Back to Journals » Journal of Hepatocellular Carcinoma » Volume 9
Transarterial Chemoebolization in Hepatocellular Carcinoma: A Binational Japanese-German Study
Authors Auer TA , Sofue K, Ueshima E, Rauer N, Yamaguchi T , Gebauer B, Hamm B, Murakami T, Althoff CE
Received 16 February 2022
Accepted for publication 23 June 2022
Published 1 August 2022 Volume 2022:9 Pages 695—705
DOI https://doi.org/10.2147/JHC.S359705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Timo A Auer,1,2 Keitaro Sofue,3 Eisuke Ueshima,3 Nina Rauer,1 Takeru Yamaguchi,3 Bernhard Gebauer,1 Bernd Hamm,1 Takamichi Murakami,3 Christian E Althoff1
1Department of Radiology, Charité - University Medicine Berlin, Berlin, Germany; 2Berlin Institute of Health (BIH), Berlin, Germany; 3Department of Radiology, Kobe University Graduate School of Medicine, Kobe, Japan
Correspondence: Timo A Auer, Department of Radiology, Campus Virchow-Klinikum, Charité – Universitätsmedizin Berlin, Augustenburger Platz 1, Berlin, 13353, Germany, Tel +49-30-450-557001, Fax +49-30-450-557901, Email [email protected]
Objective: The purpose of this study was to investigate outcomes of transarterial chemoembolization (TACE) in treating hepatocellular carcinoma (HCC) comparing the different approaches used in Germany and Japan.
Methods: This binational IRB-approved retrospective dual-center study included a total of 94 HCC patients subdivided in a German and a Japanese cohort. For each patient, liver and tumor volumetry was performed using computed tomography (CT) and magnetic resonance imaging (MRI). Furthermore, a comprehensive risk profile, including body constitution and liver and kidney function was established. Primary endpoints were progression-free and overall survival (PFS/OS).
Results: PFS in the German cohort was 168 vs 224d in the Japanese cohort (p=0.640). When subdivided by BCLC stage, no significant differences were reported (p=0.160– 0.429). OS was significantly longer in the Japanese cohort with 856 vs. 303d (p< 0.001). OS for BCLC A was significantly longer in the Japanese cohort (1960 vs. 428d; p< 0.001), while survival rates did not differ significantly in BCLC B (785 vs 330d; p=0.067) and C-stages (208 vs 302d; p=0.186). Older age (p=0.034), poorer liver/kidney function (p=0.025-0-035), and a higher liver/tumor ratio (p< 0.001) were found to correlate with shorter survival. ECOG scores were significantly higher in the German cohort (p=0.002).
Conclusion: While OS is longer in TACE-treated patients in the Japanese cohort compared to the German cohort, the two approaches seem to be equally effective as PFS does not differ significantly. The different survival rates may be caused by the different clinical performance status of the selected collectives. In very early and early stage HCC, TACE in Japan seems to be an effective treatment option while in Germany for patients in those stages TACE remains a second-line option for patients not available for surgery or ablation.
