Back to Journals » Drug Design, Development and Therapy » Volume 17
Tracking Research on Hemoglobin-Based Oxygen Carriers: A Scientometric Analysis and In-Depth Review
Authors Zhang Q, Ma YX, Dai Z, Zhang B, Liu SS, Li WX, Fu CQ, Wang QM, Yin W
Received 13 June 2023
Accepted for publication 4 August 2023
Published 24 August 2023 Volume 2023:17 Pages 2549—2571
DOI https://doi.org/10.2147/DDDT.S422770
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Qi Zhang,* Yue-Xiang Ma,* Zheng Dai, Bin Zhang, Shan-Shou Liu, Wen-Xiu Li, Chuan-Qing Fu, Qian-Mei Wang, Wen Yin
Department of Emergency, Xijing Hospital, Air Force Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wen Yin, Department of Emergency, Xijing Hospital, Air Force Medical University, No. 127 Changlexi Road, Xi’an, 710032, People’s Republic of China, Email [email protected]
Abstract: Numerous studies on the formulation and clinical applications of novel hemoglobin-based oxygen carriers (HBOCs) are reported in the scientific literature. However, there are fewer scientometric analysis related to HBOCs. Here, we illustrate recent studies on HBOCs using both a scientometric analysis approach and a scope review method. We used the former to investigate research on HBOCs from 1991 to 2022, exploring the current hotspots and research trends, and then we comprehensively analyzed the relationship between concepts based on the keyword analysis. The evolution of research fields, knowledge structures, and research topics in which HBOCs located are revealed by scientometric analysis. The elucidation of type, acting mechanism, potential clinical practice, and adverse effects of HBOCs helps to clarify the prospects of this biological agent. Scientometrics analyzed 1034 publications in this research field, and these findings provide a promising roadmap for further study.
Keywords: blood substitutes, trauma, hemorrhagic shock, organ preservation, anemia, risk management
Introduction
The clinical practice of blood transfusion typically involves component therapy, with red blood cells (RBCs) transfusion being the most widely used and prominent component in clinical conditions of severe blood disorders.1 The primary physiological function of RBCs is their unique oxygen-transport ability, with adaptive regulation in response to changes in the oxygen concentration in organic tissues, significantly improving survival.2,3
Nevertheless, RBCs for transfusion from donor blood have several limitations and inherent hazards, including the potential transmission of infectious diseases, non-hemolytic febrile reactions, and reperfusion injury.4 Additionally, blood-based products have a limited storage life of 42 days in refrigerated conditions and 1 day at room temperature. The physiology and circulation time in vivo of RBCs decrease over time due to the storage lesions.5,6 Beyond these existing challenges, the increased aging populations have led to a chronic shortage of clinical blood availability, which is falling to meet the demand for medical care. The COVID-19 pandemic has further exacerbated the imbalance between supplies and demands, as the number of eligible blood donors has decreased.7,8 As a result, the research efforts are focusing on new substitutes for RBCs to enhance the availability and applicability.
An ideal blood substitute should have the following characteristics: stable oxygen-transport capacity, minimal immunogenicity, an extended half-life, renal biocompatibility, the absence of disease transmissions, and simple metabolic clearance. Moreover, it should be amenable to ease of storage and produced at a large scale, thus having easy availability and portability. Given the inherent risks and notable drawbacks associated with blood transfusion therapy, scientists are of interests in searching for an alternative.
As a novel substitute for RBCs, artificial oxygen carriers are being developed to mimic and even enhance the functions of endogenous blood components.9–13 To date, hemoglobin-based oxygen carriers (HBOCs) have been extensively studied and being a prospective erythrocyte substitute. It makes use of the excellent oxygen carrying and delivering properties of natural hemoglobin (Hb). However, it remains necessary to scrutinize their research models and the prevailing trends to promote further progress.14
Bibliometric analysis provides a useful tool for studying the global trends in a research field, as it highlights recent advancements, critical issues, existing gaps, and collaborations between researchers and institutions.15 The purpose of this study is to conduct a visual analysis and comprehensive review, demonstrate and discuss the current status and prospective development trends of HBOCs by using various graphs, and provide a scientific foundation for promoting clinical research and application.
Methods
Database
The data utilized in the present study were obtained from the Web of Science Core Collection (WoSCC) in Science Citation Index Expanded (SCIE) database on Web of Science (WoS). The SCIE database is a widely used database in bibliometric research and recognized as a preeminent literature citation database with global reach.16,17 The number of scientific publications available in WOS database exceeds that of other analogous databases, such as Scopus, Derwent, and CNKI (China National Knowledge Infrastructure). Thus, WOS is the most frequently used database for bibliometric analysis.16–18 To investigate the subject of interest, the search term “hemoglobin-based oxygen carrier” was employed, time frame for the literature search extended from 1991 to 2022 and language “English”. Several search conditions and restrictions were implemented during the search process in order to guarantee the results’ precision and exhaustiveness. After a thorough examination of the search results, a total of 1034 relevant publications were identified. To facilitate subsequent analyses, all relevant metadata including titles, authors, journals, abstracts, and other relevant information were systematically extracted and digitally recorded. Figure 1 is a flowchart of the study depicting the research methodology.
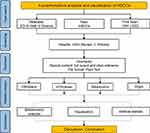 |
Figure 1 Flow chart of scientometric analysis. |
Visualization Tool
The present study employed several software tools, including VOSviewer 1.6.13, CiteSpace 6.1.1R6, Bibliometrix and Origin 2021, which are commonly employed for visualizing bibliometric data.19 We collected fundamental data, like authorship, institutional affiliations, geographical regions, publication venues, keywords, and reference lists.
VOSviewer, is a visualization program that employs a systematic analysis by creating bibliometric maps, was created by Van Eck and Waltman (Leiden University, the Netherlands).20 Compared to commonly used bibliometric software, VOSviewer concentrates on graphical presentation based on bibliometric network data, which consists of nodes and links. According to the VOSviewer’s visualization rules, each node reflects a specific parameter, such as country/region, journal, or keyword. The magnitude of a node is proportional to its frequency of occurrence, such as publication counts, keyword occurrences, or citations. The colors of nodes depict the co-occurrence analysis cluster to which the nodes belong.19
CiteSpace, a software, was created by Chaomei Chen (Drexel University, USA), which is a multi-dimensional, time-sharing, dynamic visualization analysis software.20,21 It possesses an advantage in academic searches of WoSCC in SCIE database, and some of its functionalities and key indicators facilitate more precise data processing.20 Multiple visualization methods are available in CiteSpace, particularly keyword burst detection, time span measurement and warm-cool color mapping. Keyword burst detection is used to identify sudden changes in nodes to represent that keywords were frequently cited within a specific period. Time span measurement provides a timeline view to track the research history, turning points, and research hotspots. In this study, we detected the burst of keywords and cited references by using CiteSpace 6.1.R6 to investigate the emerging literature and the recurrent keywords.22–24
Bibliometrix, a small package founded on R software, was developed by the Aria’s team.25 The data, which are exported from the WoS database and completed by the research team, are processed using the Bibliometrix package with R software for visualization. The H-index is an effective instrument for evaluating the quantity and quality of academic papers published by scientists, nations, journals, or institutions.26 In bibliometric analysis, the most frequently employed indicators are the co-authorship rate, the co-occurrence rate, and the co-citation rate. Co-authorship analysis is based on a quantitative technique that emphasizes the interrelationships between different elements, while co-occurrence analysis is a process that accentuates interconnections between different institutions, authors and countries. Citation analysis is a statistical methodology that underscores the advantages of cited articles.
