Back to Journals » Journal of Pain Research » Volume 15
Tracking the Temporal Footprint Effect of Thermonociception and Denervation on the Brain’s Pain Matrix: fMRI and BOLD Study in Rats
Authors Pellicer F, Ortega-Legaspi JM, Martín R , Solís-Nájera S , Magis-Weinberg L, León-Olea M, Graff-Guerrero A, de la Fuente-Sandoval C , Alfredo O Rodriguez
Received 17 November 2021
Accepted for publication 11 February 2022
Published 30 March 2022 Volume 2022:15 Pages 857—865
DOI https://doi.org/10.2147/JPR.S349840
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Supplementary video 1 of "Thermonociception and denervation on the brain’s pain matrix" [ID 349840].
Views: 142
Francisco Pellicer,1 Juan M Ortega-Legaspi,2 Rodrigo Martín,3 Sergio Solís-Nájera,4 Lucía Magis-Weinberg,5 Martha León-Olea,6 Ariel Graff-Guerrero,7,8 Camilo de la Fuente-Sandoval,9 Alfredo O Rodriguez3
1Laboratorio de Neurofisiología Integrativa, Dirección de Investigaciones en Neurociencias, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, CDMX, México; 2Department of Medicine, Division of Cardiovascular Medicine, University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, USA; 3Departamento de Ingeniería Eléctrica, Universidad Autónoma Metropolitana Iztapalapa, CDMX, México; 4Departamento de Física, Facultad de Ciencias, Universidad Nacional Autónoma de México, CDMX, México; 5Department of Psychology, University of Washington Guthrie Hall (GTH), Seattle, WA, USA; 6Departamento de Neuromorfología Funcional, Dirección de Investigaciones en Neurociencias, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, CDMX, México; 7Brain Health Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada; 8Department of Psychiatry, University of Toronto, Toronto, ON, Canada; 9Laboratorio de Psiquiatría Experimental, Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez, CDMX, México
Correspondence: Francisco Pellicer, Dirección de Investigaciones en Neurociencias, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz, Calzada México Xochimilco 101, San Lorenzo Huipulco, Alcaldía Tlalpan, CDMX, 14370, México, Tel +52 55 41605063, Email [email protected]
Objective: Pain constitutes an essential alarm for preserving the organism’s integrity. Damage to the nervous system produces a pathological condition known as neuropathic pain.
Purpose: Blood oxygenation level-dependent (BOLD) and functional magnetic resonance imaging (fMRI) have been widely used to map neuroanatomy and the active regions of interest (ROI) of nociceptive processing. Our study explored the brain’s BOLD response in rats after thermal noxious stimulation, immediately after sciatic nerve damage and during 75 minutes after surgical lesion of the sciatic nerve.
Methods: Nine male Wistar rats were tested; the experiments were performed on a 7-Tesla /21-cm Varian Agilent system. This approach allowed, for the first time, to measure in vivo the BOLD changes in brain regions involved with the pain process: cingulated (ACC), somatosensory (S1), and insular cortices (IC), as well as thalamus (Th) and ventral tegmental area (VTA) related with acute thermal pain and during the early stages of sciatic denervation that produce neuropathic pain.
Results: During thermonociception scan, all subjects showed BOLD activation in the ROIs determined as ACC, S1, Th, IC and VTA. After denervation, these regions continued to show activation with a slow decrement in intensity for the duration of the experiment. The results suggest that these brain structures are overactive during the genesis of neuropathic pain.
Conclusion: The study shows for the first time continuous activation of the pain matrix following an acute thermal nociceptive stimulus followed by neuropathic damage. These results have given insight into the early stages of the development of neuropathic pain in vivo.