Keywords: HCC, TACE, liver, hepatocellular carcinoma, interventional oncology
Introduction
Liver cancer is among the five most common cancers worldwide and is the second most frequent cause of cancer-related death.1 Accounting for over 90% of primary liver malignancies, hepatocellular carcinoma (HCC) is by far the most common type of liver cancer, and its incidence is increasing in all populations1,2 While in Chinese and black Africans populations HCC occurs in younger patients, the incidence of HCC in Japan is highest in patients aged 70 to 79 years.3,4
Across continents and countries, the management of HCC is based on a multidisciplinary consensus to develop therapeutic strategies, diagnostic algorithms, and surveillance programs. Both Germany and Japan have highly standardized clinical practice consensus guidelines for the treatment of HCC. Current European/German guidelines for the management of HCC were last updated in 2018 as a result of a joint effort by the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) and are known as EASL guidelines.2,5 In Japan recommendations for the management of HCC are established by the Japan Society of Hepatology (JSH) through their JSH Consensus-Based Clinical Practice Guidelines and were last updated in 2017.6,7 Although the European and Japanese Guideline have much in common, there are some key differences. According to the EASL guidelines HCCs are classified using the Barcelona Clinic Liver Classification (BCLC) system.8,9 Transarterial chemoembolization (TACE) is only considered as a first-line therapy in intermediate stage asymptomatic patients without macroscopic vascular invasion or extrahepatic spread, classified as BCLC B.2,9–12 This is in sharp contrast to Japan. While subclassification of HCCs according to JSH and EASL is similar and is based on liver function, extrahepatic spread, venous invasion, and number and size of lesions, indications for TACE are different in the two countries. In Japan though TACE can be performed in BCLC A patients (with curative intentions) based on the patients will or out of technical or physical aspects (eg, severe location for local ablative therapies like radiofrequency and microwave ablation (RFA/MWA) or surgical hepatectomy).
These differences in treatment algorithms were the rationale for comparing outcomes of TACE for treating HCC according to EASL and JSH guidelines. The purpose of this study was to discuss different approaches using TACE for treating HCC in Germany and Japan.
Materials and Methods
Patients
We performed a retrospective international IRB-approved dual-center study (Ethical Approval Germany: EA4/126/19 - Ethics committee of the Charité – Universitätsmedizin Berlin; Ethical approval Japan: No. 190,173 – Ethics committee of Kobe University). A total of 94 patients were included – 50 patients in the Japanese cohort 44 patients in the German cohort. The Japanese patients underwent TACE from January 2017 to January 2018. The German cohort was matched by age and sex. Patients were enrolled from January 2011 through September 2019. Patients who were lost to follow-up or alive in September 2019 were excluded. Both cohorts were subcategorized according to the BCLC system.9 Primary study endpoints were overall survival (OS) and progression-free survival (PFS). All patients were referred for TACE by hepatologists and/or surgeons. All treatment indications were confirmed by an interdisciplinary tumor board. Institutional ethics committee approval, and written informed consent was obtained. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
An individual comprehensive risk profile was established using the following parameters (Table 1):
- Volumetric analysis of liver and tumor volume (cm3), the liver/tumor ratio, and tumor percentage based on the baseline imaging modality before initial TACE. This could have either been CT or magnetic resonance imaging (MRI).
- Body constitution including height, weight, and body mass index (BMI).
- Cirrhosis (yes/no).
- Laboratory parameters of liver function including baseline alpha-fetoprotein (AFP), albumin, and bilirubin.2,13
- Scores of liver function including the Child-Pugh score and the albumin-bilirubin (ALBI) score.9,14,15
- Laboratory parameters of renal function including creatinine and calculated glomerular filtration rate (GFR).
 |
Table 1 Patient Characteristics |
Furthermore, patients’ ECOG (Eastern Co-operative Oncology Group) performance score was captured.16
Technique
Germany
An experienced interventional radiologist performed all TACE procedures. Either conventional TACE (cTACE) or TACE with drug-eluting beads (DEB-TACE) was performed after a consensus oncologic tumor board meeting. All transarterial procedures were carried out under sterile conditions using latest-generation digital subtraction angiography units. Local anesthesia was performed with mild conscious sedation as necessary, using 2–3 mg Midazolam (Hoffmann-La Roche, Basel, Switzerland). Superselective catheterization of feeding arteries was performed with angiographic guidance. Diagnostic catheters were either 5-F Cobra (Radiofocus® Terumo, Leuven, Belgium) or 5-F SOS Omni Selective 3 (Soft-Vu® AngioDynamics, Queensbury, USA) Superselective embolization of all feeding arteries was performed with a microcatheter (Cantata® 2.5F or MikroFerret-18® 3-F, Cook Medical, Bjaeversko, Denmark or Maestro, Merit Medical Systems, Jordan, USA). For cTACE, patients were embolized selectively in a lobar or segmental fashion or superelecetively in a subsegmental fashion. An emulsion of a 5:1 mixture of doxorubicin/mitomycin B and Lipiodol (Guerbet GmbH, Sulzbach, Germany) was administered at maximum doses of 50 mg doxorubicin, 10 mg mitomycin B, and 10 mL Lipiodol. Successful chemoembolization was verified with an unenhanced CT scan acquired no later than 24 h after the procedure. If indicated, TACE was repeated every 6 to 8 weeks based on the results of contrast-enhanced multiphasic CT examinations.