Results
Global Publishing Trends and Forecasts
The temporal evolution of the published literature on HBOCs reflects the annual fluctuation in international scholars’ interests. Figure 2 depicts the annual statistics of HBOCs-related literature data in the WOSCC between 1991 and 2022. This dataset consists of 1034 publications, including articles and reviews. The line graph representing the annual publication frequency demonstrates that the publication trend can be partitioned into three distinct sub-periods: a period of noticeable growth (1991–2009), a period of rapid decline (2009–2014), and a period of gradual growth (2014–2022). In the 1990s, blood scarcity emerged as a critical global issue, prompting significant research efforts on major research institutions and pharmaceutical ventures to develop blood substitutes that could satisfy military and civilian needs. This research fervor culminated in 2009. The emergence of significant safety concerns resulted in controversy and the halting of HBOCs-related research by the US Food and Drug Administration (FDA), leading to a sharp decline in research publications after 2009. After 2014, more evidence emerged, signaling a renewed interest in HBOCs-related research.
 |
Figure 2 Annual publications between 1991–2022. |
Distribution of Major Research Directions
Figure 3 shows the scholarly classification of publications pertaining to HBOCs, and it is noteworthy that approximately 29% of the literature is focused on the research area of biomedical engineering, biomaterials, and molecular biology. Currently, a global imbalance between supply and demand has prompted numerous countries and institutions to engage in the active research and development of artificial blood. As a biological pharmaceutical product, artificial blood requires multidimensional research on its composition, physicochemical properties, and pharmacological mechanisms, as well as a comprehensive evaluation of its efficacy and safety. Additionally, as a blood substitute, HBOCs have been extensively employed in various clinical fields, for instance, hematology, critical care medicine, anesthesiology, peripheral vascular disease, and organ transplantation, as evidenced by pertinent publications in these areas. The findings of such research unequivocally demonstrate the expansive application prospects and potential of HBOCs.
 |
Figure 3 Distribution of major research directions. |
Country and Regional Cooperation Distribution
Based on the results of international cooperation in various countries and regions, research on HBOCs-related topics has been conducted in 36 different countries and regions. According to the geographical distribution of global productivity (Figure 4A), the majority of articles were published in North America, Asia, and European nations. Table 1 shows the top ten countries/regions with the most published papers on this topic, and Figure 4B displays the annual publication counts for these nations/regions between the years 1991 and 2022. The highest number of publications (555 papers, 53.675%) came from the United States, indicating that this country has contributed most to HBOC research. The following countries are China, Japan, Italy, France, Canada, Romania, England, and the Netherlands. Meanwhile, American works were cited 6199 times, significantly surpassing China in second place (1228 times). This information also demonstrates that the United States has been a driving force in the study of HBOCs, which may be attributable to the greater emphasis on scientific investigation in developed countries.
 |
Table 1 The Top 10 Productive Countries/Regions Related to HBOCs Research |
Figure 4C indicates that there is close cooperation among countries in HBOC research, as the development of blood substitute currently becomes a global challenge, the urgent need for clinical scenarios related to artificial blood substitutes is more common, especially in developed countries, making HBOC research an urgent issue to be addressed. Consequently, nations and regions should strengthen cooperation to advance this research field.
Contributions of Institutions
Regarding publications on HBOCs, the research contributions were made by a total of 484 institutions, with Table 2 presenting the top ten high-output institutions. According to the institution rankings, it is noteworthy that the United States featured prominently, with 6 research institutions ranking among the top 10. Of these, the Ohio State University ranked first (with 67 publications), followed by the University of California, San Diego (with 58 publications). In the context of research on HBOCs, it is notable that the institutions making the largest contributions from China were Sichuan University (with 51 publications) and the University of Chinese Academy of Sciences (with 34 publications). Moreover, high citation frequency serves as a key metric indicating an increase in cited papers within a certain period. It is postulated that the University of California, San Diego, FDA, and University of California, Davis, achieved breakthrough progress in HBOCs-related research during a certain period, as evidenced by the sudden surge in citation frequency. Of the top 5 research constitutions run into the top 100 according to the global rank.
 |
Table 2 The Top 10 Productive Institutions Related to HBOCs Research |
Contributions of Authors
H-index serves as a reliable measure for assessing scientific achievements and academic contributions of a particular researcher. The H-index measures a scholar’s rate of citation and indicates the total number of articles that have been at least H times cited.27 Table 3 lists the most creative ten authors ranked by their publications related to HBOC studies. Palmer AF (55 articles, USA), Sakai H (38 articles, Japan) and Alayash AI (33 articles, USA) had published the most papers. Professor Palmer is a global leader in the development of novel blood substitute research for multiple uses in transfusion medicine and tissue engineering, and he is currently working to develop more safer and commercially viable RBC substitutes.28 Prof. Alayash has received the most total citations (8526 times) and the greatest H-index (49). Prof. Alayash’s research group is dedicated to assessing the safety and efficacy of blood substitutes, they are trying to analyze the complexities associated with the interaction of Hb and vascular system and attempting to tackle toxicities.29
 |
Table 3 The Top 10 Authors with Most Publications Related to HBOCs Research |
Analysis of Journals and Co-Cited Journals
The research on HBOCs involved a total of 389 academic journals. Table 4 lists the top twenty journals in the field of HBOC studies. Among the 389 journals that issued papers on HBOCs, Artificial Cells, Nanomedicine, and Biotechnology published the most with 108 (10.44%), followed by Shock (30, 2.90%) and Transfusion (28, 2.70%). The top ten journals included eight American publications and two British publications.
 |
Table 4 The Top 20 Journals with Most Publications Related to HBOCs Research |
Among the top twenty journals, eight had an impact factor greater than 5 scores and ranked the Q1 according to Journal citation reports criteria, including Artificial Cells Nanomedicine & Biotechnology (6.355), Anesthesia and Analgesia (6.627), American Journal of Physiology-Heart and Circulatory Physiology (5.125), Critical Care Medicine (9.296), Free Radical Biology and Medicine (8.101), Anesthesiology (8.986), Antioxidants & Redox Signaling (7.468), Biomaterials (15.304) and Clinical chemistry (12.167). Among the top twenty journals, the impact factor of Artificial Organs was the lowest at 2.663.
Table 5 shows the twenty most cited journals. Artificial Cells, Nanomedicine, and Biotechnology (857 citations) ranked first, followed by Transfusion (568 citations) and Journal of Biological Chemistry (533). JAMA – Journal of the American Medical Association (157.335), Science (63.714), Blood (25.476), Proceedings of the National Academy of Sciences of the United States of America (12.779), The New England Journal of Medicine (176.079), and Nature (69.504) were journals with an impact factor greater than 10 scores. The majority of research on HBOCs centered on the development of novel formulation, pharmacological mechanisms, as well as the evaluation of efficacy and safety. In comparison to the analysis of journals, the number of highly cited journals has significantly increased, indicating that research in these journals is more fundamental or theoretical in nature.
 |
Table 5 Top 20 Co-Cited Journals Related to HBOCs Research |
Co-Cited References Analysis
The term “co-citation” refers to the situation in which one or more references are cited in common by one or more publications.30 The top ten co-cited documents on HBOC research are presented in Table 6. The highly co-cited papers cover a range of types including meta-analysis,31 randomized controlled trials,32,33 and reviews.34,35 Within the top ten co-cited references, four articles focused on the development and synthesis of novel HBOCs, three articles focused on clinical trials, and four articles focused on its physiological toxicity. The most frequently cited article is a meta-analysis reported in 2008.36 This study summarized distinct trials from diverse populations (ie, elective surgery or trauma), different comparisons (crystalloids, colloids, or blood), and HBOC preparations with varied physicochemical properties. The conclusion revealed significant associations between HBOCs and risk of death.36 Due to concerns identified by this report, FDA mandated all ongoing and future trials of HBOCs in the United States.
 |
Table 6 The Top 10 Cited References Related to HBOCs |
Some academicians have harshly criticized the flawed methodological and conclusive validity of the study.37–39 Consequently, numerous commercial enterprises shifted their target indication or relocated their operations outside the United States. This probably be the reason why a sharp decline on HBOC research during 2009–2014. The co-citation timeline view demonstrates that all references form 14 clusters with active co-citations between them (Figure 5).
