Back to Journals » Journal of Pain Research » Volume 16
Transcutaneous Electrical Acupoint Stimulation Accelerates the Recovery of Patients Undergoing Laparoscopic Myomectomy: A Randomized Controlled Trial
Authors Pan Y , Shao Y , Chi Z , Jin S, Wang J
Received 13 December 2022
Accepted for publication 4 March 2023
Published 10 March 2023 Volume 2023:16 Pages 809—819
DOI https://doi.org/10.2147/JPR.S399249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Yuanyuan Pan,1,* Yifan Shao,2,* Zhanghuan Chi,3,* Shenhui Jin,1 Junlu Wang1
1Department of Anesthesia, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, People’s Republic of China; 2Department of Anesthesia, Sir Run Run Shaw Hospital, Affiliated with the Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 3Department of Anesthesia, Wenzhou People’s Hospital, Wenzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junlu Wang, Departments of Anesthesiology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China, Tel +86 13806689854, Fax +86 0577-55578999-689854, Email [email protected]
Purpose: To evaluate transcutaneous electrical acupoint stimulation (TEAS) on the perioperative rehabilitation of patients undergoing laparoscopic myomectomy.
Patients and Methods: One hundred and five women undergoing laparoscopic hysteromyomectomy were randomly divided into TEAS group (Group T) and control group (Group C). Propofol and remifentanil were used to stabilize patient blood pressure and keep BIS between 40 and 60. Group T patients received TEAS at LI4/PC6 30 minutes before the operation and lasting until the end of anesthesia, followed by TEAS at ST36/SP6 for 30 minutes in PACU. All required indicators were recorded.
Results: Group T patients required lower dosages of remifentanil and vasoactive drugs, and had a reduced incidence of propofol injection pain and intraoperative hypotension compared to Group C. Group T also had a lower maximum NRS score in PACU, lower NRS scores at 1 hour and 24 hours, and a lower incidence of vomiting within 24 hours. In addition, the QoR-40 score for Group T at 24 hours after operation was higher in terms of physical comfort, emotional state, pain and total score.
Conclusion: TEAS can reduce the amount of anesthetic, maintain hemodynamic stability, reduce postoperative pain, reduce postoperative vomiting, enhance the recovery of gastrointestinal function, increase the quality of postoperative recovery and thus accelerate overall patient recovery.
Keywords: TEAS, laparoscopic myomectomy, QoR-40
Introduction
Uterine leiomyoma is an abnormal hyperplasia of uterine smooth muscle cells. Women aged between 30 and 50 years have a high incidence of this condition, with 20–40% having obvious symptoms, menstrual disorders, dysmenorrhea, abdominal and pelvic pain, and even infertility, abortion and other diseases.1,2 This can have major adverse effects on their physical and mental health, as well as on their quality of daily life. For patients with serious clinical symptoms and ineffective conservative treatment with drugs, the early treatment plan often involves surgery. Laparoscopic myomectomy is more easily accepted by patients, with less trauma and less intraoperative bleeding. It is a well established and simple operation that is widely performed.3,4 However, various adverse reactions often occur during the perioperative period, including stress reactions caused by long-term pneumoperitoneum, head low position, anesthesia and surgical stimulation during the operation, the use of drugs to promote uterine contraction, and the use of anesthesia and analgesics after the operation. These can cause postoperative nausea and vomiting, pelvic pain, dizziness and other complications that affect the recovery of patients.5–9
Transcutaneous electrical acupoint stimulation (TEAS) is a non-invasive operation whereby the acupoint electrode piece is pasted onto the skin of the corresponding acupoint of the patient. Once connected to the corresponding instruments and equipment, a specified intensity of electrical stimulation is given in order to achieve the effect of traditional electric acupuncture.10 Various studies have demonstrated that TEAS has analgesic and sedative effects.11–13 Application of TEAS during surgery can protect vital functions of the heart, brain, liver, stomach and other organs.14,15 TEAS can also reduce inflammatory reactions, improve immune function, and lower the frequency of postoperative complications.16,17 The goal of this work was to evaluate TEAS with regard to the perioperative rehabilitation of patients undergoing laparoscopic myomectomy.