Japan
The choice of cTACE or DEB-TACE was made in consensus by interventional radiologists and hepatologists according to the number, size, and distribution of lesions and the patient’s global liver function. Basically, HCC lesions within 4 tumors of 7 cm criterion were treated with cTACE, and lesions over the 4 tumors of 7 cm criterion were treated with DEB-TACE.17 Regardless of the number and size of tumors, DEB-TACE was selected when treating lesions in more than one lobe.
All procedures were performed by board-certified interventional radiologists with 14–18 years of experience. Local anesthesia was performed with 1.0% procaine. A 4-F catheter of suitable shape was advanced into the celiac trunk or common hepatic artery over the 0.035-inch guidewire through an introducer sheath inserted via the common femoral artery. A co-axial microcatheter was then advanced into the feeding arterial branches after the subsequent angiograms using iodinated contrast agent.
cTACE was performed with epirubicin mixed with Lipiodol (Guerbet, Paris, France) using a maximum dose of 50 mg epirubicin and 8 mL Lipiodol. Following chemotherapy injection, the feeding artery was embolized with gelatin sponge particles (Gelpart: Nippon-Kayaku, Tokyo, Japan). Superselective embolization was performed whenever possible, and parasitic blood vessels including inferior phrenic, intercostal, and renal capsular arteries were embolized if necessary. DEB-TACE was performed with 100–300 µm DC beads (BTG, London, UK) loaded with 50 mg of epirubicin or 50–100 µm Hepasphere (Nippon-Kayaku, Tokyo, Japan) loaded with 50 mg of fine-powder cisplatin (IA-call; Nippon-Kayaku) according to each manufacturer’s instruction.18 Embolization was performed until the angiogram showed stasis in the tumor-feeding vessels, but preserved flow in the segmental and lobar arteries. Repeated TACE was carried out until out of indication for TACE or refractory to TACE, only when follow-up multiphasic CECT/MR examinations performed every 3–4 months revealed recurrent lesions.
Follow-Up
Baseline was defined as the contrast-enhanced MRI or CT scan obtained before the interventional procedure. All patients underwent regular follow-up including clinical visits and liver MRI with Gd-EOB-DTPA or multiphasic contrast-enhanced (CE) CT, initially every six to eight weeks and afterwards every three months till recurrence. Based on the findings, the indication for repeat TACE was established. Chest CT was not routinely performed but recommended in the time period of twelve months after initial therapy. Nevertheless, when distant metastases were suspected additional chest CTs were performed. Usually, patients received routinely done chest radiographs during their clinical visit. Of course, if suspicious findings were detected chest CTs were added to rule out pulmonary metastases.
Definitions
Response to TACE was assessed using the Modified Response Evaluation Criteria in Solid Tumors (mRECIST).19 PFS was defined as the interval from the date of first TACE to any intra- or extrahepatic tumor progression. OS was defined as the interval from first TACE to the date of death.
Volumetric Assessment
Images were postprocessed with the Visage Software Tool (Visage Imaging/Pro Medicus Limited, Version 7.1.10). Tumor volume was determined by semi-automatic volumetric measurement by two readers in consensus (T.A.A. and N.R.) (Figure 1).