 |
Figure 5 Reference timeline view. |
Keywords Analysis
Keywords are a fundamental component of scientific articles, and high-frequency keywords reflect current issues and frontiers in a specific research field.40 The statistical analysis of keywords can effectively and rapidly identify essential topics within specific academic disciplines.41 Co-occurrence keyword analysis of HBOCs shows in Figure 6. The VOSviewer software clustered keywords into five main clusters and used different colors to represent these clusters (Figure 6A). In the literature on HBOC studies, the most frequently appearing keywords are “hemoglobin” (106), followed by “blood substitutes” (77) and “hemoglobin-based oxygen carriers” (59). The keywords that appear in citation bursts indicate that their citation frequency has significantly increased over a certain period of time, which can confirm whether a research field is in a popular stage during a certain period and also highlights emerging topics.42 As shown in Figure 6B, we were able to obtain the top 25 keywords with the strongest citation using keyword burst analysis. These keywords cover a wide range of HBOC-related research subjects, including animal models of vitro trials, chemical modification mechanism, evaluating outcomes, and clinical applications.
 |
Figure 6 (A) VOSviewer network map of co-occurrence analysis of all keywords of HBOCs-related studies. (B) Top 25 keywords with the strongest citation bursts on HBOCs from burst analysis of keywords. |
However, this is not sufficient to provide readers with a full understanding of the research area. The analysis of data can reveal the overall structure and interdependencies of linked field and literature.43 We subsequently categorized these keywords into four dimensions: HBOC types, physiological mechanism, clinical scenario, and adverse event, respectively. Results after manually placing the keywords in each dimension are shown in Figure 7. The modification frequently appeared most was cross-linked Hb, followed by liposomal-encapsulated Hb and polymerized bovine Hb (Figure 7A). The physical actions of HBOCs and measured indicators briefly summarized from keywords analysis are demonstrated in Figure 7B. Based on the efficacy of such complexes, this category set primarily includes oxygen delivery and oxygen consumption. Regarding clinical scenario, HBOCs are typically applied to hypovolemic and ischemic-hypoxic diseases, with hemorrhagic shock being the most common disease in the field (Figure 7C). Adverse events are focused on the safety of HBOCs in pre-clinical and clinical trials, with nitric-oxide scavenging induced hypertension being the most frequent one (Figure 7D).
 |
Figure 7 (A) Type of HBOCs. (B) Physiological mechanism. (C) Clinical scenario. (D) Adverse event. |
Discussion
Hb plays an essential role for life activities and its deficiency or disorders may lead to severe syndrome conditions. Researchers have been interested in the topic of modifying Hb and improving its oxygen transport function over the three decades. Here, we conducted various bibliometric approaches to visualize the studies for HBOCs from different perspectives, and thoroughly summarize the historical development and research frontiers. In the following sections, we will develop the discussion of this review in detail in accordance with the classification of keywords.
Sources of Hb for HBOCs
The Hb for most HBOC products sourced from expired human blood, fresh bovine or porcine blood, and some marine invertebrates. The fundamental principle of HBOCs design is to regulate the oxygen loading and unloading capacity of the Hb utilized. Human Hb relies on regulatory effector molecule, 2,3-diphosphoglycerate, to maintain its P50 within a specific range (26–28 mmHg).44 Bovine Hb, in contrast, has chloride-dependent oxygen affinity and is more thermally stable during separation and processing.45 Moreover, Johnstone et al46 suggested that bovine RBCs are less prone to hemolysis, exhibit less fragility, and produce fewer serum reactions in vitro tests than swine RBCs. Hb molecules derived from marine invertebrates are not packed within RBCs or other types of membranes. They exhibit intrinsic superoxide dismutase (SOD)-like activity, allowing them to deplete reactive oxygen species,47 while also demonstrating significant oxygen transport efficiency.48,49 Considering the availability, processing, and efficacy, bovine blood has emerged as the preferred commonly used raw material for HBOCs.50
Types of HBOCs
The initial attempt at developing HBOCs involved stoma cell-free Hb, which, when removed from the protective environment of RBCs, rapidly dissociates from tetramer into dimers, ultimately resulting in glomerular damage and kidney failure.51,52 Meanwhile, the free radicals generate by heme portion inflicted severe harm to vital organs. As a consequence of these deficiencies, considerable effort has been devoted to stabilizing and optimizing the functionality of the Hb molecule.
To circumvent inherent limitations, Hb must be chemically modified or encapsulated within the protective carrier shell to reduce its toxicity (Figure 8). Internal cross-linking, polymerization, conjugation, and surface modification are specific chemical modification strategies for cell-free Hb. Internal cross-linking involves connecting Hb monomers with cross-linking agents to form oligomers; polymerization entails the aggregation of Hb molecules into larger polymer complexes; conjugation involves attaching Hb with other molecules (such as polyethylene glycol, PEG) to soluble non-hemoglobin polymers. These strategies increase Hb’s stability, decrease its toxicity, and lengthen its half-life. Encapsulation aims to simulate the in vivo environment of RBCs by encapsulating Hb and enzymes inside artificial RBCs with lipid or other biodegradable membranes.
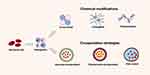 |
Figure 8 Hemoglobin modifications mechanisms. |
The Typical Chemically Modified HBOCs
Despite the fact that several HBOCs products have progressed to clinical phase, only Hemopure® (HBOC-201, HbO2 Therapeutics Corporation, USA) is commercially available for human use on a global scale.38,53 Cross-linked bovine Hb was polymerized with glutaraldehyde to create this product, which after extensive purification contains 13.6 g/dL of Hb in a Ringer’s solution with a pH of 7.6–7.9.54 Instead of a single Hb molecule, these polymers consist of four or five Hb molecules with a total molecular weight of 250 kDa. The half-life of HBOC-201 is 16 to 20 hours, slightly reduced to 8.5 hours in patients with liver disease. Additionally, it can be kept for three years at ambient temperature. In clinical trials, HBOC-201 was found to substantially improve patient survival and decrease the requirement for blood transfusions during cardiac surgery.55
Encapsulation Strategies
The conception of Hb-encapsulated delivery system was initially proposed by Chang et al in the 1950s and 1960s.56,57 Encapsulated HBOCs products are manufactured in a manner that maximizes their resemblance to RBCs, which was accomplished by utilizing various effector molecules or reductive enzymes within micro- or nano-sized (up to 500nm) encapsulation platforms. The encapsulated Hb products demonstrated more advantages than acellular Hb products, including relief from hypertension, increased half-life, and extended shelf life.58
The frequently mentioned HBOC refers to Hb encapsulated in liposomes, which involves entrapping Hb within the bilayer structure of liposomes.59 Liposomes are stable vesicles composed of phospholipid molecules that can encapsulate a variety of nanoscale biological molecules. Liposomes can shield Hb from immune system attacks, prolong its half-life, and modulate its oxygen affinity and release to better suit the requirements. In addition, liposomes surface-modified with PEG can lessen toxicity, increase circulation lifetime up to 60 hours, and decrease nitric oxide (NO) scavenging. PEG increases their half-life but decreases their antigenicity, thereby enhancing their targeting specificity to certain sites and imparting water-solubility. They are also compatible with the human immune system, thereby protecting it from immune system assaults and extending its half-life within the body.60 Moreover, encapsulation diminished Hb’s high viscosity and high colloidal osmotic pressure.61,62
Furthermore, to better simulate the intracellular environment of RBCs and control oxidative damage, Hb molecules are cross-linked with superoxide dismutase, catalase, and carbonic anhydrase, forming a soluble poly-nanobiotechnological complex.9,13 This novel soluble nanobiotechnological complex not only eliminates oxygen radicals and prevents ischemia-reperfusion injury but enhances the function of RBCs.9 Other enzymes, such as adenosine and reduced glutathione, can be added to alleviate hypertensive reactions.11 However, due to the exorbitant cost of commercially purified enzymes, the cost-benefit of using them must still be considered in practical applications. Despite this, there are some challenges, including the difficulty in controlling the rate of Hb release, immune system response, and the high production cost. This technique is therefore still in research phase and requires further investigation to demonstrate its safety and efficacy.