Keywords: fMRI, resting state, neuropathic pain, pain matrix, chronic pain, acute pain
Introduction
Pain is a complex experience that encompasses sensory, motor, autonomic and affective spheres. The experience of pain constitutes an essential alarm necessary to preserve body integrity and is key for survival. However, pain can become pathological when it stops working as an alarm and turns into an illness on its own. In this category belongs neuropathic pain, which is caused by direct damage to the somatosensory nervous system, and also involves time as a factor in the evolution to chronicity.1 This kind of pathological pain is highly disabling and represents a major distress to the individual and an important burden to the healthcare system, both socially and economically.2,3
Pain is encoded and processed in the central nervous system by a series of structures that activate or deactivate in relationship with the anticipation and processing of a harmful stimulus, instead of the concept of only one that would play the role of a pain center in the brain. The evidence obtained by pain activation in specific regions of interest (ROI) comes first from human brain neuroimaging which led to the concept of a pain matrix. Such matrix is defined as a group of brain structures that are activated with painful stimuli. The level of activation or contribution of structures in the phenomenon of pain varies, however, these structures consistently include the prefrontal cortex (PFC), the mid and anterior insulae (IC), the anterior cingulate (ACC), prefrontal and posterior parietal areas.4,5 Other areas have been described related to the affective, cognitive and somatic components of the painful experience. Some of these include the thalamus (Th), the primary (S1) and secondary (S2) somatosensory cortices, and the dorsolateral prefrontal cortex.6–11 These same structures have also been reported to be activated in neuropathic pain.12 Furthermore, there is evidence from a neuropathic pain model in the rat that identifies the role of the ventral tegmental area (VTA) as a site of inhibitory modulation that is exerted through its monosynaptic projections in structures such as ACC, IC. by this. We, therefore, included the VTA as a region of interest in this study.13,14
In experiments developed later, the same brain structures have been observed in animal models of pain with acute diverse noxious stimuli (electrical, chemical, thermal and mechanical) employing functional magnetic resonance imaging (fMRI),15–23 in combination with blood oxygenation level-dependent (BOLD), have been a fundamental tool for the study of brain areas related to the processing of different sensory stimulation.24 It has been documented by Zhao’s group21–23 that the increase in the level of oxygenation detected in the different brain ROIs is related to the frequency of nociceptive neuronal stimulation, which weighs and validates with high specificity and sensitivity these changes in intensity related to the stimulation.
To the best of our knowledge, no research has studied brain changes associated with the transition from acute pain to neuropathic pain in vivo with fMRI and BOLD. Therefore, our current study was aimed at describing the dynamics of activation of the pain matrix over time after an acute thermal stimulus and after the induction of neuropathic pain by sciatic denervation in the rat.
Materials and Methods
Subjects
Nine male Wistar rats were tested but only five (mean weight 250 g) were included in the analysis due to excessive head motion in the scanner in the other four rats.
Animal Preparation
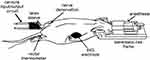
All experiments were conducted adhering to the Ethical Guideline Regulation of the International Association for the Study of Pain (IASP) and approved by the local ethical and scientific institutional review board at the Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz in Mexico City; (Project: INPRFM-NC-3230 for FP). Vital signs were constantly monitored: body temperature was measured by a rectal probe and maintained at 37 ± 0.5 °C during all measurements by means of a thermal isolation device, respiration was monitored by a balloon secured on the rat’s chest and a pressure transducer as well as an electrocardiogram were used to monitor (model 1025, SA Instruments, Inc., Stony Brook, NY., USA). The animals were continuously anesthetized with isoflurane 2.5% in a mixture of O2 via a nosecone for the entirety of the experiment. Once anesthetized, they were fixed in a prone position in a rat cradle equipped with a stereotactic-like head holder. Before positioning the animals in the scanner, a nylon filament (3–0) was fixed around the left sciatic nerve to perform the denervation. Also, in the same ankle, an input/output cannula circuit to a latex sleeve enveloping the left rear ankle was placed.
Scanner
The experiments were performed on a 7-Tesla/21-cm Varian Agilent system (Agilent, Palo Alto, CA), equipped with Direct Drive technology and a transceiver 16 rung birdcage coil (16 cm long and a 6.5 cm diameter).
fMRI and Forepaw Stimulation Protocol
Subjects participated in a single 3-hour scanning session. During each run, the animal was subjected to a non-stimulating period, for 5 min (Resting); thermal noxious stimulation, sciatic denervation, and followed for 75 minutes thereafter. Thermonociception and denervation were performed inside the fMRI bore. For thermonociception, a bolus of petrolatum at 60°C was applied to the left hind paw through the input/output cannula circuit. The denervation was carried out 30 minutes following thermonociception by pulling the previously placed nylon filament in the same hind paw (Figure 1).
Image Pre-Processing
The data were pre-processed and analyzed using SPM Mouse. All functional images were realigned to the first session volume using a six-parameter rigid body transformation, and a mean image was created. Data from animals that showed movement of greater than 2 mm on any axis were discarded (4 animals discarded). The mean image generated was employed as the template (source) for the co-registration into each animal anatomical image. Computed transformation parameters were applied to all functional images, interpolated to isotropic voxels of 0.5 x 0.5×0.5 mm, and the resulting images were smoothed using an 0.3 mm full-width half-maximum (FWHM) isotropic Gaussian kernel.