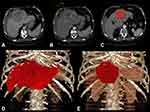 |
Figure 1 Semiautomatic volumetric assessment of a patient with a single 12cm, BCLC C HCC lesion in the left liver lobe. |
Analysis and Statistics
All statistical analyses were performed using XLSTAT (Version 2011.0.01; Addinsoft SARL, New York, USA). PFS and OS after first TACE were assessed as described above. Statistical analysis included the x2-test and contingency tables for proportional distribution. OS and PFS were assessed by means of survival plots and Kaplan–Meier curves. All p-values were calculated using the Log rank test. The Cox proportional hazard model was used for multivariate analysis of the influence of individual risk factors on survival A p-value less than 0.05 was considered statistically significant.
Results
Patients
In the total study population (n = 94), mean age was 70 years (range: 48–91); and 73.5% (69/94) patients were male and 26.5% (25/94) patients female. Nearly half of all patients, 49% (46/94), had BCLC stage B HCC, while 31% (29/94) were BCLC A and 20% (19/94) BCLC C. The proportions subclassified according to the BCLC scheme did not differ significantly between the German and the Japanese cohort (p=0.593). The TACE procedures (cTACE or DEB-TACE) did not differ significantly while the majority of both cohorts were treated with cTACE (p=0.901) (Table 1).
Survival Rates
Median OS of all patients was 502d (95% CI 372–755). OS was significantly longer in the Japanese than in the German cohort (858d; 95% CI 582d-1207d vs 303d; 95% CI 152d-430d) (p<0.001). When subdivided into the different BCLC stages, Japanese BCLC A patients had significantly longer OS than German patients (1960d; 95% CI 1273d-2643d vs 177d; 95% CI 110d-626d) (p<0.001). For BCLC B and C subgroups, no significant difference was reported (p = 0.067–0.186). Median PFS was 205d for all patients (95% CI: 168–250) and did not differ significantly between the two cohorts (224d; 95% CI: 175d-371d vs 168d; 95% CI: 123d-250d) (p<0.640). There were also no significant differences between subgroups with different BCLC stages (p=0.160–0.429) (Figure 2).
Comprehensive Risk Profile Analysis
Comprehensive risk profiles were established to assess the influence of individual risk factors on OS and PFS. The influence of each risk factor was tested by means of the Cox proportional hazard model. In the entire collective the following parameters were associated with shorter OS: older age (p=0.034; HR: 4.49); an elevated Child-Pugh score (p=0.029; HR: 4.77); low serum albumin (p=0.035; HR: 4.44); a restricted GFR (p=0.025; HR: 4.99); and a lower liver/tumor ratio (p<0.001; HR: 12.43). The following parameters were associated with shorter PFS: older age (p=0.017; HR: 5.96); absence of cirrhosis (p=0.027; HR: 4.89); low serum albumin (p=0.011; HR: 6.46); a low ALBI score (p=0.029; HR: 4.75); low serum creatinine (p=0.049; HR: 3.89); and a restricted GFR (p=0.035; HR: 4.46). When multivariate Cox proportional hazard model analysis was performed separately for each cohort, the following parameters differed:
OS
- Absence of cirrhosis: Japanese cohort: p=0.043 (HR: 4.10); German cohort: p=0.503 (HR: 0.45).
- Low serum albumin: Japanese cohort: p=0.964 (HR: 0.02); German cohort: p=0.047 (HR: 3.94).
PFS
- ALBI score: Japanese cohort: p=0.538 (HR: 0.38)); German cohort: p=0.026 (HR: 4.94)).
- Low GFR: Japanese cohort: p=0.181 (HR: 1.79)); German cohort: p=0.029 (HR: 4.75)).
- Liver volume: Japanese cohort: p=0.895 (HR)); German cohort: p=0.026 (HR: 3.50)).
- Tumor volume: Japanese cohort: p=0.617 (HR: 0.26)); German cohort: p=0.022 (HR: 5.24)).