Physicochemical Parameters and Brief Evaluation System
Over the past three decades, studies related to HBOCs have primarily focused on optimizing their physicochemical properties to improve efficacy and safety. The primary objective of developing HBOCs was to restore volumetric oxygen-carrying capacity following acute blood loss.63 Specifically, whether a particular HBOC improves microvascular perfusion of vital organs and fully restores oxygen to hypoxic tissues, thereby correcting any accumulated oxygen debt, depends on their physicochemical properties.
Physicochemical Parameters
The critical properties include molecular size, Hb concentration, osmolarity, viscosity, oxygen affinity, pH value and purity. The possible active mechanisms of these variables are presented in Table 7. The molecular size and Hb concentration of HBOCs not only influence the viscosity, oncotic pressure, and dosage of final product, they also affect the total volume of therapeutic fluids administrated to patients. It has been demonstrated that HBOCs with smaller molecular weight and greater oxygen diffusion coefficients cause vasoconstriction and confine blood to the capillary bed.64,65
 |
Table 7 Physiochemical Parameters and their Active Mechanisms on HBOCs |
In macroscopic circulation, viscosity is an essential factor in the delivery of oxygen from organ to tissues. Basically, HBOCs have a lower viscosity than whole blood, and hemodilution consequently occurs during resuscitation treatment. However, hemodilution decreases the viscosity of whole blood and systemic vascular resistance, thereby enhancing the state of blood flow in systemic circulation.66
In microcirculation, many factors are involved in this regulatory process. The shear stress experienced by endothelial cells of small vessels is proportional to viscosity and osmotic pressure.67 A decrease in microcirculatory shear stress triggers endothelial cell downregulation leading to NO production.68,69 HBOCs with high osmolarity theoretically keep the microcirculation, while its hemodilution effect to some extent balances the high colloidal osmolarity.70,71 Polymerized tetramers have lower colloidal osmolality than stable Hb tetramers or surface-modified tetramers and therefore may be less effective in maintaining microcirculation.
The small terminal arteries modulate the microcirculation according to the partial oxygen pressure, thereby adapting to oxygen demand of tissues.72 Factors that determine oxygen delivery to the small arteries include the oxygen concentration of the blood, the ability for Hb to unload oxygen and to diffuse into vascular endothelium. Oxygen affinity is an important parameter measured by P50 value to determine the ability of oxygen transporting and releasing in tissues.9 A right shift observed in oxygen dissociation curve facilitates oxygen release to tissues and improves resistance to changes in oxygen consumption or blood pressure, whereas the oxygen equilibrium curve only exhibits an advantage under conditions of severe hypoxia. The oxygen-transport efficiency of human RBCs is 23%.72 RBC substitutes, therefore, only improve oxygen-transport efficiency and facilitate release when its oxygen affinity is equal to or lower than that of RBCs.
In addition to the factors mentioned above, the pH formulation of HBOCs can be shown to have a measurable effect on the blood’s pH, with the fact that Hb has some buffering capability within the physiologic range.73 Lastly, formulations of toxic residuals and endotoxin-contaminated materials that may induce cardiac dysfunction have been reported.74,75
The Brief Evaluation System for HBOCs
From resuscitation perspectives, fluid therapy following hypovolemia requires immediate restoration of blood volume which firstly recovers hemodynamics and function and finally tissue oxygenation. We theoretically propose a concise evaluation system to illustrate its effectiveness and safety (Figure 9). Hemodynamics and tissue oxygenation are main dimensions to demonstrate effectiveness. Hemodynamics is mainly affected by blood vessel flow and vascular resistance. A series of significant clinical indicators can be determined through techniques. The conditions of tissue oxygenation are calculated by oxygen delivery and consumption, which are primarily illustrated by partial oxygen pressure, oxygen saturation, and oxygen tension. Nonetheless, the absence of methods for measuring partial oxygen pressure was a significant limitation. Regarding safety, we consider that toxicity and injury should comprise endothelia, tissue and organ injury ranging from macro to micro perspective. These injury-related conditions are obtained from laboratory tests, histopathological sections, and molecular biology techniques respectively.
 |
Figure 9 The brief evaluation system for HBOCs in vivo and in vitro trials. |
Potential Clinical Practice
Trauma and Hemorrhagic Shock
Hemorrhagic shock (HS) resulting from uncontrolled blood loss is the leading cause of mortality in trauma patients following blunt or penetrating injuries, both in military and civilian contexts.76,77 The administration of whole blood as a resuscitation fluid for traumatic hemorrhage is widely recognized in tactical combat casualty care. Yet, the transportation of whole blood to remote units has been a logistical challenge due to the rigorous cold chain requirements.78 HS is characterized by inadequate blood perfusion, which results in a deficiency of cellular substrates and hypoxia to vital organs.78 Blood viscosity is significantly altered in HS due to hemodilution and decreased hematocrit, which directly affects microvascular function. Damage to the microcirculation caused by severe shock delays the return to adequate perfusion, consequently contributing to the development of lactic acidosis and further exacerbating the associated outcomes.79,80
Several HBOC agents have been tested its efficacy in preclinical animal model of HS. In hypoxic tissue environment, they facilitate oxygen diffusion from erythrocytes to vascular endothelial cells, enhance oxygen transport in microcirculation and increase the uniformity of oxygen flux cross capillaries.81,82 In an animal model of HS combined with traumatic brain injury, SanFlow significantly improved MAP and heart rate and decreased intracranial edema, acidosis, and hyperkalemia.83 It provided brain protection and tackles the toxicity associated with cardiovascular and renal failure.84 Despite the early HBOCs demonstrated efficacy in preclinical animal models, they did not yield clinical benefits in subsequent human trauma trials.85 Clinical investigations pertaining to HBOCs conducted in the context of trauma have not exhibited a universal advantage in relation to the avoidance or reduction of RBC transfusion or 28-day mortality.86
Recently, a multi-institutional clinical trial has been initiated by experts from the US Army Institute of Surgical Research and Emergency Medicine and the University of Terrebonne in South Africa to evaluate the efficacy of resuscitating trauma victims with synthetic blood products prior to hospital admission. The project aims to assess the impact of HBOC-201 in comparison to conventional fluid resuscitation on the mortality rate within a 24-hour period.87 Meanwhile, researchers from the University of Maryland are currently spearheading a research initiative aimed at assessing the safety and efficacy of a new blood substitute in the context of multiple trauma scenarios.88 Thi Modern experimental platforms, sophisticated modelling methods, machine learning algorithms and multiple complementary animal models are all to be used in this project. The goal is to modify the product to suit environmental climate applications, guaranteeing its durability in harsh conditions for extended periods. This project is anticipated to enhance treatment alternatives for trauma patients globally, particularly those affected by war, natural calamities, and other emergency situations. The execution of these initiatives would yield novel perspectives towards the management of trauma.
Organ Preservation and Transplantation
One of the common and inherent complications in harvesting and organ transplantation is ischemia-reperfusion injuries.89,90 The contemporary preservation techniques involve hypothermic storage in a solution that comprises electrolytes, amino acids, and other substances with the aim of maintaining cell integrity. Even though there are techniques for reducing aimed at reducing enzyme activity that may reduce the risk of ischemia, an efficient oxygen delivery system is still required. Hemo2-life (M101) is a biotechnological product that consists of unaltered cell-free Hb derived from a marine worm.47 The assessment of the M101 has been conducted in both organ preservation and resuscitation contexts.