Materials and Methods
Study Design and Participant Recruitment
In this double-blind randomized control-group clinical trial, we studied about Transcutaneous electrical acupoint stimulation accelerates the recovery of patients undergoing laparoscopic myomectomy. The Ethics Committee of Wenzhou Medical University (WMU) approved this study (clinical trial number: ChiCTR2100045173; Registry URL:YJLCYJ-2020-148). The study was conducted in accordance with the Declaration of Helsinki. Included in the study cohort were 120 women (ASA I and II) aged 18 to 60 years and who received laparoscopic myomectomy at the First Affiliated Hospital, WMU, Zhejiang, China, from May 2021 to December 2021. Patients were assigned to TEAS group (Group T) and control group (Group C) using random number tables. All participants provided informed consent.
Exclusion criteria included: incision or surgical scar at acupoint; infection of acupoint; nerve injury in any limb; prior spinal surgery; allergic reaction to any of the drugs used in this study; sinus bradycardia; severe neurological disease; severe respiratory disease; smoking; severe cardiovascular disease; addiction to an anaesthetic; recent history of opioid use.
Types of Outcomes
Transcutaneous Electrical Acupoint Stimulation (TEAS)
Patients in Group T received TEAS. The anesthesiologist selected acupoints of LI4 (Hegu) and PC6 (Neiguan) on both hands of the patients, stuck the electrodes, and connected Han’s Acupoint Nerve Stimulator (Nanjing Jinan Xinsheng Technology, Jinan, Nanjing, China) capable of producing a dilatational wave with a frequency of 2/100 HZ, which is the maximum tolerable for patients receiving −1 mA of electrical stimulation for 30 minutes before the operation and lasting until the end of anesthesia. Using the same method at bilateral acupoints of ST36 (Zusanli) and SP6 (Sanyinjiao) for 30 minutes while in PACU (Postanesthesia care unit). Patients in Group C did not undergo TEAS. The acupoint locations are shown in Figure 1.
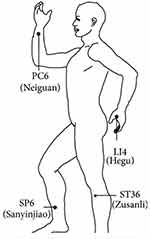 |
Figure 1 Illustration of the acupoints. |
Numerical Rating Scale (NRS)
1h and 24h after the procedure, a PACU nurse, who was unaware of the group of each patient, assisted patients to evaluate their pain level by using a numerical rating scale (NRS: 0–10,0 = none, 10 = most severe, 1–3 mild pain, 4–6 moderate pain, 7–10 severe pain).
Grade of Abdominal Distension Severity
Grade 0: No or transient abdominal distension; Grade 1: mild abdominal distension, or temporary sudden abdominal distension, gas movement in the abdomen, but does not affect rest and sleep, mild abdominal bulge, abdominal wall tension; Grade 2: moderate abdominal distension, nausea, rest, sleep, moderate abdominal bulge, large abdominal tension; Grade 3: severe abdominal distension, severe nausea, vomiting, irritability, serious sleep, significant abdominal swelling, and percussion.
Anesthesia, Surgical Procedures, and Analgesia
A 22-gauge trocar was used to obtain venous access on the dorsal side of the non-dominant hand prior when the patient is in the preoperative preparation room. After the infusion channel was connected to the extension tube, 500 mL Ringer’s solution was infused through the channel, with an infusion speed of 20 mL/min. No medications were given. Blood pressure, oxygen saturation and electrocardiography were monitored while in operating theatre. The anesthesia induction sequence was started with intravenous sufentanil (0.5 µg/kg) (Yichang Renfu, Yichang, Hubei, China) over two seconds, propofol (2mg/kg) (Aspen Pharma Trading Limited) and cisatracurium (2mg/kg) (Jiangsu Hengrui, Jiangsu, China) were given and endotracheal intubation was performed. Propofol and remifentanil (Yichang Renfu, Yichang, Hubei, China) were injected by electronic pump during anesthesia maintenance to kept the patient’s blood pressure stable during operation and BIS(Bispectral Index) maintained between 40 and 60. Add the cisatracurium or sufentanil and record the dosage according to the actual situation. Both groups were given tropisetron 5mg immediately at the start of surgery to prevent postoperative nausea and vomiting and flurbiprofen 100mg for postoperative analgesia. The surgeon was instructed to administer ropivacaine for local infiltration analgesia around the incision at the end of the procedure. At the end of surgery, the muscle relaxation antagonism (neostigmine 1mg, atropine 0.5mg) was given immediately, and 20mg was given to promote the recovery of spontaneous breathing. When the patient recovered spontaneous breathing, tidal volume was >6 mL/kg, respiratory waveform was regular, he could respond to oral instructions, and the tracheal tube was removed after sputum suction and the patient was taken to the PACU. Norepinephrine(4ug) was administered intravenously every time when systolic blood pressure fell to <90 mmHg or diastolic blood pressure fell to <60 mmHg. Atropine (0.5 mg) was administered when the heart rate fell below 50 per minute (Figure 2).
