Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
Transarterial Chemoembolization Combined with Atezolizumab Plus Bevacizumab or Lenvatinib for Unresectable Hepatocellular Carcinoma: A Propensity Score Matched Study
Authors Zhao C, Xiang Z, Li M, Wang H , Liu H, Yan H, Huang M
Received 4 May 2023
Accepted for publication 20 July 2023
Published 25 July 2023 Volume 2023:10 Pages 1195—1206
DOI https://doi.org/10.2147/JHC.S418256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Chenghao Zhao,* Zhanwang Xiang,* Mingan Li, Haofan Wang, Huan Liu, Huzheng Yan, Mingsheng Huang
Department of Interventional Radiology, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, 510630, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingsheng Huang; Huzheng Yan, Tel/Fax +86-20-85253416, Email [email protected]; [email protected]
Purpose: Combined transarterial chemoembolization (TACE) and Lenvatinib (LEN) treatment (LEN-TACE) has been shown to be beneficial. We aimed to evaluate retrospectively Atezolizumab plus Bevacizumab (Atezo/Bev)-TACE compared with LEN-TACE as a first-line therapy for unresectable HCC.
Patients and Methods: From October 2020 to October 2022, data from 98 consecutive HCC patients were analyzed. After propensity score matching, two cohorts of 34 patients who received either Atezo/Bev-TACE or LEN-TACE were studied. We compared overall survival (OS), progression-free survival (PFS), duration of response, objective response rate (ORR) and disease control rate (DCR) based on RECIST 1.1 and mRECIST, as well as safety outcome between the two cohorts.
Results: The 6-month and 12-month OS rates were 85.3% (95% CI 73.5– 97.0) and 75.4% (95% CI 53.6– 85.7) in the Atezo/Bev-TACE group, and 88.2% (95% CI 76.5– 97.1) and 79.2% (95% CI 63.6– 90.9) in the LEN-TACE group, respectively. The hazard ratio for death in the Atezo/Bev-TACE group compared to the LEN-TACE group was 1.09 (95% CI 0.47– 2.51; P = 0.837). The median PFS was 7.03 months (95% CI 3.89– 10.17) in the Atezo/Bev-TACE group and 6.03 months (95% CI 0– 14.14) in the LEN-TACE group (HR 1.21; 95% CI 0.66– 2.21; P = 0.545). No significant difference in ORR and DCR between the two groups was observed either according to RECIST 1.1 or mRECIST standards. Incidence rates of hand-foot skin reaction (35.3% vs 5.9%, P = 0.003) and proteinuria (17.9% vs 2.9%, P = 0.046) were significantly higher in the LEN-TACE group.
Conclusion: Atezo/Bev-TACE and LEN-TACE showed comparable efficacy and safety as first-line therapies for unresectable HCC patients.
Keywords: atezolizumab plus bevacizumab, lenvatinib, transarterial chemoembolization, hepatocellular carcinoma, efficacy
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, the third leading cause of cancer-related death and 44% of HCC cases were attributable to chronic HBV infection, but the majority of cases occurred particularly in East Asia.1,2 More than 80% of HCC patients are diagnosed with intermediate or advanced stage disease.3,4 Lenvatinib (LEN) is one of the first-line treatments for advanced HCC and is also recommended for some intermediate HCC.5 However, efficacy of targeted treatment alone including by tyrosine kinase inhibitors (TKIs) such as LEN still have room for improvement.