A biological substance may be incorporated into preservation fluids for organs to regulate oxygen levels in hypoxic environments and mitigate the decline of oxygen concentration in tissues. Preclinical animal studies have demonstrated that M101 improves the quality, function, and prognosis of diverse organs in kidney, lung, and heart transplantations.47,90,91 Kaminski et al89 observed that M101 enhances long-term kidney function under refrigerated conditions. Additionally, the study showed the progression of interstitial fibrosis and tubular atrophy in the kidney under mechanical perfusion.89 Prior study found that the effects of M101 on porcine kidney transplantation were similar to those of pure oxygen and that M101 supplementation helped to restore post-transplant renal function and reduced post-transplant adverse effects, regardless of whether pure oxygen was added.92 A multicenter study evaluating the safety of the effects of M101 in the preservation of 60 deceased donor kidneys showed better renal function after kidney transplantation with an organ preservation solution containing HBOC agents and significantly reduced the occurrence of delayed graft function recovery and shortened the time to recovery of renal function.93 Phase I clinical studies for kidney transplantation have demonstrated the safety of M101 for both patients and grafts.94
In an ongoing investigation, M101 is added to the conventional organ preservation solution for patients with end-stage renal disease undergoing kidney transplantation to prevent delayed graft function. This study is in a clinical Phase II study and is expected to be used in the clinical organ transplantation discipline.95
Anemia
HBOC-201 is currently being administrated in “compassionate use” initiatives for Jehovah’s Witness individuals who suffered from severe autoimmune hemolytic anemia or refusal of natural blood.55,94,96 For clinicians, the prompt recognition of patient requirements and comprehension of attributes of HBOCs are crucial for ensuring safety and expeditious administration, thereby potentially preserving lives. Davis reported97 that three critically ill patients with sickle cell disease suffering from multiple organ failure were successfully saved after the use of HBOC-201. The present study suggests that it has the capacity to offer oxygen support in the management of individuals experiencing sickle cell crisis, until the Hb levels have been replenished to meet the metabolic demands.
Gomez et al98 observed that a Jehovah’s Witness who was diagnosed with acute lymphoblastic leukemia experienced significant anemia (with a Hb level as low as 3.1 g/dl) during chemotherapy. However, during HBOC-201 infusion, the patient’s Hb level remained within the range of 3.6–5.3 g/dl, and there were no observed instances of ischemia or compromised organ function.98 Gomez et al also documented instances of double solid organ transplant recipients with critical drops in Hb levels (2 g/dl) in both renal and pancreatic cases. However, following HBOC-201 infusion, Hb levels increased to 6.8 g/dl.99 The results indicate that HBOC-201 offers sufficient oxygen supply to the tissues during the period of bone marrow erythrocyte recovery.100 Even these studies show that HBOC-201 is safe and feasible for use in anemia, larger randomized clinical trials with more high-quality data are required to validate the effectiveness, safety, and acceptability.
Potential Applications in Other Fields
Numerous clinical applications proposed the utilization of HBOCs for the prevention or treatment of acute ischemic diseases.101 This encompasses a variety of prospective uses. Tumor hypoxia represents a significant characteristic of the microenvironment of solid tumors within the context of tumor therapy.102 The oxygen-deficient environment enhances further tumor deterioration, reduces the efficacy of therapeutic treatments, thus leading to drug resistance, etc.103,104 HBOCs possess a greater capacity to efficiently perfuse the anomalous microcirculatory system of neoplastic growths, thereby facilitating the provision of essential oxygen for the purpose of chemotherapy and radiation therapy.105,106 Additionally, HBOCs are expected to be applied to treat ischemic and hypoxic diseases such as limb ischemia,102 wound healing,107 sepsis,108 etc.
Adverse Events and Treatment Strategies
It is critical to comprehend how HBOCs affect vital organs and their physiological processes in patients as well as in healthy groups. HBOCs have various safety concerns: vasocontraction,109 gastrointestinal complications ranging from abdominal pain and gastroesophageal reflux syndrome to necrotizing pancreatitis,110 hemoglobinuria,111 volume overload,112 elevated liver enzymes,113,114 oxidation distress,115 coagulation disorders116 and iron deposition, etc.117
The traditional explanation for hypertension as an adverse reaction is the scavenging of NO in endothelial cells, which reduces their vasodilatory capacity.118,119 Additionally, free Hb affects the blood osmolarity, leading to alterations of blood volume and subsequent cardiovascular complications.120 To mitigate this effect, a variety of strategies have been clinically suggested, for instance, employing inhaled NO during HBOCs infusion, and antihypertensive therapies have been suggested to mitigate this effect.121
In the absence of antioxidant enzymes in cell-free Hb binds to oxygen, forming free radicals like superoxide, hydrogen peroxide, hydroxyl radicals, and oxidized iron-based porphyrins.122 The heme iron of HBOCs undergoes auto-oxidation, resulting in the production of superoxide. Oxidative stress exacerbates NO scavenging, leading to vasoconstriction and reduced oxygen delivery. Improper regulation of cell-free Hb increases the risk of ischemia-reperfusion injury, thereby increasing potential risks.123
Furthermore, the susceptibility of extracellular Hb to oxidation and conversion to high-iron Hb is increased as a result of NADPH reductase depletion. Hemoglobinemia with high-iron Hb levels exceeding 50% may affect heart, metabolism, and coagulopathy.124 However, high-iron hemoglobinemia rarely occurs after the infusion of HBOC-201, and its levels are easily treatable when they do usually not exceeding 15–20%. Treatment with ascorbic acid and methylene blue has been successfully to avoid oxidation effects of free Hb.125
There have also been reports of more severe adverse events like myocardial infarction, heart failure, arrhythmias, and cerebrovascular accident.126 It is worth considering that certain situations where complementary therapies are unavailable, patients may face higher risks than adverse reactions associated with HBOCs. Although current HBOCs may not be equivalent to allogeneic RBC transfusions, they still serve as a temporary source of oxygen for critically injured patients who require transfusions (eg, battlefield, emergency settings, or cases where patients refuse transfusions) until their hematopoietic system can generate enough RBCs.55,127,128 Nonetheless, conducting more trials to assess the effectiveness and safety of HBOCs is necessary to determine the most suitable clinical situations and to balance the benefits and risks of HBOCs.110
Due to the current unfavorable environment and lack of government support, the development of new HBOCs is facing funding shortages.129 However, in such circumstances, it is essential to coordinate the efforts of academia, industry, and government to promote the development and clinical application of HBOCs.129 Academia provides technical support and theoretical foundations for HBOC development through fundamental research and attracts investment from both industry and government. Industry can offer experience and expertise in large-scale production, assist in translating laboratory research into marketable products, and generate commercial profits. The government can provide start-up capital and regulatory support, such as streamlining approval processes and providing regulatory guidance, thus to accelerate the entry of HBOCs into the market. It is anticipated that the new generation of HBOCs will breakthrough current restrictions and revolutionize the practices in the wide range of environmental conditions, thereby making significant contributions to human health.
Conclusion
The study utilized scientometric analysis method to examine the SCIE database’s literature on HBOCs over the three decades, with the aim of elucidating the prevailing trends in current research hotspots. Our results demonstrated the research trends, major research directions, productive countries, and institutions that have focused on HBOCs. Through statistical analysis of scientometrics, the most productive country, institute, field, journal and most-cited journal, co-cited reference, and keywords were identified. Meanwhile, the review provides a comprehensive summary of the type, physiological mechanism, clinical application scenarios, potential adverse events and its management strategies for HBOCs based on the analysis of the most frequent keywords. These not only provide the historical development and current research hotspots on HBOCs across different stages but also the grey areas that require further investigation.