Observation Indexes
The main observation indexes included the 40 items in QoR-40 24 hours after surgery to evaluate the quality of postoperative rehabilitation. The secondary outcome measures: the general patient condition, dosage of remifentanil, propofol, any vasoactive drugs, etc used during the operation, incidence of pain caused by propofol injection during the operation, and the incidence of cough induced by sufentanil during anesthesia induction. Ten recordings of the patients’ mean arterial pressure (MAP), HR (Heart Rate), SaO2 (oxygen saturation), BIS were recorded at the following times: in the operating room and patient calmed (T0), immediately before tracheal intubation (T1), one min after tracheal intubation (T2), two min after tracheal intubation (T3), immediately at the beginning of operation (T4), pneumoperitoneum at 5 min (T5), pneumoperitoneum at 30 min (T6), suture of the operation (T7), immediately before extubation (T8), one min after extubation (T9). Records were also made of bleeding, urine volume, liquid, the time of anesthesia, the time of operation, the incidence of intraoperative hemodynamic instability, the utilization rate of vasoactive drugs, the postoperative respiratory recovery time, the conscious time, the extubation time, Aldrete score, highest pain score in PACU, the retention time in PACU, the utilization rate of remedial drugs in PACU, NRS (Numerical Rating Scale) at 1 h and 24 h after the operation, the occurrence of adverse reactions including nausea, vomiting, abdominal distention, the utilization rate of analgesic and antiemetic drugs in 24h, the time to first postoperative anal exhaust, the time to first postoperative water intake, the time to solid food tolerance, the time to catheter removal, the time to first postoperative implantation activity, the time of postoperative hospitalization.
Statistical Analysis
The necessary sample size was estimated based on an improvement of 4 points in the QoR-40 evaluation. With a = 0.05 and β = 0.20, at least 49 patients were needed in each group. All data were analyzed by SPSS 23.0. The mean ± standard deviation of measurement data that had a normal distribution was used in statistical descriptions. The median (with interquartile spacing) was used for statistical description of measurement data not conforming to a normal distribution. Two-sample independent t-test was employed to evaluate measurement indexes with normal distribution and to test the homogeneity of variance. Rank sum test was employed to evaluate non-conforming normal distribution. Numerical data were expressed as a percentage (%) and was compared using chi-square test. Statistical tests were bilateral, with p < 0.05 considered as statistically significant.
Results
The overall study cohort was comprised of 105 patients, with 52 in Group T and 53 in Group C. Six patients used analgesic drugs other than those in the experimental plan, 6 patients underwent a different surgical procedure, two patients used PCIA (Patient-controlled intravenous analgesia), and one patient had intraoperative bleeding (Figure 3).
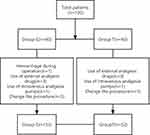 |
Figure 3 Flowchart for inclusion, exclusion and missing interviews. |
General Characteristics
The general preoperative (Table 1) and intraoperative (Table 2) conditions were compared between Groups T and C. No statistically significant differences were observed for ASA, age, weight, BMI, MAP, HR, BIS, temperature, duration of anesthesia, duration of operation, intraoperative blood loss, intraoperative urine volume, dosage of sufentanil, propofol, or atracurium cisbenzenesulfonate. However, the dosage of remifentanil used in Group T was significantly lower by almost 15% relative to Group C.
 |
Table 1 Preoperative General Conditions Between Two Groups |
 |
Table 2 Intraoperative General Conditions in Between Two Groups |
Intraoperative Evaluation Index and PACU Evaluation Index
The incidence of propofol injection pain was significantly lower in Group T (Table 3). Group T also showed a lower incidence of sufentanil-induced cough response, although this was not statistically different. Several significant differences in hemodynamics were apparent between Groups T and C at all of the T0-T9 times (Table 4). The frequency of intraoperative hypotension and the use of vasoactive drugs were also lower in Group T (Table 5). No significant difference was observed between the two Groups for the anesthesia recovery index (Table 6). The maximum NRS score for Group T was significantly less than that of Group C. Remedial drug use in PACU was lower in Group T, but this was not significantly different. No significant differences between the two groups were seen for PACU aldrete score and retention time (Table 6).