- Tumor volume percentage: Japanese cohort: p=0.440 (HR: 0.60)); German cohort: p=0.032 (HR: 4.58).
P-values for all parameters and the Cox proportional models performed for each cohort are displayed in Table 2 (Table 2).
 |
Table 2 Multivariate Cox Regression Analysis |
Distribution of ECOG-scores differed significantly (p=0.002) between the two cohorts (Table 1):
Japanese cohort: ECOG: 0=28x; 1=17x; 2=3x; 3=1; 4=0x; 5=0x.
German cohort: ECOG: 0=11x; 1=16x; 2=11; 3=5; 4=0x; 5=0x.
Discussion
In both Germany and Japan, clinicians can rely on highly standardized clinical practice consensus guidelines for the management of HCC. In this study, we found a significantly longer overall survival in the Japanese cohort than in the German cohort. When subdivided by the different BCLC stages, only BCLC A patients in Japan had a significant longer survival rate. BCLC B and C subgroups showed no significant difference, although survival was generally longer in the Japanese patients. PFS rates did not differ significantly between the total cohorts of German and Japanese patients or when subdivided by BCLC stage.
Survival rates in general when subdivided into BCLC stages are controversial. In patients with intermediate-stage HCC (BCLC B), our results are in line with published studies, which reported survival rates of approx. 16 months for multinodular liver tumors without vascular invasion or extrahepatic tumor manifestation. At this point, it should be mentioned that overall survival rates may be influenced negatively in this study as death was the primary endpoint accepted.11,20
According to the Japanese Society of Hepatology (JSH) consensus guidelines, TACE can be performed in HCC patients who would have been classified stage A according to the BCLC system. Our results are equal when compared to the data reported in the EASL guidelines, indicating TACE to be a treatment option also in early HCC.2,7,21
In the German cohort, median survival of the BCLC A subgroup was superior to that reported elsewhere (eg, EASL criteria).2 There are several reasons for this, which may also explain why median OS in general, also in BCLC B and C patients, was longer in the Japanese as compared to the German cohort. According to the EASL guidelines, patients with BCLC A are most likely to achieve optimal outcomes and are treated with the purpose of achieving long-term cure. The first-line therapies for these early-stage patients are resection, ablation, and transplantation.2 Accordingly, when TACE is performed in BCLC A patients in the German cohort, this means a pre-selection of a high-risk multimorbid subpopulation not suitable for resection or ablation and no chance for a liver transplant.2 This might explain the low survival rate of 428d compared to nearly 2000d in the Japanese cohort where TACE seems to be performed more frequently at this stage of disease. It still remains challenging though to compare TACE at this stage to RFA or hepatic surgery as there are no large number of randomized trials. The median survivals though in patients staged BCLC 0 and A range from three years to over five and more than seven in patients treated with either ablation or surgery.2,22,23 As our cohort summarized early and very early staged HCCs, the reported median survival of 1969d (about 5,3 years) in the Japanese cohort has led to the consumption that TACE is in effective treatment alternative, although this study's aim was not to compare TACE to ablation or hepatic resection. This data could be an interesting impulse for treatment regimes in Germany where TACE in those stages remains a second-line option for patients not available for surgery or ablation.
Yet, although TACE is indicated as a first-line therapy in intermediate BCLC B patients, according to EASL, we may expect that, in Germany, these are also patients with a higher risk than BCLC B patients who undergo TACE in Japan. Here, TACE is not indicated as curative treatment but to downstage patients and make them eligible for resection or transplantation.2,24 These assumptions are substantiated by our finding that PFS did not differ significantly across all BCLC stages in both cohorts. Our results suggest that TACE is also highly accurate and effective in early HCC as performed in Japan.