Acknowledgments
This work was financially supported from the Key Research Project of Application Foundation of Logistics Support Department of Central Military Commission [BWS21J002].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Liu WL, Liu T, Zou MZ, et al. Aggressive man‐made red blood cells for hypoxia‐resistant photodynamic therapy. Adv Mater. 2018;30(35):1802006. doi:10.1002/adma.201802006
2. Sen Gupta A, Doctor A. Oxygen carriers. Damage Control Resuscit. 2020;2020:197–222.
3. Bialas C, Moser C, Sims CA. Artificial oxygen carriers and red blood cell substitutes: a historic overview and recent developments toward military and clinical relevance. J Trauma Acute Care Surg. 2019;87(1S):S48–S58. doi:10.1097/TA.0000000000002250
4. Coll-Satue C, Bishnoi S, Chen J, Hosta-Rigau L. Stepping stones to the future of haemoglobin-based blood products: clinical, preclinical and innovative examples. Biomater Sci. 2021;9(4):1135–1152. doi:10.1039/D0BM01767A
5. D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55(1):205–219. doi:10.1111/trf.12804
6. Devine DV, Serrano K. The platelet storage lesion. Clin Lab Med. 2010;30(2):475–487. doi:10.1016/j.cll.2010.02.002
7. Okamoto W, Hasegawa M, Usui T, et al. Hemoglobin-albumin clusters as an artificial O2 carrier: physicochemical properties and resuscitation from hemorrhagic shock in rats. J Biomed Mater Res. 2022;110(8):1827–1838. doi:10.1002/jbm.b.35040
8. Prokopchuk‐Gauk O, Petraszko T, Nahirniak S, Doncaster C, Levy I. Blood shortages planning in Canada: the national emergency blood management committee experience during the first 6 months of the COVID −19 pandemic. Transfusion. 2021;61(11):3258. doi:10.1111/trf.16661
9. Bian Y, Chang TMS. A novel nanobiotherapeutic poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a rat model with 90 minutes of sustained severe hemorrhagic shock with loss of 2/3 blood volume. Artif Cells Nanomed Biotechnol. 2015;43(1):1–9. doi:10.3109/21691401.2014.964554
10. Guo C, Chang TMS. Long term safety and immunological effects of a nanobiotherapeutic, bovine poly-[hemoglobin-catalase-superoxide dismutase-carbonic anhydrase], after four weekly 5% blood volume top-loading followed by a challenge of 30% exchange transfusion. Artif Cells, Nanomed Biotechnol. 2018;46(7):1349–1363. doi:10.1080/21691401.2018.1476375
11. Bian Y, Guo C, Chang TM. Temperature stability of Poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] in the form of a solution or in the lyophilized form during storage at− 80° C, 4° C, 25° C and 37° C or pasteurization at 70° C. Artif Cells, Nanomed Biotechnol. 2016;44(1):41–47. doi:10.3109/21691401.2015.1110871
12. Guo C, Gynn M, Chang T. Extraction of superoxide dismutase, catalase, and carbonic anhydrase from stroma-free red blood cell hemolysate for the preparation of the nanobiotechnological complex of polyhemoglobin–superoxide dismutase–catalase–carbonic anhydrase. Artif Cells, Nanomed Biotechnol. 2015;43(3):157–162. doi:10.3109/21691401.2015.1035479
13. Chang TMS. Translational feasibility of soluble nanobiotherapeutics with enhanced red blood cell functions. Artif Cells, Nanomed Biotechnol. 2017;45(4):671–676. doi:10.1080/21691401.2017.1293676
14. Cebrino J, Portero de la Cruz S. A worldwide bibliometric analysis of published literature on workplace violence in healthcare personnel. PLoS One. 2020;15(11):e0242781. doi:10.1371/journal.pone.0242781
15. Gall M, Nguyen KH, Cutter SL. Integrated research on disaster risk: is it really integrated? Int J Disaster Risk Reduct. 2015;12:255–267. doi:10.1016/j.ijdrr.2015.01.010
16. Chen X, Yang K, Xu Y, Li K. Top-100 highest-cited original articles in inflammatory bowel disease: a bibliometric analysis. Medicine. 2019;98(20):1.
17. Perazzo MF, Otoni ALC, Costa MS, Granville‐Granville AF, Paiva SM, Martins‐Júnior PA. The top 100 most‐cited papers in Paediatric Dentistry journals: a bibliometric analysis. Int J Paediatr Dent. 2019;29(6):692–711. doi:10.1111/ipd.12563
18. Xu D, Wang Y-L, Wang K-T, et al. A scientometrics analysis and visualization of depressive disorder. Curr Neuropharmacol. 2021;19(6):766–786. doi:10.2174/1570159X18666200905151333
19. Wu H, Tong L, Wang Y, Yan H, Sun Z. Bibliometric analysis of global research trends on ultrasound microbubble: a quickly developing field. Front Pharmacol. 2021;12:646626. doi:10.3389/fphar.2021.646626
20. Van Eck N, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
21. Chen C. Science mapping: a systematic review of the literature. J Data Inf Sci. 2017;2(2):1–40. doi:10.1515/jdis-2017-0006
22. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000–2014). Expert Opin Biol Ther. 2014;14(9):1295–1317. doi:10.1517/14712598.2014.920813
23. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. Am Med Inform Assoc. 2005;2005:724.
24. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. 2004;101(suppl_1):5303–5310. doi:10.1073/pnas.0307513100
25. Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. 2017;11(4):959–975. doi:10.1016/j.joi.2017.08.007
26. Shen Z, Wu H, Chen Z, et al. The global research of artificial intelligence on prostate cancer: a 22-year bibliometric analysis. Front Oncol. 2022;12:843735. doi:10.3389/fonc.2022.843735
27. Bornmann L, Daniel HD. What do we know about the h index? J Am Soc Inf Sci. 2007;58(9):1381–1385. doi:10.1002/asi.20609
28. The Ohio State University. Andre Palmer Biography. Available from: https://cbe.osu.edu/people/palmer.351.
29. US Food & Drug Administration. Evaluating the Safety and Efficacy of Hemoglobin-based Blood Substitutes. Available from: https://www.fda.gov/vaccines-blood-biologics/biologics-research-projects/evaluating-safety-and-efficacy-hemoglobin-based-blood-substitutes.