 |
Table 3 The Incidence of Propofol Injection Pain and Sufentanil-Induced Cough in the Two Groups |
 |
Table 4 Intraoperative Hemodynamic Comparison Between the Two Groups |
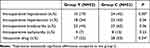 |
Table 5 The Incidence of Intraoperative Hemodynamic Instability and the Utilization Rate of Vasoactive Drugs Were Compared in the Two Groups |
 |
Table 6 Comparison of Anesthesia Indicators and the Indicators of PACU in the Two Groups |
Postoperative Recovery Evaluation Index
The NRS score while in the mobile state was significantly lower in Group T at 1 hour and at 24 hours after operation (Table 7). Within the first 24 hours, vomiting was significantly less in Group T than in Group C. Analgesic and antiemetic drug use at 24 h were also both less frequent in Group T. However, these did not reach statistical significance (Table 7). Some mild and moderate abdominal distension was recorded for Group T (Table 8), but this was not significantly different to Group C (P = 0.24). Group T patients had significantly shorter times for first postoperative anal exhaust and solid food tolerance. However, the times for first postoperative water intake, removal of the catheter, first postoperative implantation activity, and postoperative hospitalization were not statistically different between the two Groups (Table 9). At 24 hours after operation, Group T had significantly higher QoR-40 scores for physical comfort, emotional state, pain, and total score. However, no significant differences were observed for self-care ability and psychological support (Table 10).
 |
Table 7 Comparison of NRS, the Incidence of Vomiting and Utilization Rate of Analgesic and Antiemetic Drugs in 24h Between the Two Groups |
 |
Table 8 Abdominal Distension at 24h After Surgery Between Both Groups |
 |
Table 9 Postoperative Gastrointestinal Function Recovery Between the Two Groups |
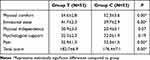 |
Table 10 Comparison of QoR-40 Recovery Quality Score Scale at 24h Between the Two Groups |
Discussion
This study found that TEAS was associated with a reduced dosage of anesthetic, better hemodynamic stability, a lower postoperative pain NRS score, reduced incidence of postoperative vomiting, better recovery of gastrointestinal function, improved quality of postoperative recovery, and an accelerated recovery process.
TEAS is a non-invasive operation compared to traditional electroacupuncture (EA). The stimulation intensity is more moderate, stable and continuous, and similar clinical results to traditional EA can be obtained.18 A large number of clinical and animal studies have shown numerous beneficial effects of TEAS, and there is now consensus both at home and abroad regarding the advantages of TEAS.19 However, the parameters and acupoints used for stimulation have varied in different studies. In the present study, Hegu (LI4), Neiguan (PC6), Zusanli (ST36) and Sanyinjiao (SP6) were used (Figure 1). Some studies have reported that the analgesic effect of TEAS derives from release of the endogenous opioids enkephalin, β-endorphin, and dendorphine.20,21 The analgesic effect obtained from high-frequency (100 Hz) EA occurs after the release of dynorphins from the central nervous system. The levels of endorphins and of mandorphins in human CSF both increase after 30 minutes of low (2 Hz) and high frequency (100 Hz) electroinjection.
TEAS can significantly reduce propofol injection pain and TEAS may activate sympathetic nerve fibers, inhibit inflammatory mediators, reduce vascular permeability (directly and indirectly), and reduce the contact opportunity between propofol and peripheral nerves, thus lowering the injection pain. Moreover, TEAS is able to increase the patient pain threshold and produce a reliable endogenous opioid effect by inducing the central release of multiple opioid peptides.22 This could also explain why the highest NRS score for PACU, as well as postoperative NRS at 1 h and 24 h, were lower in Group T than Group C.