Transarterial chemoembolization (TACE) is the standard treatment for intermediate HCC and the most widely administered treatment for intermediate and advanced HCC according to the BRIDGE study.6,7 Several studies combining systemic therapy with TACE have been carried out,8–10 but the LUNCH trial was the first to demonstrate in a Phase III clinical trial that systemic therapy in combination with locoregional treatment improved overall survival (OS) in patients with advanced HCC, exhibiting longer median OS and progression-free survival (PFS) (approximately 6.3 months and 4.2 months, respectively) in LEN with TACE group compared to LEN alone group.11
Atezolizumab, a programmed death-ligand 1 (PD-L1) inhibitor, plus bevacizumab, an anti-vascular endothelial growth factor (VEGF) drug, was recently added to the list of first-line treatments for advanced HCC after results of IMbrave150 proved superior efficacy and safety of such treatment compared to sorafenib alone.12 In theory, TACE could augment immune stimulation because tumor tissue necrosis that ensued embolization should induce antigen release which could lead to phenotypic adjustment among peripheral immune cells.13,14 But TACE could also lead to immune suppression by inducing hypoxic microenvironment and upregulating hypoxia-inducible factor-1α (HIF-1α) and programmed cell death protein 1 (PD-1) expression.15 Atezo/Bev treatment, on the other hand, potentially could promote tumor vascular normalization and immune motivation in a synergistic fashion, which may positively impact effect of TACE in a combined treatment scenario. Meanwhile, as huge tumor burden, portal vein tumor thrombus (PVTT), and poor liver function often observed in unresectable HCC, monotherapy tends to have limited efficacy. Given these assumptions, since LEN plus TACE has been shown to significantly improve the survival benefit of HCC patients, Atezo/Bev plus TACE may be a promising combination treatment strategy. Unfortunately, clinical effect of combined Atezo/Bev and TACE treatment has not yet been demonstrated.
Therefore, we aimed to evaluate the efficacy and safety of Atezo/Bev with TACE (Atezo/Bev-TACE) versus LEN with TACE (LEN-TACE) as a first-line therapy for unresectable HCC in a well-balanced case series.
Materials and Methods
Study Design
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University and complied with the Declaration of Helsinki 1975 (Ethical number: II2023-118-01). As it was a retrospective study, informed consent was waived, which does not compromise the welfare and rights of the patients. Non-identified information was utilized to protect patient confidentiality. We retrospectively screened the electronic medical records of 201 consecutive patients with HCC who received Atezo/Bev-TACE or LEN-TACE in our hospital from October 2020 to October 2022. These patients were considered ineligible for resection, ablation, or transplantation by our Multidisciplinary Team (MDT).
The eligibility criteria for this study included: (a) age 18–75 years; (b) diagnosis of HCC according to the European Association for the Study of the Liver (EASL), with no prior treatment or initial recurrence after radical resection without any postoperative treatment; (c) Child-Pugh classification grade A; (d) Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–1; (e) Barcelona Clinic Liver Cancer (BCLC) stages B-C; (f) receiving Atezo/Bev or LEN treatment; (g) interval between initial TACE and systemic treatment not exceeding 30 days; (h) at least one measurable lesion; and (i) no high-risk factors for esophageal-gastric variceal bleeding confirmed by endoscopy. The exclusion criteria were as follows: (a) prior systemic therapy; (b) history of hepatic decompensation, such as hepatic encephalopathy, refractory ascites, and esophageal or gastric variceal bleeding; (c) application of Atezo/Bev or LEN as second- or third-line therapy; (d) combination with other treatments; and (e) other concomitant malignancies.
Systemic Treatment
Treatment plan was determined by the MDT. Patients in the LEN-TACE group received a daily dose of 8mg (weight <60 kg) or 12mg (weight ≥60 kg), while patients in the Atezo/Bev-TACE group received an injection of Atezolizumab 1200mg and Bevacizumab 15mg/kg every three weeks. Symptomatic treatment without dose adjustment or suspension was prescribed when grade 1–2 adverse events occurred. For grade 3–4 adverse events in LEN-TACE group, dose reduction or temporary suspension was recommended depending on safety consideration and patient tolerability. Atezo/Bev was maintained at the initial dose as much as possible without dosage adjustment. Medication would be temporarily suspended though if patient could not tolerate it. When follow-up examination indicated disease progression, the treating physician determines whether to continue the initial treatment or change to second-line treatment.
TACE Treatment
The initial TACE treatment should be performed within 30 days before or after systemic treatment. Since no significant difference in efficacy between conventional TACE and drug-eluting beads-TACE (DEB-TACE) has been reported,16,17 specific method was chosen by experienced interventional radiologists based on tumor burden, macrovascular invasion, liver function, and patient tolerance from either (1) DEB-TACE: Epirubicin was loaded into 100–300 μm or 300–500 μm HepaSphere (Merit Medical, USA) or CalliSpheres (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) microspheres; or (2) Conventional TACE: Ethiodized oil (Jiangsu Hengrui Medicine Co., Ltd., Jiangsu, China) and epirubicin were thoroughly mixed into a lipiodol emulsion in a ratio of 2:1. Gelatin sponge particle was used for embolization in either case. The tumor feeding artery was catheterized super-selectively, and the chemoembolization agents were injected until the tumor feeding artery was completely embolized. Lenvatinib was suspended 1–3 days before and after TACE, and then resumed to original level once liver function has recovered. Atezolizumab/Bevacizumab was administered according to the treatment schedule without being affected by TACE.