Whole-Brain Data Analysis
Before all stimulation, resting-state BOLD brain activity was obtained for each subject and ROI (vide infra). All comparisons were done against this measurement. Fifteen minutes after the resting-state scan, the animals received thermonociception (vide supra) and a second acquisition time was obtained at the same time (Thermo scan). Fifteen minutes after thermonociception, a third acquisition time was taken to see the state of activation of all the ROIs previous to sciatic denervation (Pre-denerv scan). Following this acquisition, the subjects were denervated with acquisition times at 0, 15, 30, 45, 60 and 75 minutes. A 1-sample t-test was performed for each subject at each acquisition time, to detect the voxels with higher signal intensity over the mean for the 11 volumes encompassing each acquisition.
ROI Analysis
The raw fMRI signal intensity for the right ACC, right S1, right Th, right IC and VTA was extracted. ROIs were defined from significant clusters in the pain vs plain contrast, for the purpose of signal characterization, with the anatomical reference of Paxinos atlas.25
The T-images obtained from this analysis were overlaid into the structural MRI images of each subject for anatomical localization. ROIs were defined from the activation maps as the highest T-values voxels of activation (those which pass the criteria p <0.05) circumscribed in 2 mm3 cubes encompassing each locus (ACC, Th, S1, IC, VTA).
Statistical Analysis
The statistical threshold for the a priori regions (pain neuromatrix) was set at p = 0.03 level of significance (t > 1.88) and the peak survived a family-wise error (FWE) correction for multiple comparisons of p = 0.05. Behavioral normal data were analyzed using repeated measures (ANOVA) in R. For non-normal data (VTA and S1), a Friedman Repeated Measures Analysis of Variance on Ranks was used.
Results
All results were compared to BOLD activity, in the resting state, prior to thermonociception stimulation. We did not find BOLD activity during resting state in ACC, Th, IC and VTA, with the exception of S1, in 2 subjects, that have activated in this scan (see Figure 2, IC Resting). During the thermonociception scan, all subjects showed BOLD activation in the ROIs determined as ACC, S1, Th, IC and VTA (see Figure 2, Thermo).
The pre-denervation scan showed, in general, a decrease in BOLD activity with the exception of ACC that remained activated in this trial (see Figure 2, ACC Pre-denerv). The BOLD activity in post-denervation trials depicted an increase in ACC and S1 which was maintained and tended to decrease discreetly and progressively up to 75 min. For the remaining structures, a progressive decrease was observed after denervation (see Figure 2, T = 0-T = 75). The numerical values of the BOLD activation along the time for each structure can be seen in Figure 3. The only statistical significance within group in the post-denervation trials was present in the ACC and Th (ACC, F (5) = 5.31, p= 0.003, S1, Chi Sq (6) = 13.75, p = 0.032 and Th, F (5) = 4.55, p = 0.003). In these cases, there was a significant difference between t = 0 and t = 75.
The BOLD activation was predominantly present in the right hemisphere of the subjects, contralateral to the left hind limb which received the noxious stimuli (Figure 4). In particular, the ACC showed bilateral activation; see the reconstruction of the volume in 3D motion (Supplementary Materials).
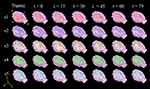 |
Figure 4 Cerebral volume reconstructions of BOLD activation maps in response to noxious thermonociception and sciatic denervation Cerebral reconstruction in 3D of false colour images of changes in BOLD signal of each subject (subject 1 to 5, in rows), thermal nociception (t = −15), immediate sciatic denervation (t = 0) and post-denervation every 15 minutes (t =15, 30, 45, 60 and 75). The BOLD activation appeared predominantly in the right hemisphere; except for the ACC, which occurred bilaterally. Representative videos for times −15, 0, 30 and 75 min may be accessed in Supplementary materials. Abbreviations:A, anterior; P, posterior; R, right; L, left. |
Interestingly, the number of activated voxels was highly consistent for the thermal noxious stimulation, acute nerve lesion, and post-denervation state.
Discussion
The temporal course of pain activation, induced by injury in the nervous system, is a key component to define the transition from acute to early phases of neuropathic pain. Our study illustrates the brain changes in BOLD signal intensity after nerve damage in the pain matrix and other related structures. The approach employed in this study, BOLD and fMRI techniques, allowed us to compare these changes between acute thermonociception and acute nerve damage (denervation).26,27
The results showed, in the resting state, discrete activity in S1, We believe that this is due to the surgical intervention performed in the experimental subjects for the dissection of the sciatic nerve; the other structures did not show BOLD activity. This situation gave us a stable resting platform to compare the activity produced by thermonociception and denervation.