Endogenous opioid peptides induced by TEAS can positively and negatively regulate blood pressure through the blood pressure regulation zone. Central blood pressure regulation is somewhat related to the central analgesic mechanism of TEAS.23–25 The reduction in MAP at 2 min after intubation was less in Group T, whereas differences in intraoperative MAP, HR, oxygen saturation, and BIS between the two groups at other T0-T9 times were not statistically significant. However, the dosage of remifentanil required for intraoperative maintenance was reduced by about 15% in Group T relative to Group C. Intraoperative hypotension was also significantly less frequent in Group T, as well as the need for vasoactive drugs. These results indicate that TEAS plays a role in maintaining the stability of intraoperative circulation, thus reducing the need for drug intervention.
LI4 and PC6 have been shown in many clinical trials to reduce the incidence of PONV (Postoperative Nausea and Vomiting).26–28 In the present study, Group T showed less frequent vomiting within 24 h than Group C. However, the incidence of vomiting within 1 h was not significantly different between the two groups, possibly because preoperative antiemetic drug use effectively prevented the occurrence of early postoperative nausea and vomiting. ST36 is the classic acupuncture point of traditional Chinese medicine used in the treatment of gastrointestinal diseases. It stimulates the parasympathetic nerve and improves gastrointestinal smooth muscle excitability, gastrointestinal microcirculation, and intestinal and endocrine function, thereby promoting the recovery of gastrointestinal function.29–31 In the current study, the times for first postoperative anal exhaust and postoperative solid food tolerance were significantly shorter in Group T patients, indicating that TEAS promotes the recovery of gastrointestinal function after surgery.
Previous studies found that TEAS could reduce the dosage of intraoperative propofol used in craniotomy patients,32 as well as accelerating analepsia.33 In the present study, however, no significant differences were observed between Groups T and C for the use of propofol, respiratory recovery time, anesthesia awake time, and tracheal catheter extraction time. This may have been due to the short operation time, or because the benefits of TEAS are acupoint-specific.
QoR-40 is an objective measure of the quality of anesthesia and of postoperative recovery, and has been widely used to evaluate postoperative rehabilitation. It consists of 40 questions and provides a total score from five dimensions of subitem scores, comprised of psychological support, physical comfort, emotional status, self-care ability and pain.34–36 This study indicates that TEAS can improve the quality of recovery after laparoscopic myomectomy and concur with those of Chen et al32 who studied patients treated with gynecological laparoscopic surgery. The improvements are probably due to the reduction of postoperative pain, a lower frequency of adverse reactions (nausea, vomiting, abdominal distension), reduced complications from high-dose opioid use, and improved patient satisfaction and comfort. In addition, it was easier for patients to turn over in bed, get out of bed, and to perform other activities after surgery, thus helping with gastrointestinal recovery. Postoperative gastrointestinal nutritional support can be carried out in advance to further accelerate the recovery of patients.
In the current study, we evaluated patient recovery only within 24 h of surgery, and continuous postoperative recovery thereafter was not assessed. Nevertheless, this limitation was partially compensated by observing multiple indicators for each patient, which is likely to have more clinical significance.
Conclusion
TEAS promotes rapid perioperative recovery of patients who undergo laparoscopic uterine myomectomy by reducing the incidence of postoperative complications. TEAS is a simple operation with no obvious toxic or side effects and with high economic benefits, thereby justifying its wide use in clinical practice.
Data Sharing Statement
Once received, the clinical datasets generated during and/or analyzed during the current study are available from Junlu WANG ([email protected]) on reasonable request within 6 months.
Acknowledgment
We thank all the individuals who contributed to this study. This work was supported by the National Foundation of Natural Science of China (No.81573742 and 82104622) and the Wenzhou Municipal Science and Technology Bureau (No:Y20180568),the Yuhuan Municipal Science and Technology Bureau (No:Y202211).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol. 2016;34:3–12. doi:10.1016/j.bpobgyn.2015.11.018
2. Lewis TD, Malik M, Britten J, et al. A comprehensive review of the pharmacologic management of uterine leiomyoma. Biomed Res Int. 2018;2018:2414609. doi:10.1155/2018/2414609
3. Takmaz O, Ozbasli E, Gundogan S, et al. Symptoms and health quality after laparoscopic and robotic myomectomy. JSLS. 2018;22:4.