Follow-Up and Assessments
The initial diagnosis of HCC was made based on multiphase enhanced CT or magnetic resonance imaging (MRI) according to EASL guidelines. Enhanced CT or MRI examination was performed for efficacy evaluation and laboratory test results (mainly alpha-fetoprotein, routine blood cell count, and liver-renal function panels) were recorded 4–6 weeks after the first treatment and then every 2–3 months. OS was defined as the time between first application of Atezo/Bev or LEN to the date of death from any cause or the last follow-up (March 31, 2023). PFS was defined as the time from the first use of Atezo/Bev or LEN to tumor progression or death. During the follow-up period, we also evaluated the best overall response, which was defined as the best efficacy during the period from the first use of Atezo/Bev or LEN until disease progression or recurrence, and was categorized as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to RECIST1.1 and mRECIST criteria.18,19 Two radiologists independently evaluated the response, and in cases of discordance, a third, more senior radiologist made the final determination. Accordingly, duration of response (DOR) was defined as the time from the first record of CR or PR to disease progression or death. It should be noted that the following situations were considered as PD: (a) new intrahepatic lesions or macrovascular invasion, (b) new extrahepatic metastases, and (c) increase in maximal diameter of the enhanced area of more than 20% the recorded sum of minimal diameter of target lesions. However, to be considered as CR, all target lesions and non-target lesions (including tumor thrombus) during the combined treatment period should simultaneously meet the following criteria: disappearance of arterial phase enhancement, pathological lymph nodes <10mm, and normal serum AFP. Adverse events were graded according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0.
Statistical Analysis
Quantitative variables were described as mean (standard deviation [SD]) or medians (interquartile range [IQR]) and data from the two groups were compared by Student’s t test and the nonparametric Mann–Whitney U-test. Categorical variables were described as percentages and compared between the two groups using Pearson’s χ2-test or Fisher’s exact test. To minimize the impact of confounding factors, a set of covariates that were deemed to potentially influence treatment decisions and important prognostic factors in univariate analysis were utilized (Data Supplement 1). Propensity score matching (PSM) was performed using a logistic regression model with the following variables: gender, age, etiology, ECOG, tumor number and size, extent of PVTT, extrahepatic metastasis, ALBI grade, creatinine level, platelet count, international normalized ratio (INR), and AFP level (≤200/>200 ng/mL). The nearest-neighbor matching method (caliper = 0.1) was used for 1:1 matching between the groups. Data comparison after PSM was performed based on 34 patients per matched group. The Wilcoxon paired signed rank sum test was used to analyze differences in continuous variables from baseline to follow-up. OS, PFS, and DOR were calculated using the Kaplan–Meier method, and differences were compared using the Log rank test. Subgroup analysis was based on baseline characteristics and factors potentially affecting survival outcomes. The statistical analysis was performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-sided, with p < 0.05 considered statistically significant.
Results
The study flow chart is shown in Figure 1. Among 98 consecutive patients included, 38 were in the Atezo/Bev-TACE group and 60 in the LEN-TACE group. Baseline characteristics of the patients are shown in Table 1. No significant difference was observed in baseline features between the two groups. These characteristics were further balanced in the 34-pair propensity score-matched cohorts as shown in Table 2. At the start of combination therapy, the Child-Pugh grade of both groups was grade A, with 36 patients (52.9%) in ALBI grade 1 and 32 patients (47.1%) in ALBI grade 2. In each of the two cohorts, there were 23 patients (67.6%) with PVTT. During PSM, we further matched the location of PVTT according to the Liver Cancer Study Group of Japan staging system to balance the impact of the site and extent of PVTT on treatment outcomes.20
 |
Table 1 Baseline Characteristics of Patients Before Propensity Score Matching |
 |
Table 2 Baseline Characteristics of Patients After Propensity Score Matching |
 |
Figure 1 Flowchart of the study. |
As of March 31, 2023, the median follow-up time for all patients was 18.5 months (IQR 17.5–19.5). Mortality rate was 29.4% (10) in the Atezo/Bev-TACE group and 41.2% (14) in the LEN-TACE group (HR 1.09; 95% CI 0.47–2.51; P = 0.837). Overall, there was no significant difference in OS between the two groups, as shown in Figure 2A; the 6-month and 12-month OS rates were 85.3% (95% CI 73.5–97.0) and 75.4% (95% CI 53.6–85.7) in the Atezo/Bev-TACE group and 88.2% (95% CI 76.5–97.1) and 79.2% (95% CI 63.6–90.9) in the LEN-TACE group, respectively.
