS on patients. Just as a recent study calling on optimizing postoperative brain health in surgical patients,30 the application of TEAS with more sessions might be a good measure. Furthermore, the analgesic effect of TEAS was also confirmed in this study, as well as the results showed that TEAS significantly reduced VAS scores on the first postoperative day, which was consistent with the findings of a previous meta-analysis on the analgesic effect of TEAS.31 Considering that unchanged VAS scores were assessed for hip surgery on second postoperative day, we speculate that TEAS could not exhibit a long-term effect of analgesic in such surgery. In addition, we also detected that the intraoperative consumption of propofol and remifentanil was significantly decreased in the TEAS group,32 which confirmed that TEAS could be an effectively assisted technique for anesthesia. Finally, although six included studies measured the serum biomarkers such as NSE, IL-6, MMP-9, and so on perioperatively. The detection time was inconsistent among these studies. The result of this meta-analysis did not reveal that TEAS could significantly change their serum values. As a recent study indicated, to identify pathophysiologic pathways to prioritize the development of diagnostic and therapeutic regimens for POD, an associative, predictive, and systems analysis should be conducted,33 the ideas of which could also be applied to guide future studies on the mechanism of TEAS on POD.
In the present study, we tried to make some recommendations on the application of TEAS on preventing POD. As the Neiguan (PC6), Hegu (LI4), and Baihui (GV20) have been frequently selected as combined acupoints in the included studies and exhibited good effectiveness, and the three acupoints were mainly targeted for systemic analgesia and neuropsychiatric diseases in Chinese medicine.34 Therefore, we suggested that stimulation on these acupoints might show superiority in preventing POD in surgical patients. Meanwhile, according to the included studies in this study, we suggested that the intraoperative intervention (30 min before induction, until the end of the surgery) and more treatment sessions with TEAS might result in better benefits on surgical patients’ postoperative recovery. Moreover, as no serious adverse events of TEAS were reported in any of the included studies, it is expected to apply TEAS as a family therapy that could benefit the long-term prognosis of various surgical patients in the future.
The findings of this meta-analysis should be interpreted with caution because of the limitations. Although TEAS has obvious advantages in preventing the incidence of POD, the influence of bias in this analysis could not be completely excluded, because the surgical procedures of included studies were different which also potentially affected the internal heterogeneity among the studies. As a former study observed, the prevalence of delirium is 5–10% in non-cardiac surgery, whereas 36–40% of adult patients develop POD in cardiac surgery.1 It could be explained as a profound inflammatory response to cardiopulmonary bypass and surgery, which is thought to uniquely contribute to the higher risk of POD in this population.35 In addition, the sample size of our meta-analysis was limited considering the massive population and the multiple ethnic groups in the world. Consequently, there is no denying that single-factor interventions such as TEAS could not eliminate the occurrence of POD given the wide range of influencing factors, and the functional mechanism of TEAS is not clear. Future research should focus on the mechanism of the technology. Further studies with stricter designs and larger samples from different races are needed.
Conclusion
TEAS might be an effective and safe way to prevent POD within postoperative 7 days. This study again confirmed the effect of TEAS on analgesics, reducing intraoperative consumption of propofol and remifentanil. We highly recommend that this technology could be widely used in clinics.
Data Sharing Statement
The original contributions presented in this study are included in the article, further inquiries can be directed to the corresponding author Changjun Gao.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81971225) and the Natural Science Basic Research Program of Shaanxi Province (Grant No. 2023-JC-ZD-52), and the Clinical research project of Air Force Medical University (Grant No. 2021LC2207).
Disclosure
The authors declare that they have no competing interests.
References
1. Swarbrick CJ, Partridge J. Evidence-based strategies to reduce the incidence of postoperative delirium: a narrative review. Anaesthesia. 2022;77(Suppl 1):92–101. doi:10.1111/anae.15607
2. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi:10.1016/S0140-6736(13)60688-1
3. Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112(5):1202–1211. doi:10.1213/ANE.0b013e3182147f6d
4. Oh ES, Fong TG, Hshieh TT, Inouye SK. Delirium in older persons: advances in diagnosis and treatment. JAMA. 2017;318(12):1161–1174. doi:10.1001/jama.2017.12067
5. Matsuki M, Tanaka T, Takahashi A, et al. Incidence and risk factors of postoperative delirium in elderly patients undergoing urological surgery: a multi-institutional prospective study. Int J Urol. 2020;27(3):219–225. doi:10.1111/iju.14172
6. Yang Z, Wang XF, Yang LF, et al. Prevalence and risk factors for postoperative delirium in patients with colorectal carcinoma: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35(3):547–557. doi:10.1007/s00384-020-03505-1
7. Migirov A, Chahar P, Maheshwari K. Postoperative delirium and neurocognitive disorders. Curr Opin Crit Care. 2021;27(6):686–693. doi:10.1097/MCC.0000000000000882
8. Xu Y, Ma Q, Du H, Yang C, Lin G. Postoperative delirium in neurosurgical patients: recent insights into the pathogenesis. Brain Sci. 2022;12(10):1371. doi:10.3390/brainsci12101371
9. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP series. PLoS One. 2015;10(6):e0123090. doi:10.1371/journal.pone.0123090
10. Xi L, Fang F, Yuan H, Wang D. Transcutaneous electrical acupoint stimulation for postoperative cognitive dysfunction in geriatric patients with gastrointestinal tumor: a randomized controlled trial. Trials. 2021;22(1):563. doi:10.1186/s13063-021-05534-9
11. Li J, Hao M, Liu M, et al. Transcutaneous electrical acupoint stimulation pretreatment alleviates cerebral ischemia-reperfusion injury in rats by modulating microglia polarization and neuroinflammation through Nrf2/HO-1 signaling pathway. Neurochem Res. 2023;48(3):862–873. doi:10.1007/s11064-022-03797-5
12. Meng D, Mao Y, Song QM, et al. Efficacy and safety of Transcutaneous Electrical Acupoint Stimulation (TEAS) for postoperative pain in laparoscopy: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:9922879. doi:10.1155/2022/9922879
13. Chen X, Kong D, Du J, Ban Y, Xu H. Transcutaneous electrical acupoint stimulation affects older adults’ cognition after general anesthesia: a meta-analysis. Geriatr Nurs. 2022;46:144–156. doi:10.1016/j.gerinurse.2022.05.010
14. Glumac S, Kardum G, Karanovic N. Postoperative cognitive decline after cardiac surgery: a narrative review of current knowledge in 2019. Med Sci Monit. 2019;25:3262–3270. doi:10.12659/MSM.914435
15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
16. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
17. Yu B, Ding L, Lu B, Wang Q. Study on brain protection and oxidative stress on general anesthesia patients of laparoscopic surgery by TEAS. Shaanxi J Trad Chin Med. 2020;41(02):241–244.