Another aspect to be considered in interpreting our results for the German patients is that at the German center TACE in combination with CT-guided high-dose rate brachytherapy (CT-HDBRT)25 is performed as an alternative to TACE alone. These patients had to be excluded from our present analysis, further negatively pre-electing the German cohort investigated in this retrospective study. This is supported by evaluation of the ECOG scores as the German cohort averages significantly higher ECOG scores as the Japanese cohort. An explicit consensus regarding the chemotherapeutic agents and embolic agents used for TACE is still lacking.2,26,27 Furthermore, there is an ongoing discussion regarding the potential benefits of cTACE vs DEB-TACE. Recent studies suggest that there is no significant difference in overall survival. However, the significantly lower number of treatments needed in the DEB-TACE group makes it a more appealing treatment option than cTACE for appropriately selected patients with unresectable HCC.28 According to the EASL criteria, the most common drugs used for conventional TACE, either as single agents or in combination, are doxorubicin, epirubicin, cisplatin, and miriplatin.2,26 In Japan, the number of chemotherapeutic agents is limited due to strict approval processes. Nevertheless, the main chemotherapeutic drugs used in this study are comparable as both are anthracyclines. In the German cohort, TACE was performed with an additional low dose of mitomycin.
Secondly, we investigated a comprehensive risk profile to identify clinical prognostic factors affecting survival. Beside the tumor volume, liver function, and alpha-fetoprotein, we assessed the ALBI score and parameters which may indicate end-stage liver disease such as body constitution and kidney function.2,9,13–15 As expected, the ALBI score was associated with shorter PFS as it is well established that the degree of underlying liver dysfunction is an important factor determining both OS and PFS.29
Limitations
Our study has several limitations including the retrospective study design and small numbers of patients. Moreover, all readers of imaging datasets were aware of this design, introducing a potential detection bias. Furthermore, the multivariate analysis may be not free for bias as some parameters (Child-Pugh Score, Albi-Score, etc.) correlated within themselves. It is worth mentioning that the overall survival rates may be influenced negatively as death was the primary endpoint accepted.
Conclusion
The main difference in the management of HCC between Europe and Japan is that TACE in Europe is most widely used in unresectable intermediate HCC for downstaging, so that patients can then be operated on, while TACE in Japan can be performed routinely at nearly any stage of disease and primarily with curative intention. Accordingly, OS is longer in TACE-treated patients in Japan than in patients undergoing TACE in Germany. The clinical performance status of Japanese patients is superior as those in Germany and could affect OS significantly. Nevertheless, both approaches seem to be equally effective as PFS after TACE does not differ between the two countries. Moreover, our study suggests that TACE in Japan is also effective in very early and early stage HCC and represents an effective treatment alternative. The results could be an interesting impulse for treatment regimes in Germany, where TACE in those stages remains a second-line option for patients not available for surgery or ablation.
Abbreviations
AFP, alpha-fetoprotein; ALBI score, albumin and bilirubin score; BCLC, Barcelona clinic liver classification; BMI, body mass index; CT, computed tomography; CT-HDRBT, CT-guided high-dose-rate brachytherapy; DSA, digital subtraction angiography; HCC, hepatocellular carcinoma; EASL, European Association for the Study of the Liver; EORTC, European Organization for Research and Treatment of Cancer; GFR, glomerular filtration rate; JSH, Japanese Society of Hepatology; MWA, microwave ablation; MRI, magnetic resonance imaging; mRECIST, modified response evaluation criteria in solid tumors; RFA, radio frequency ablation; TACE, transarterial chemoembolization; cTACE, conventional TACE; DEB-TACE, drug-eluting beads TACE; OS, overall survival; PFS, progression-free survival.
Acknowledgments
- The project was supported by the exchange program by the German Japanese Radiological Affiliation (GJRA).
- The project was part of the Doctoral Thesis from cand. med. Nina Rauer.
- Dr. med. Timo A. Auer is participant in the BIH-Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health.
- The authors thank Bettina Herwig for language editing.
- The abstract of this paper was presented at the CIRSE Summit 2020 as an electronical abstract. The abstract was published in the CIRCE Summit 2020 Book of Abstracts: CIRSE 2020 Summit – Book of Abstracts. Cardiovasc Intervent Radiol 43, 127–459 (2020) https://doi.org/10.1007/s00270-020-02606-2.