Thermonociception induced an important BOLD activity in both regions of the ACC (right and left) as well as in the rest of the structures, but predominantly in the right side, that is, contralateral to the stimulation side. It is important to highlight that practically all the structures, except for the ACC, returned to the baseline condition exemplified in the Pre-denerv scan, as can be seen in Figure 2. This indicates that the end of this noxious stimulation also ends the BOLD activity in these regions, with the exception of the ACC, which remains activated and starts actively at the time of denervation.
Denervation produced a significant increase in BOLD activity in all structures compared to the resting state and also presented sustained activity over 75 minutes with a tendency to gradually decrease. This suggests that the damage to the sciatic nerve generates a long-term activation spot.
A possible explanation for this physiology is the ectopic generation of nerve activity as a result of the accumulation of Na+ voltage-dependent channels in the proximal cut ending of a peripheral nerve (neuroma).28 It is also noteworthy that the time period between acute thermonociceptive stimulation and denervation showed increased BOLD signal in the ACC and S1 but decreased practically to zero in the Th, IC and VTA. This denotes the more defined role of a consistent structure of the pain matrix as opposed to those that could be activated by other stimuli.4 After denervation, all structures were continuously activated for 75 minutes which shows the different physiology behind acute and neuropathic/chronic processes. Although the thalamus and the VTA are not considered to be part of the pain matrix, these structures were significantly activated post denervation. In previous work,14 our group provided evidence of participation of the VTA and its monosynaptic dopaminergic projections involved in nociception and pain modulation. In the same way, it has been reported that the pulvinar nucleolus of the thalamus is a locus of multisensory integration including touch, proprioception and pain.29 Certainly, our experiment does not have the anatomical resolution to identify parts of the thalamus or sectors of the VTA to determine the sub-regions that are activated, but it is important to take into account these structures for future studies related to types of pain. Moreover, receptor expression changes have been documented in structures belonging to the pain matrix in a similar neuropathic model. However, these changes have been seen after seven days and the temporal rate of these changes is not fully understood.30–33 Our results show immediate and long activation (at least 75 minutes) of the pain matrix after denervation. There are several propositions as well as experimental evidence that could explain the installment processes of neuropathic pain after denervation. Neuronal activity immediately following nerve damage and the consequent nociception is also not fully understood. In order to explain this phenomenon, a spontaneous ectopic discharge caused by the traumatic event has been proposed. There is evidence that suggests that this early activity may be due to the activation of Aβ fibers, which normally do not codify to nociception transmission, but the over-activation could produce a “painful” sensation.34 Despite this, there is no definitive experimental evidence that gives an answer to the genesis of the phenomenon. On the other hand, our group has provided experimental evidence of the role of cholinergic pathways and receptors related to what has been coined as “pain memory”35 and its process of consolidation. We showed a functional relationship between nociception-related memory and ACC is susceptible to being modified by a scopolamine microinjected into ACC in a long-lasting animal pain mode.36 Additionally, there is evidence of receptor expression changes after long-term nociception in the ACC using a similar neuropathic pain model in the rat.31
Another important finding is that acute nerve damage matches that of a thermonociception topological substrate. It is worth noting that the quantitative ROI analysis shows that the increase in intensity is not related to the physical expansion of the cerebral area involved in different types of pain processing. In this line of evidence, a study by Zhao23 showed that BOLD response had a direct relationship with electric nociceptive stimulation frequency. Considering that our results showed a sustained increase in BOLD values along 75 minutes, it can be inferred that denervation activates a frequency generator that is fairly constant and maintains the nociceptive state.37 Further research is needed to support this statement.
Until now, nociception used BOLD and fMRI techniques studies in humans have been extensively documented.6,7,10,11 In animals, BOLD and fMRI studies have been performed to explore brain changes immediately after injury.16,20,23,38 Our study evaluated changes in BOLD response during and throughout the first minutes after direct and experimentally controlled nerve damage. Our study is the first that provides a resting state approach to the early stages of neuropathic pain development.
The available treatments for neuropathic pain are still partially effective and are expensive. Our experimental approach opens the possibility to study the genesis of neuropathic pain conditions such as phantom pain and to evaluate alternative and more effective treatment targets for chronic and neuropathic pain.