4. Bhave Chittawar P, Franik S, Pouwer AW, et al.Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2014;(10):CD004638. doi:10.1002/14651858.CD004638.pub3
5. Buckley VA, Nesbitt-Hawes EM, Atkinson P, et al. Laparoscopic myomectomy: clinical outcomes and comparative evidence. J Minim Invasive Gynecol. 2015;22(1):11–25. doi:10.1016/j.jmig.2014.08.007
6. Suarez-Pierre A, Terasaki Y, Magruder JT, et al. Complications of CO 2 insufflation during endoscopic vein harvesting. J Card Surg. 2017;32(12):783–789. doi:10.1111/jocs.13249
7. Son JS, Oh JY, Ko S. Effects of hypercapnia on postoperative nausea and vomiting after laparoscopic surgery: a double-blind randomized controlled study. Surg Endosc. 2017;31(11):4576–4582. doi:10.1007/s00464-017-5519-8
8. Saccardi C, Gizzo S, Vitagliano A, et al. Peri-incisional and intraperitoneal ropivacaine administration: a new effective tool in pain control after laparoscopic surgery in gynecology: a randomized controlled clinical trial. Surg Endosc. 2016;30(12):5310–5318. doi:10.1007/s00464-016-4881-2
9. Kwack JY, Ahn KH, Kwon YS. Postoperative pain control with ropivacaine following laparoscopic myomectomy: a randomized double-blind, pilot study. J Obstet Gynaecol Res. 2019;45(4):871–876. doi:10.1111/jog.13910
10. Han JS, Ho YS. Global trends and performances of acupuncture research. Neurosci Biobehav Rev. 2011;35(3):680–687. doi:10.1016/j.neubiorev.2010.08.006
11. Hou Y, Yan Q, An H, et al. The use and protective effects of transcutaneous electrical acupoint stimulation during abdominal surgery: study protocol for a multicenter randomized parallel controlled trial. Trials. 2019;20(1):462. doi:10.1186/s13063-019-3558-2
12. Grech D, Li Z, Morcillo P, et al. Intraoperative low-frequency electroacupuncture under general anesthesia improves postoperative recovery in a randomized trial. J Acupunct Meridian Stud. 2016;9(5):234–241. doi:10.1016/j.jams.2016.03.009
13. Chen L, Tang J, White PF, et al. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesth Analg. 1998;87(5):1129–1134.
14. Mo Y, Zhang A, Zheng B, et al. 经皮穴位电刺激对手术患者胃排空的影响 [Effect of transcutaneous electrical acupoint stimulation on gastric emptying in patients undergoing surgery]. Zhongguo Zhen Jiu. 2017;37(12):1261–1264. Chinese. doi:10.13703/j.0255-2930.2017.12.002
15. Wang JL, Ren QS, Pei SL. 经皮穴位电刺激对颅脑围手术期脑氧及糖代谢的影响 [Effect of transcutaneous acupoint electrical stimulation on brain oxygen and glucose metabolism in the perioperative period of the craniocerebral operation]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2008;28(3):201–205. Chinese.
16. Shan JG, Xue S, Xu G-X, et al. 针药复合麻醉对心脏手术患者围术期炎性因子的影响 [Effects of acupuncture-drug compound anesthesia on perioperative inflammatory factors in patients undergoing cardiac surgery]. Zhongguo Zhen Jiu. 2010;30(7):585–588. Chinese.
17. Lin SY, Yin Z-L, Gao J, et al. 针药复合麻醉对老年患者术后早期认知功能障碍及炎性细胞因子TNF-α、IL-1β、IL-6的影响 [Effect of acupuncture-anesthetic composite anesthesia on the incidence of POCD and TNF-alpha, IL-1beta, IL-6 in elderly patients]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34(7):795–799. Chinese.
18. Gao YQ, Jia Q, Xie S, et al. 不同穴位不同刺激方式针刺辅助麻醉用于甲状腺手术的临床研究 [Clinical trials for thyroidectomy under acupuncture-aided anesthesia by using electroacupuncture or transcutaneous acupoint electrical stimulation of different acupoints]. Zhen Ci Yan Jiu. 2017;42(4):332–337. Chinese.