As shown in Figure 2B, the median PFS was 7.03 months (95% CI 3.89–10.17) in the Atezo/Bev-TACE group and 6.03 months (95% CI 0–14.14) in the LEN-TACE group (HR 1.21; 95% CI 0.66–2.21; P = 0.545). The 6-month PFS rate was 55.9% (95% CI 38.2–73.5) in the Atezo/Bev-TACE group and 52.9% (95% CI 35.4–70.6) in the LEN-TACE group. The duration of response in both groups is shown in Figure 2C, and there was no significant difference between the two groups (HR 1.03; 95% CI 0.42–2.54; P = 0.952).
As neither OS nor PFS presented statistically significant difference between the two cohorts, we further compared the best overall response (Table 3, Figure 3). According to RECIST 1.1, the objective response rate (ORR) and disease control rate (DCR) of the Atezo/Bev-TACE group were 26.5% (95% CI 11.8–41.2) and 76.5% (95% CI 61.8–91.2), respectively, while those of the LEN-TACE group were 14.7% (95% CI 2.9–29.4) and 85.3% (95% CI 70.6–97.1), respectively (P values for ORR and DCR were P = 0.230 and P = 0.355, respectively). According to mRECIST standards, the ORRs were 61.8% (95% CI 47.1–76.5) and 58.8% (95% CI 41.3–73.5), respectively (P = 0.804); while the DCRs were 82.4% (95% CI 67.6–94.1) and 85.3% (95% CI 73.5–97.0), respectively (P = 0.742). Subgroup analysis depicted in Figure 4 also revealed no significant difference between the subgroups.
 |
Table 3 Summary of Best Overall Response |
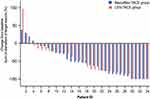 |
Figure 3 Changes in sum of diameter of target lesions according to mRECIST criteria. |
Adverse Events
Common adverse events are summarized in Table 4. No treatment-related death occurred in either group. After propensity score matching, common adverse events of any grade in the Atezo/Bev-TACE group compared to LEN-TACE mainly included elevated AST (82.4% vs 55.9%, P = 0.018), elevated ALT (61.8% vs 50.0%, P = 0.329), and abdominal pain (58.8% vs 50.0%, P = 0.465). In addition, incidence rate of hand-foot skin reaction (35.3% vs 5.9%, P = 0.003) and that of proteinuria (17.9% vs 2.9%, P = 0.046) were significantly higher in the LEN-TACE group than in the Atezo/Bev-TACE group. Notably, the incidence of hypertension (29.4% vs 26.5%, P = 0.787) and gastrointestinal bleeding (11.8% vs 17.6%, P = 0.493) were not significantly different between the two groups. Because of severe adverse events, 4 patients (11.8%) in the Atezo/Bev-TACE group suspended treatment, of whom two were due to immune thrombocytopenia. The LEN-TACE group showed a 26.5% incidence of dose reduction or interruption.
 |
Table 4 Safety Profiles and Adverse Events |
Discussion
The LAUNCH trial demonstrated that LEN-TACE improved OS compared to LEN alone.11 Such result was particularly encouraging because studies such as the STAH trial on combined TACE and targeted therapy had tried many different drugs, including sorafenib, brivanib and orantinib, but had until then failed to prove that combination therapy could improve survival.8–10,21 To our knowledge, this was the first study that reported the efficacy and safety of Atezo/Bev-TACE in comparison to LEN-TACE as a first-line therapy for unresectable HCC. After strict inclusion criteria and PSM, Atezo/Bev-TACE and LEN-TACE groups showed comparable OS, PFS, DOR, and response rates, and both demonstrated favorable and acceptable safety.