Conclusion
The study shows for the first time continuous activation of the pain matrix following an acute thermal nociceptive stimulus followed by neuropathic damage (sciatic denervation). Both of these activations were similar topographically but it was proven that neuropathic nociception would trigger a constant long-lasting activation, rather than one that would wean down over 75 minutes. These results have given insight into the early stages of the development of neuropathic pain in vivo.
Abbreviations
fMRI, functional magnetic resonance imaging; BOLD, blood oxygen level-dependent; ACC, anterior cingulate cortex; PCC, posterior cingulate cortex; IC, insular cortex; Th, thalamus; VTA, ventral tegmental area; S1, primary somatosensory cortex; S2, secondary somatosensory cortex.
Acknowledgments
This project was partially supported by CONACyT Grant 62433 and INPRF Grant NC 3230 for F P.
We want to thank Suneeta Singh Carbone for the preparation for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Merskey H, Bogduk N. Pain terms a current list with definitions and notes on usage. In: Merskey H, Bogduk N, editors. Pain. Vol. 24. Seattle: IASP Press; 1986: S215–S221. doi:10.1016/0304-3959(86)90113-2.
2. Doth AH, Hansson PT, Jensen MP, Taylor RS. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149(2):338–344. doi:10.1016/j.pain.2010.02.034
3. O’Connor AB. Neuropathic pain: quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27(2):95–112. doi:10.2165/00019053-200927020-00002
4. Garcia-Larrea L, Peyron R. Pain matrices and neuropathic pain matrices: a review. Pain. 2013;154(SUPPL. 1):S29–S43. doi:10.1016/j.pain.2013.09.001
5. Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. Neurophysiol Clin. 2000;30(5):263–288. doi:10.1016/S0987-7053(00)00227-6
6. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463. doi:10.1016/j.ejpain.2004.11.001
7. Davis KD. The neural circuitry of pain as explored with functional MRI. Neurol Res. 2000;22(3):313–317. doi:10.1080/01616412.2000.11740676
8. Graff-Guerrero A, González-Olvera J, Fresán A, Gómez-Martín D, Méndez-Núñez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Cogn Brain Res. 2005;25(1):153–160. doi:10.1016/j.cogbrainres.2005.05.002
9. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Hypn Theory Res Appl. 2017;277:345–348. doi:10.4324/9781315252858-35
10. Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15(4):478–487. doi:10.1016/j.conb.2005.06.010
11. Treede RD, Kenshalo DR, Gracely RH, Jones AKP. The cortical representation of pain. Pain. 1999;79(2–3):105–111. doi:10.1016/S0304-3959(98)00184-5
12. Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37(SUPPL. 1):80–88. doi:10.1016/j.neuroimage.2007.03.054
13. López-Avila A, Coffeen U, Ortega-Legaspi JM, Del Ángel R, Pellicer F. Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain. 2004;111(1–2):136–143. doi:10.1016/j.pain.2004.06.010
14. Pellicer F, Ortega-Legaspi J, López-Avila A, Coffeen U, Jaimes O. Dopamine pathways and receptors in nociception and pain. In: Pharmacology Pain. IASP Press; 2010:241–253.
15. Endo T, Spenger C, Hao J, et al. Functional MRI of the brain detects neuropathic pain in experimental spinal cord injury. Pain. 2008;138(2):292–300. doi:10.1016/j.pain.2007.12.017
16. Hess A, Sergejeva M, Budinsky L, Zeilhofer HU, Brune K. Imaging of hyperalgesia in rats by functional MRI. Eur J Pain. 2007;11(1):109. doi:10.1016/j.ejpain.2006.01.005
17. Luo Z, Yu M, Smith SD, et al. The effect of Intravenous Lidocaine on brain activation during non-noxious and acute noxious stimulation of the forepaw: a functional magnetic resonance imaging study in the rat. Anesth Analg. 2009;108(1):334–344. doi:10.1213/ane.0b013e31818e0d34.