30. Liu H, Chen H, Hong R, Liu H, You W. Mapping knowledge structure and research trends of emergency evacuation studies. Saf Sci. 2020;121:348–361. doi:10.1016/j.ssci.2019.09.020
31. Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299(19):2304–2312. doi:10.1001/jama.299.19.jrv80007
32. Levy JH, Goodnough LT, Greilich PE, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124(1):35–42. doi:10.1067/mtc.2002.121505
33. Sprung J, Kindscher JD, Wahr JA, et al. The use of bovine hemoglobin glutamer-250 (Hemopure®) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesth Analg. 2002;94(4):799–808. doi:10.1097/00000539-200204000-00006
34. Chen J-Y, Scerbo M, Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics. 2009;64(8):803–813. doi:10.1590/S1807-59322009000800016
35. Alayash AI. Blood substitutes: why haven’t we been more successful? Trends Biotechnol. 2014;32(4):177–185. doi:10.1016/j.tibtech.2014.02.006
36. Levien LJ, Hodgson RE, James MF. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300(11):1295. doi:10.1001/jama.300.11.1295-A
37. Mackenzie CF, Pitman AN, Hodgson RE, et al. Are hemoglobin-based oxygen carriers being withheld because of regulatory requirement for equivalence to packed red blood cells? Am J Ther. 2015;22(4):e115–21. doi:10.1097/MJT.0000000000000009
38. Mer M, Hodgson E, Wallis L, et al. Hemoglobin glutamer-250 (bovine) in South Africa: consensus usage guidelines from clinician experts who have treated patients. Transfusion. 2016;56(10):2631–2636. doi:10.1111/trf.13726
39. Shander A, Javidroozi M, Thompson G. Letter to the editor (2008): hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300(11):1296–1297. doi:10.1001/jama.300.11.1296-b
40. Li H, An H, Wang Y, Huang J, Gao X. Evolutionary features of academic articles co-keyword network and keywords co-occurrence network: based on two-mode affiliation network. Phys a Stat Mech Appl. 2016;450:657–669. doi:10.1016/j.physa.2016.01.017
41. Liu X, Zhan FB, Hong S, Niu B, Liu Y. A bibliometric study of earthquake research: 1900–2010. Scientometrics. 2012;92(3):747–765. doi:10.1007/s11192-011-0599-z
42. Yan W, Zheng K, Weng L, et al. Bibliometric evaluation of 2000–2019 publications on functional near-infrared spectroscopy. Neuroimage. 2020;220:117121. doi:10.1016/j.neuroimage.2020.117121
43. Bai B, Bai X, Wang C. Mapping research trends of temporomandibular disorders from 2010 to 2019: a bibliometric analysis. J Oral Rehabil. 2021;48(5):517–530. doi:10.1111/joor.13143
44. Muir WW, Wellman ML. Hemoglobin solutions and tissue oxygenation. J Vet Intern Med. 2003;17(2):127–135. doi:10.1111/j.1939-1676.2003.tb02423.x
45. Sakai H, Masada Y, Takeoka S, Tsuchida E. Characteristics of bovine hemoglobin as a potential source of hemoglobin-vesicles for an artificial oxygen carrier. J Biochem. 2002;131(4):611–617. doi:10.1093/oxfordjournals.jbchem.a003141
46. Johnstone JE, MacLaren LA, Doucet J, McAlister VC. In vitro studies regarding the feasibility of bovine erythrocyte xenotransfusion. Xenotransplantation. 2004;11(1):11–17. doi:10.1111/j.1399-3089.2004.00070.x
47. Mallet V, Dutheil D, Polard V, et al. Dose-ranging study of the performance of the natural oxygen transporter HEMO2 Life in organ preservation. Artif Organs. 2014;38(8):691–701. doi:10.1111/aor.12307
48. Cao M, Wang G, He H, et al. Hemoglobin-based oxygen carriers: potential applications in solid organ preservation. Front Pharmacol. 2021;12:760215. doi:10.3389/fphar.2021.760215
49. Lupon E, Lellouch AG, Zal F, Cetrulo CL, Lantieri LA. Combating hypoxemia in COVID-19 patients with a natural oxygen carrier, HEMO(2)Life® (M101). Med Hypotheses. 2021;146:110421. doi:10.1016/j.mehy.2020.110421
50. Rogers DM, Crookston KP. The approach to the patient who refuses blood transfusion. Transfusion. 2006;46(9):1471–1477. doi:10.1111/j.1537-2995.2006.00947.x
51. Bunn HF, Esham WT, Bull RW. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969;129(5):909–923. doi:10.1084/jem.129.5.909
52. Buehler PW, D’Agnillo F, Schaer DJ. Hemoglobin-based oxygen carriers: from mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 2010;16(10):447–457. doi:10.1016/j.molmed.2010.07.006
53. Ortiz D, Barros M, Yan S, Cabrales P. Resuscitation from hemorrhagic shock using polymerized hemoglobin compared to blood. Am J Emerg Med. 2014;32(3):248–255. doi:10.1016/j.ajem.2013.11.045
54. Jahr JS, Moallempour M, Lim JC. HBOC-201, hemoglobin glutamer-250 (bovine), Hemopure (Biopure Corporation). Expert Opin Biol Ther. 2008;8(9):1425–1433. doi:10.1517/14712598.8.9.1425
55. Weiskopf RB, Beliaev AM, Shander A, et al. Addressing the unmet need of life-threatening anemia with hemoglobin-based oxygen carriers. Transfusion. 2017;57(1):207–214. doi:10.1111/trf.13923
56. Chang TM. Semipermeable microcapsules. Science. 1964;146(3643):524–525. doi:10.1126/science.146.3643.524
57. Chang TM. Blood replacement with nanobiotechnologically engineered hemoglobin and hemoglobin nanocapsules. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2(4):418–430. doi:10.1002/wnan.95
58. Moritz ED, Winton CS, Tonnetti L, et al. Screening for Babesia microti in the US blood supply. N Engl J Med. 2016;375(23):2236–2245. doi:10.1056/NEJMoa1600897
59. Li S, Nickels J, Palmer AF. Liposome-encapsulated actin–hemoglobin (LEAcHb) artificial blood substitutes. Biomaterials. 2005;26(17):3759–3769. doi:10.1016/j.biomaterials.2004.09.015
60. Kaneda S, Ishizuka T, Sekiguchi A, Morimoto K, Kasukawa H. Efficacy of liposome‐encapsulated hemoglobin in a rat model of cerebral ischemia. Artif Organs. 2014;38(8):650–655. doi:10.1111/aor.12358
61. Sakai H, Sou K, Horinouchi H, Kobayashi K, Tsuchida E. Review of hemoglobin-vesicles as artificial oxygen carriers. Artif Organs. 2009;33(2):139–145. doi:10.1111/j.1525-1594.2008.00698.x
62. Qin Y, Cheng C, Geng H, et al. Efficient ambipolar transport properties in alternate stacking donor-acceptor complexes: from experiment to theory. Phys Chem Chem Phys. 2016;18(20):14094–14103. doi:10.1039/C6CP01509C
63. Intaglietta M, Cabrales P, Tsai AG. Microvascular perspective of oxygen-carrying and -noncarrying blood substitutes. Annu Rev Biomed Eng. 2006;8:289–321. doi:10.1146/annurev.bioeng.8.061505.095713
64. McCarthy MR, Vandegriff KD, Winslow RM. The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem. 2001;92(1–2):103–117. doi:10.1016/S0301-4622(01)00194-6
65. Sakai H, Hara H, Yuasa M, et al. Molecular dimensions of Hb-based O(2) carriers determine constriction of resistance arteries and hypertension. Am J Physiol Heart Circ Physiol. 2000;279(3):H908–15. doi:10.1152/ajpheart.2000.279.3.H908
66. Winslow RM, Gonzales A, Gonzales ML, et al. Vascular resistance and the efficacy of red cell substitutes in a rat hemorrhage model. J Appl Physiol. 1998;85(3):993–1003. doi:10.1152/jappl.1998.85.3.993
67. Karmakar N, Dhar P. Effect of steady shear stress on fluid filtration through the rabbit arterial wall in the presence of macromolecules. Clin Exp Pharmacol Physiol. 1996;23(4):299–304. doi:10.1111/j.1440-1681.1996.tb02827.x
68. de Wit C, Von bismarck P, Bolz -S-S, Pohl U, Scähfer C. Elevation of plasma viscosity induces sustained NO-mediated dilation in the hamster cremaster microcirculation in vivo. Pflugers Arch. 1997;434(4):354–361. doi:10.1007/s004240050408
69. Intaglietta M, Johnson PC, Winslow RM. Microvascular and tissue oxygen distribution. Cardiovasc Res. 1996;32(4):632–643. doi:10.1016/S0008-6363(96)00110-1
70. Fischer SR, Burnet M, Traber DL, Prough DS, Kramer GC. Plasma volume expansion with solutions of hemoglobin, albumin, and Ringer lactate in sheep. Am J Physiol. 1999;276(6):H2194–203. doi:10.1152/ajpheart.1999.276.6.H2194
71. Migita R, Gonzales A, Gonzales ML, Vandegriff KD, Winslow RM. Blood volume and cardiac index in rats after exchange transfusion with hemoglobin-based oxygen carriers. J Appl Physiol. 1997;82(6):1995–2002. doi:10.1152/jappl.1997.82.6.1995
72. Duling BR, Berne RM. Longitudinal gradients in periarteriolar oxygen tension. A possible mechanism for the participation of oxygen in local regulation of blood flow. Circ Res. 1970;27(5):669–678. doi:10.1161/01.RES.27.5.669
73. Chen G, Duan Y, Liu J, Wang H, Yang C. Antioxidant effects of vitamin C on hemoglobin-based oxygen carriers derived from human cord blood. Artif Cells Nanomed Biotechnol. 2016;44(1):56–61. doi:10.3109/21691401.2015.1111239
74. Chagnon F, Bentourkia M, Lecomte R, Lessard M, Lesur O. Endotoxin-induced heart dysfunction in rats: assessment of myocardial perfusion and permeability and the role of fluid resuscitation. Crit Care Med. 2006;34(1):127–133. doi:10.1097/01.CCM.0000190622.02222.DF
75. Berkowitz DE. Myocyte nitroso-redox imbalance in sepsis: NO simple answer. Circ Res. 2007;100(1):1–4. doi:10.1161/01.RES.0000255898.65901.9d
76. Spinella PC. Zero preventable deaths after traumatic injury: an achievable goal. J Trauma Acute Care Surg. 2017;82(6S):S2–S8. doi:10.1097/TA.0000000000001425
77. Eastridge BJ, Hardin M, Cantrell J, et al. Died of wounds on the battlefield: causation and implications for improving combat casualty care. Journal of Trauma: Injury, Infection & Critical Care. 2011;71(1):S4–S8. doi:10.1097/TA.0b013e318221147b
78. Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9(Suppl 5):S1–9. doi:10.1186/cc3779
79. Abramson D, Scalea TM, Hitchcock R, Trooskin SZ, Henry SM, Greenspan J. Lactate clearance and survival following injury. J Trauma. 1993;35(4):584–588. doi:10.1097/00005373-199310000-00014
80. Manikis P, Jankowski S, Zhang H, Kahn RJ, Vincent J-L. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med. 1995;13(6):619–622. doi:10.1016/0735-6757(95)90043-8
81. Régnier M-A, Raux M, Le Manach Y, et al. Prognostic significance of blood lactate and lactate clearance in trauma patients. Anesthesiology. 2012;117(6):1276–1288. doi:10.1097/ALN.0b013e318273349d
82. Keipert PE. Hemoglobin-Based Oxygen Carrier (HBOC) development in trauma: previous regulatory challenges, lessons learned, and a path forward. Adv Exp Med Biol. 2017;977:343–350.