In the present study, patients in LEN-TACE group showed a median PFS of only 6.03 months, which seemed shorter than in the LAUNCH trial. It could be because the latter excluded patients with tumor diameter >10 cm, number >10, or tumor burden ≥50%. In our study, maximum tumor diameter was 97.9±42.3 mm and PVTT grade Vp-3/4 was observed in 39.7% of our patients. According to the up-to-7 criteria, 92.6% of our patients had high tumor burden, which could better reflect the real-world conditions. The median PFS of patients who met the inclusion criteria for the LAUNCH trial (ie, tumor diameter ≤10cm and ≤10 tumors) was 11.3 months, and the 6-month OS rate was 94.4% (Data Supplement 2), which was comparable to the 10.6 months and 95.9% outcome of the LAUNCH trial. These findings could be cautiously interpreted as showing insufficiency of clinical trials due to their strict inclusion criteria to represent all patients with advanced HCC seeking help and information in the real world.
Our investigation demonstrated no significant difference in OS with an HR of 1.09 (95% CI 0.47–2.51; P = 0.837) for Atezo/Bev-TACE compared with LEN-TACE. The 6-month OS rate and PFS rate in the Atezo/Bev-TACE group were 85.3% and 55.9% while in the LEN-TACE group they were 88.2% and 52.9%, respectively, which were also not significantly different. We originally deduced that the former might be more effective because the REFLECT trial did not demonstrate significant difference in OS between LEN treatment and sorafenib,22 while Atezo/Bev was shown to provide better survival benefits compared to sorafenib.12 However, other large multicenter retrospective studies have demonstrated that both have comparable clinical efficacy and safety in the real world.23,24
Previous research has demonstrated that the tumor burden impacts the survival outcomes of patients with advanced HCC.25 TACE can provide earlier disease control compared to systemic therapy, resulting in tumor debulking, although not local cure. By controlling the intrahepatic tumor burden, there is a potential to improve liver function, PFS, and allow for a longer duration of systemic therapy, ultimately enhancing the effectiveness of overall treatment.8,26 As research on anti-VEGF drug and immune checkpoint inhibitors deepens, the rationality of TACE-based combination therapy is gradually being accepted. Firstly, after TACE treatment, tumor cells induce upregulation of HIF-1α in the hypoxic environment, leading to upregulation of VEGF and platelet-derived growth factor (PDGF) expression.27,28 This may be one of the reasons why residual HCC tissue survives after TACE treatment and is rich in neovascularization. Bevacizumab, as an anti-VEGF antibody, could promote vascular normalization and improve tumor microenvironment, which could in turn promote maturation of the tumor vascular system and form a positive feedback loop.29 Secondly, ischemia post-TACE results in increased Tregs cell numbers and PD-1/PD-L1 expression, and PD-1 supports immune tolerance by inhibiting T cell activation via phosphatase SHP2 and altering the contact time of T cells with APCs or target cells.30,31 Atezolizumab, as an anti-PD-L1 antibody, activates functionally exhausted T lymphocytes and increases the density of CD4+ and CD8+ cytotoxic T cells while upregulating IFN-γ secretion and reducing the amount of VEGF. This implies that the application of Atezo/Bev after TACE could have a synergistic effect in tumor vascular normalization and stimulation of immune activation. The largest multicenter study to date (CHANCE001 study) has demonstrated enhanced local tumor control and systemic anti-tumor effects of this combined treatment modality.32
Limitations of this study included firstly that the retrospective nature of it could introduce selection bias even after we included variables potentially affecting outcomes in PSM whenever possible. Secondly, this study included patients first admitted from October 2020 to October 2022, and we evaluated PFS, 6-month, and 12-month survival, but this might have led to bias in the final results because the follow-up time was not sufficient to fully assess mortality. Thirdly, this study focused mainly on Child-Pugh A unresectable HCC patients with HBV infection, but the sample size was limited, while the applicability of our results to patients with non-viral hepatitis or poor liver function remained unknown. The preliminary results of this study were reported here, hoping to provide clinically meaningful information. We will update the long-term follow-up study data and include more sample sizes at an appropriate time. In the meantime, it is necessary to conduct additional prospective studies to further verify our findings.
In conclusion, Atezo/Bev-TACE and LEN-TACE have comparable efficacy and safety as first-line therapies for unresectable HCC in the real world. These results need to be further validated with longer follow-up time and large-scale prospective studies.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University and complied with the Declaration of Helsinki 1975 (Ethical number: II2023-118-01). Informed consent was waived in consideration of its retrospective nature and simultaneous confirmation that the data was anonymized or maintained with confidentiality.