18. Shih YYI, Chen YY, Chen CCV, Chen JC, Chang C, Jaw FS. Whole-brain functional magnetic resonance imaging mapping of acute nociceptive responses induced by formalin in rats using atlas registration-based event-related analysis. J Neurosci Res. 2008;86(8):1801–1811. doi:10.1002/jnr.21638
19. Thompson SJ, Bushnell MC. Rodent functional and anatomical imaging of pain. Neurosci Lett. 2012;520(2):131–139. doi:10.1016/j.neulet.2012.03.015
20. Tuor UI, Malisza K, Foniok T, et al. Functional magnetic resonance imaging in rats subjected to intense electrical and noxious chemical stimulation of the forepaw. Pain. 2000;87(3):315–324. doi:10.1016/S0304-3959(00)00293-1
21. Zhao F, Wang P, Hendrich K, Kim S. Spatial specificity of cerebral blood volume-weighted fMRI responses at columnar resolution. Neuroimage. 2005;27(2):416–424. doi:10.1016/j.neuroimage.2005.04.011
22. Zhao F, Wang P, Hendrich K, Ugurbil K, Kim S. Cortical layer-dependent BOLD and CBV responses measured by spin-echo and gradient-echo fMRI: insights into hemodynamic regulation. Neuroimage. 2006;30(4):1149–1160. doi:10.1016/j.neuroimage.2005.11.013
23. Zhao F, Welsh D, Williams M, et al. fMRI of pain processing in the brain: a within-animal comparative study of BOLD vs. CBV and noxious electrical vs. noxious mechanical stimulation in rat. Neuroimage. 2012;59(2):1168–1179. doi:10.1016/j.neuroimage.2011.08.002
24. Ogawa S, Tank DW, Menon R, et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci U S A. 1992;89(13):5951–5955. doi:10.1073/pnas.89.13.5951
25. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. London: Academic Press; 1998.
26. Coderre TJ, Grimes RW, Melzack R. Deafferentation and chronic pain in animals: an evaluation of evidence suggesting autotomy is related to pain. Pain. 1986;26(1):61–84. doi:10.1016/0304-3959(86)90174-0
27. Coderre TJ, Melzack R. Procedures which increase acute pain sensitivity also increase autotomy. Exp Neurol. 1986;92(3):713–722. doi:10.1016/0014-4886(86)90311-0
28. Persson AK, Thun J, Xu XJ, et al. Autotomy behavior correlates with the DRG and spinal expression of sodium channels in inbred mouse strains. Brain Res. 2009;1285:1–13. doi:10.1016/j.brainres.2009.06.012
29. Froesel M, Cappe C, Ben Hamed S. A multisensory perspective onto primate pulvinar functions. Neurosci Biobehav Rev. 2021;125:231–243. doi:10.1016/j.neubiorev.2021.02.043
30. Coffeen U, Ortega-Legaspi JM, de Gortari P, et al. Inflammatory nociception diminishes dopamine release and increases dopamine D2 receptor mRNA in the rat’s insular cortex. Mol Pain. 2010;6(1):75. doi:10.1186/1744-8069-6-75
31. Ortega-Legaspi J, León-Olea M, De Gortari P, et al. Expression of muscarinic M1 and M2 receptors in the anterior cingulate cortex associated with neuropathic pain. Eur J Pain. 2010;14(9):901–910. doi:10.1016/j.ejpain.2010.02.007
32. Ortega-Legaspi J, de Gortari P, Garduño-Gutiérrez R, et al. Expression of the dopaminergic D1 and D2 receptors in the anterior cingulate cortex in a model of neuropathic pain. Mol Pain. 2011;7:1–10. doi:10.1186/1744-8069-7-97
33. Wall PD, Devor M, Inbal R, et al. Autotomy following peripheral nerve lesions: experimental anesthesia dolorosa. Pain. 1979;7(2):103–113. doi:10.1016/0304-3959(79)90002-2
34. Devor M. Ectopic discharge in Aβ afferents as a source of neuropathic pain. Exp Brain Res. 2009;196(1):115–128. doi:10.1007/s00221-009-1724-6
35. Katz J, Melzack R. Pain “memories” in phantom limbs: review and clinical observations. Pain. 1990;43(3):319–336. doi:10.1016/0304-3959(90)90029-D
36. Ortega-Legaspi JM, López-Avila A, Coffeen U, Del Angel R, Pellicer F. Scopolamine into the anterior cingulate cortex diminishes nociception in a neuropathic pain model in the rat: an interruption of “nociception-related memory acquisition”? Eur J Pain. 2003;7(5):425–429. doi:10.1016/S1090-3801(02)00147-7
37. Latremoliere A, Woolf CJ. Central S of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi:10.1016/j.jpain.2009.06.012
38. Westlund K, Vera-Portocarrero L, Zhang L, Wei J, Quast M, Cleeland C. fMRI of supraspinal areas after morphine and one week pancreatic inflammation in rats. Neuroimage. 2009;44(1):23–34. doi:10.1016/j.neuroimage.2008.07.048.fMRI
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.