Disclosure
Bernhard Gebauer reports personal fees from Parexel/CALXY, personal fees from BD, personal fees from COOK, personal fees from AngioDynamics, personal fees from Pharmcept, personal fees from Ewimed, personal fees from Novartis, personal fees from Inari, personal fees from Roche, personal fees from Merck, personal fees from MSD, personal fees from ICON, personal fees from IPSEN, personal fees from Bayer, personal fees from Pfizer, personal fees from Guerbet, personal fees from Terumo, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3(12):1683–1691. doi:10.1001/jamaoncol.2017.3055.
2. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
3. Tanaka H, Imai Y, Hiramatsu N, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med. 2008;148(11):820–826. doi:10.7326/0003-4819-148-11-200806030-00004
4. Qiu D, Katanoda K, Marugame T, Sobue T. A Joinpoint regression analysis of long-term trends in cancer mortality in Japan (1958-2004). Int J Cancer. 2009;124(2):443–448. doi:10.1002/ijc.23911
5. European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi:10.1016/j.jhep.2011.12.001
6. Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339–364. doi:10.1159/000327577
7. Kudo M, Matsui O, Izumi N, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014;3(3–4):458–468. doi:10.1159/000343875
8. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. doi:10.1016/S0140-6736(11)61347-0
9. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
10. Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11(9):525–535. doi:10.1038/nrclinonc.2014.122
11. Raoul JL, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212–220. doi:10.1016/j.ctrv.2010.07.006
12. Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi:10.1111/liv.12818
13. Ikai I, Arii S, Kojiro M, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101(4):796–802. doi:10.1002/cncr.20426
14. Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–558. doi:10.1200/JCO.2014.57.9151
15. Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–346. doi:10.1016/j.jhep.2016.09.008
16. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. doi:10.1097/00000421-198212000-00014
17. Yamakado K, Miyayama S, Hirota S, et al. Prognosis of patients with intermediate-stage hepatocellular carcinomas based on the Child-Pugh score: subclassifying the intermediate stage (Barcelona Clinic Liver Cancer stage B). Jpn J Radiol. 2014;32(11):644–649. doi:10.1007/s11604-014-0358-1
18. Seki A, Hori S. Switching the loaded agent from epirubicin to cisplatin: salvage transcatheter arterial chemoembolization with drug-eluting microspheres for unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2012;35(3):555–562. doi:10.1007/s00270-011-0176-0
19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
20. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi:10.1053/jhep.2003.50047
21. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30(1):61–74. doi:10.1055/s-0030-1247133
22. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010;51(4):1284–1290. doi:10.1002/hep.23466
23. Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol. 2010;10:78. doi:10.1186/1471-230X-10-78
24. Schlesinger S, Aleksandrova K, Pischon T, et al. Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol. 2013;24(9):2449–2455. doi:10.1093/annonc/mdt204
25. Collettini F, Schreiber N, Schnapauff D, et al. CT-guided high-dose-rate brachytherapy of unresectable hepatocellular carcinoma. Strahlentherapie Onkologie. 2015;191(5):405–412. doi:10.1007/s00066-014-0781-3
26. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
27. Brown DB, Pilgram TK, Darcy MD, et al. Hepatic arterial chemoembolization for hepatocellular carcinoma: comparison of survival rates with different embolic agents. J Vascular Interventional Radiol. 2005;16(12):1661–1666. doi:10.1097/01.RVI.0000182160.26798.A2
28. Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15:465. doi:10.1186/s12885-015-1480-x
29. Waked I, Berhane S, Toyoda H, et al. Transarterial chemo-embolisation of hepatocellular carcinoma: impact of liver function and vascular invasion. Br J Cancer. 2017;116(4):448–454. doi:10.1038/bjc.2016.423
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