18. Shi H, Li W, Zhang Y, Wang Y. Effect of transcutaneous electrical acupoint stimulation on sleep after spinal surgery in elderly. Orthop J China. 2022;30(22):2045–2049.
19. Jia B, Zhao Y, Ling Q, Ding D, Li X. Effect of transcutaneous electrical acupoint stimulation on postoperative delirium in patients undergone heart valve replacement surgery with cardiopulmonary bypass. N Chin Med. 2022;54(07):176–180.
20. Xu X, Huang X, Yu Y, Wang W, Xia P. Effects of percutaneous acupoint electrical stimulation assisted anesthesia on stress response and delirium after lumbar surgery in the elderly. Med Innov China. 2022;19(16):53–57.
21. Chang M, Long Q, Lin S, et al. Effect of transcutaneous electrical acupoint stimulation on postoperative fatigue and delirium in elderly patients with sleep disorder undergoing total hip arthroplasty. J Clin Anesthesiol. 2021;37(10):1013–1017.
22. Wei C, Hao N, Liu J, Li J. Observation on the effect of electrical stimulation at ghost points combined with an endotracheal general anesthesia on delirium in elderly patients after a total knee arthroplasty. Jilin J Chin Med. 2022;42(03):362–365.
23. Ding L, Ning J, Guo Y, et al. The preventive effect of transcutaneous electrical acupoint stimulation on postoperative delirium in elderly patients with time factors: a randomized trial. J Integr Complement Med. 2022;28(8):689–696. doi:10.1089/jicm.2021.0141
24. Wei L, Luo W, Huang J, et al. The effect of transcutaneous electrical acupoint stimulation of Shenmen and Neiguan points on sleep quality and postoperative delirium in elderly patients undergoing Hip replacement. Int J Anesthesiol Resusc. 2021;42(10):1056–1060.
25. Zhang Y, Gong L, Zhang Y, et al. Effect of transcutaneous acupoint electrical stimulation on urinary retention and urinary ATP in elderly patients after laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. Clin Interv Aging. 2022;17:1751–1760. doi:10.2147/CIA.S382912
26. Gao F, Zhang Q, Li Y, et al. Transcutaneous electrical acupoint stimulation for prevention of postoperative delirium in geriatric patients with silent lacunar infarction: a preliminary study. Clin Interv Aging. 2018;13:2127–2134. doi:10.2147/CIA.S183698
27. Wang Q, Zhou R, Ding L, et al. Effect of electroacupuncture and transcutaneous electrical acupoint stimulation on postoperative delirium in elderly patients. Chin J Surg Integr Trad West Med. 2022;28(4):485–490.
28. Wu H, Gao H, Mi Z, Lin S, Gao J. Effect of transcutaneous electrical acupoint stimulation on postoperative delirium in frail elderly patients. Chin J Anesthesiol. 2021;41(6):723–726.
29. Kong H, Xu LM, Wang DX. Perioperative neurocognitive disorders: a narrative review focusing on diagnosis, prevention, and treatment. Cns Neurosci Ther. 2022;28(8):1147–1167. doi:10.1111/cns.13873
30. O’Gara BP, Gao L, Marcantonio ER, Subramaniam B. Sleep, Pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology. 2021;135(6):1132–1152. doi:10.1097/ALN.0000000000004046
31. Wang D, Shi H, Yang Z, et al. Efficacy and safety of transcutaneous electrical acupoint stimulation for postoperative pain: a meta-analysis of randomized controlled trials. Pain Res Manag. 2022;2022:7570533. doi:10.1155/2022/7570533
32. Huang S, Peng W, Tian X, et al. Effects of transcutaneous electrical acupoint stimulation at different frequencies on perioperative anesthetic dosage, recovery, complications, and prognosis in video-assisted thoracic surgical lobectomy: a randomized, double-blinded, placebo-controlled trial. J Anesth. 2017;31(1):58–65. doi:10.1007/s00540-015-2057-1
33. Tripp BA, Dillon ST, Yuan M, et al. Targeted metabolomics analysis of postoperative delirium. Sci Rep. 2021;11(1):1521. doi:10.1038/s41598-020-80412-z
34. Yildiz M, Kozanhan B. Transcutaneous electric acupoint stimulation reduces rocuronium injection-related pain: a prospective randomized controlled study. Eur Rev Med Pharmacol Sci. 2022;26(17):6215–6220. doi:10.26355/eurrev_202209_29639
35. Rengel KF, Pandharipande PP, Hughes CG. Postoperative delirium. Presse Med. 2018;47(4 Pt 2):e53–e64. doi:10.1016/j.lpm.2018.03.012
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