Acknowledgments
The patients participating in this study are sincerely acknowledged.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Baecker A, Liu X, La Vecchia C, Zhang ZF. Worldwide incidence of hepatocellular carcinoma cases attributable to major risk factors. Eur J Cancer Prevention. 2018;27(3):205–212. doi:10.1097/CEJ.0000000000000428
3. Villanueva A. Hepatocellular carcinoma. N Engl, J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
4. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
5. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
6. Galle PR, Forner A, Llovet JM, et al. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
7. Park J, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
8. Park JW, Kim YJ, Kim DY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi:10.1016/j.jhep.2018.11.029
9. Kudo M, Cheng AL, Park JW, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, Phase 3 study. Lancet Gastroenterol Hepatol. 2018;3(1):37–46. doi:10.1016/S2468-1253(17)30290-X
10. Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial: HEPATOLOGY. Hepatology. 2014;60(5):1697–1707. doi:10.1002/hep.27290
11. Peng Z, Fan W, Zhu B, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase iii, randomized clinical trial (LAUNCH). J Clin Oncol. 2012;5:12.
12. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
13. Takaki H, Imai N, Contessa TT, et al. Peripheral blood regulatory T-Cell and Type 1 Helper T-Cell population decrease after hepatic artery embolization. J Vascular Interventional Radiol. 2016;27(10):1561–1568. doi:10.1016/j.jvir.2016.01.150
14. Hickey RM, Kulik LM, Nimeiri H, et al. Immuno-oncology and its opportunities for interventional radiologists: immune checkpoint inhibition and potential synergies with interventional oncology procedures. J Vascular Interventional Radiol. 2017;28(11):1487–1494. doi:10.1016/j.jvir.2017.07.018
15. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi:10.1084/jem.20131916
16. Sacco R, Bargellini I, Bertini M, et al. Conventional versus Doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vascular Interventional Radiol. 2011;22(11):1545–1552. doi:10.1016/j.jvir.2011.07.002
17. Facciorusso A. Transarterial chemoembolization: evidences from the literature and applications in hepatocellular carcinoma patients. WJH. 2015;7(16):2009. doi:10.4254/wjh.v7.i16.2009
18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
19. Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(01):052–060. doi:10.1055/s-0030-1247132
20. Ikai I, Kudo M, Arii S, et al. Report of the 18th follow-up survey of primary liver cancer in Japan: 18th follow-up survey of primary liver cancer. Hepatol Res. 2010;40(11):1043–1059. doi:10.1111/j.1872-034X.2010.00731.x
21. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
22. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
23. Kim BK, Cheon J, Kim H, et al. Atezolizumab/Bevacizumab vs. Lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers. 2022;14(7):1747. doi:10.3390/cancers14071747
24. Casadei-Gardini A, Rimini M, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: a large real-life worldwide population. Eur J Cancer. 2023;180:9–20. doi:10.1016/j.ejca.2022.11.017
25. Tanaka K, Yabushita Y, Nakagawa K, et al. Debulking surgery followed by intraarterial 5-fluorouracil chemotherapy plus subcutaneous interferon alfa for massive hepatocellular carcinoma with multiple intrahepatic metastases: a pilot study. Eur J Surg Oncol. 2013;39(12):1364–1370. doi:10.1016/j.ejso.2013.10.007
26. Yang C, Gen LY, Cai YH, Hang YZ, Li X. Effects of early TACE refractoriness on survival in patients with hepatocellular carcinoma: a real-world study. JHC. 2022;9:621–631. doi:10.2147/JHC.S373112
27. Liu K, Yang L, Zhang XM, et al. HIF-1α and VEGF levels for monitoring hepatocellular carcinoma treatment response to transcatheter arterial chemoembolization. Transl Cancer Res. 2017;6(6):1043–1049. doi:10.21037/tcr.2017.08.32
28. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi:10.1080/02841850801958890
29. Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. doi:10.1152/physrev.00038.2010
30. Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the Pd-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi:10.1084/jem.192.7.1027
31. Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR–induced stop signal. Nat Immunol. 2009;10(11):1185–1192. doi:10.1038/ni.1790
32. Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Sig Transduct Target Ther. 2023;8(1):58. doi:10.1038/s41392-022-01235-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