19. Wu MS, Chen K-H, Chen I-F, et al. The efficacy of acupuncture in post-operative pain management: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150367. doi:10.1371/journal.pone.0150367
20. Mayor D. An exploratory review of the electroacupuncture literature: clinical applications and endorphin mechanisms. Acupunct Med. 2013;31(4):409–415. doi:10.1136/acupmed-2013-010324
21. Jiang QY, Wang M-Y, Li L, et al. Electroacupuncture relieves labour pain and influences the spinal dynorphin/kappa-opioid receptor system in rats. Acupunct Med. 2016;34(3):223–228. doi:10.1136/acupmed-2015-010951
22. Huang L, Pan Y, Chen S, et al. Prevention of propofol injection-related pain using pretreatment transcutaneous electrical acupoint stimulation. Turk J Med Sci. 2017;47(4):1267–1276. doi:10.3906/sag-1611-35
23. Yu HC, Geng WJ, Tang HL. 经皮穴位电刺激对乳腺癌改良根治术患者全麻围拔管期应激反应的影响 [Preventive effect of transcutaneous electro-acupuncture on the intratracheal extubation stress response in general anesthesia of patients with breast cancer undergoing modified radical mastectomy]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2009;29(11):990–992. Chinese.
24. Wu JC, Ziea ET, Lao L, et al. Effect of electroacupuncture on visceral hyperalgesia, serotonin and fos expression in an animal model of irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16(3):306–314. doi:10.5056/jnm.2010.16.3.306
25. Min Y, Zhu Y, Zhou H, et al. 微创埋线药物复合麻醉替代传统针麻用于肺切除手术的临床研究 [Clinical research of lung resection surgery with microinjection acupuncture and drug anesthesia instead of traditional acupuncture anesthesia]. Zhongguo Zhen Jiu. 2015;35(4):367–371. Chinese.
26. Kim KH, Kim DH, Bae JM, et al. Acupuncture and PC6 stimulation for the prevention of postoperative nausea and vomiting in patients undergoing elective laparoscopic resection of colorectal cancer: a study protocol for a three-arm randomised pilot trial. BMJ Open. 2017;7(1):e013457. doi:10.1136/bmjopen-2016-013457
27. Li QW, Yu M-W, Yang G-W, et al. Effect of acupuncture in prevention and treatment of chemotherapy-induced nausea and vomiting in patients with advanced cancer: study protocol for a randomized controlled trial. Trials. 2017;18(1):185. doi:10.1186/s13063-017-1927-2
28. Sridharan K, Sivaramakrishnan G. Interventions for treating nausea and vomiting in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Expert Rev Clin Pharmacol. 2018;11(11):1143–1150. doi:10.1080/17512433.2018.1530108
29. Wu GJ, Xu F, Sun XM, et al. Transcutaneous Neuromodulation at ST36 (Zusanli) is more effective than transcutaneous tibial nerve stimulation in treating constipation. J Clin Gastroenterol. 2019;54(6):536–544.
30. Chao HL, Miao S-J, Liu P-F, et al. The beneficial effect of ST-36 (Zusanli) acupressure on postoperative gastrointestinal function in patients with colorectal cancer. Oncol Nurs Forum. 2013;40(2):E61–8. doi:10.1188/13.ONF.E61-E68
31. Lee CH, Kim DK, Yook TH, et al. Effectiveness of electroacupuncture at Zusanli (ST36) on the immunohistochemical density of enteroendocrine cells related to gastrointestinal function. J Acupunct Meridian Stud. 2012;5(2):63–71. doi:10.1016/j.jams.2012.01.002
32. Chen B, Zhang F, Zhang J, et al. Effect of acupuncture on quality of recovery during early period after gynecological laparoscopic surgery: quality of Recovery-40 questionnaire. Chin Asian J Anesthesiol. 2015; 12: 1428–1430
33. Mi Z, Gao J, Chen X, et al. Effects of transcutaneous electrical acupoint stimulation on quality of recovery during early period after laparoscopic cholecystectomy. Zhongguo Zhen Jiu. 2018;38(3):256–260. doi:10.13703/j.0255-2930.2018.03.007
34. Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth. 2000;84(1):11–15. doi:10.1093/oxfordjournals.bja.a013366
35. Gornall BF, Myles PS, Smith CL, et al. Measurement of quality of recovery using the QoR-40: a quantitative systematic review. Br J Anaesth. 2013;111(2):161–169. doi:10.1093/bja/aet014
36. Guimaraes-Pereira L, Costa M, Sousa G, et al. Quality of recovery after anaesthesia measured with QoR-40: a prospective observational study. Braz J Anesthesiol. 2016;66(4):369–375. doi:10.1016/j.bjane.2014.11.010
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