83. Zhang J, Cao S, Ma L, Hsia C, Koehler R. Abstract 154: protection from transient focal cerebral ischemia by transfusion of polynitroxylated pegylated hemoglobin. Stroke. 2013;44(suppl_1). doi:10.1161/str.44.suppl_1.A154
84. Shellington DK, Du L, Wu X, et al. Polynitroxylated pegylated hemoglobin: a novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit Care Med. 2011;39(3):494–505.
85. Jahr JS, Akha AS, Holtby RJ. Crosslinked, polymerized, and PEG-conjugated hemoglobin-based oxygen carriers: clinical safety and efficacy of recent and current products. Curr Drug Discov Technol. 2012;9(3):158–165. doi:10.2174/157016312802650742
86. Cohen MJ, Kutcher M, Redick B, et al. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(Suppl Supplement 1):S40–7. doi:10.1097/TA.0b013e31828fa43d
87. Defence Visual Information Distribution Service. U.S. Army medical researchers partnering with South African experts on synthetic blood project. Available from: https://www.dvidshub.net/news/311421/us-army-medical-researchers-partnering-with-south-african-experts-synthetic-blood-project.
88. The University of Maryland, Baltimore. Artificial Blood One Step Closer to Reality. Available from: https://www.umaryland.edu/news/archived-news/february-2023/artificial-blood-one-step-closer-to-reality.php.
89. Kaminski J, Hannaert P, Kasil A, et al. Efficacy of the natural oxygen transporter HEMO 2 life® in cold preservation in a preclinical porcine model of donation after cardiac death. Transpl Int. 2019;32(9):985–996. doi:10.1111/tri.13434
90. Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int. 2015;28(6):657–664.
91. Teh ES, Zal F, Polard V, Menasché P, Chambers DJ. HEMO 2 life as a protective additive to Celsior solution for static storage of donor hearts prior to transplantation. Artif Cells, Nanomed Biotechnol. 2017;45(4):717–722. doi:10.1080/21691401.2016.1265974
92. Kasil A, Giraud S, Couturier P, et al. Individual and combined impact of oxygen and oxygen transporter supplementation during kidney machine preservation in a porcine preclinical kidney transplantation model. Int J Mol Sci. 2019;20(8):1992. doi:10.3390/ijms20081992
93. Le Meur Y, Badet L, Essig M, et al. First-in-human use of a marine oxygen carrier (M101) for organ preservation: a safety and proof-of-principle study. Am J Transplant. 2020;20(6):1729–1738. doi:10.1111/ajt.15798
94. Ferenz KB, Steinbicker AU. Artificial oxygen carriers—past, present, and future—a review of the most innovative and clinically relevant concepts. J Pharmacol Exp Ther. 2019;369(2):300–310. doi:10.1124/jpet.118.254664
95. National Library of Medicine. ClinicalTrials.gov. NCT04181710. Available from: https://clinicaltrials.gov/.
96. Njoku M, St Peter D, Mackenzie CF. Haemoglobin-based oxygen carriers: indications and future applications. Br J Hosp Med. 2015;76(2):78–83. doi:10.12968/hmed.2015.76.2.78
97. Davis JM, El-Haj N, Shah NN, et al. Use of the blood substitute HBOC-201 in critically ill patients during sickle crisis: a three-case series. Transfusion. 2018;58(1):132–137. doi:10.1111/trf.14386
98. Donahue LL, Shapira I, Shander A, Kolitz J, Allen S, Greenburg G. Management of acute anemia in a Jehovah’s Witness patient with acute lymphoblastic leukemia with polymerized bovine hemoglobin-based oxygen carrier: a case report and review of literature. Transfusion. 2010;50(7):1561–1567. doi:10.1111/j.1537-2995.2010.02603.x
99. Gomez MF, Aljure O, Ciancio G, Lynn M. Hemoglobin-based oxygen carrier rescues double-transplant patient from life-threatening anemia. Am J Transplant. 2017;17(7):1941–1944. doi:10.1111/ajt.14226
100. Gannon CJ, Napolitano LM. Severe anemia after gastrointestinal hemorrhage in a Jehovah’s witness: new treatment strategies. Crit Care Med. 2002;30(8):1893–1895. doi:10.1097/00003246-200208000-00036
101. Winslow RM. Clinical Indications for Blood Substitutes and Optimal Properties. London: Elsevier; 2006:115–125.
102. Smani Y. Hemospan: a hemoglobin-based oxygen carrier for potential use as a blood substitute and for the potential treatment of critical limb ischemia. Curr Opin Investig Drugs. 2008;9(9):1009–1019.
103. Fustier C, Chang TM. PEG-PLA nanocapsules containing a nanobiotechnological complex of polyhemoglobin-tyrosinase for the depletion of tyrosine in melanoma: preparation and in vitro characterisation. J Nanomedine Biotherapeutic Discov. 2012;2(1):1–9. doi:10.4172/2155-983X.1000103
104. Wang Y, Chang TM. Nanobiotechnological nanocapsules containing polyhemoglobin-tyrosinase: effects on murine B16F10 melanoma cell proliferation and attachment. J Skin Cancer. 2012;2012:673291. doi:10.1155/2012/673291
105. Han J, Yu M, Dai M, Li H, Xiu R, Liu Q. Decreased expression of MDR1 in PEG-conjugated hemoglobin solution combined cisplatin treatment in a tumor xenograft model. Artif Cells Blood Substit Immobil Biotechnol. 2012;40(4):239–244. doi:10.3109/10731199.2012.663385
106. Shoemaker SA, Gerber MJ, Evans GL, Archer-Paik LE, Scoggin CH. Initial clinical experience with a rationally designed, genetically engineered recombinant human hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 1994;22(3):457–465. doi:10.3109/10731199409117874
107. Zhang X, Chen G, Liu Y, Sun L, Sun L, Zhao Y. Black phosphorus-loaded separable microneedles as responsive oxygen delivery carriers for wound healing. ACS Nano. 2020;14(5):5901–5908. doi:10.1021/acsnano.0c01059
108. Kuang L, Zhu Y, Zhang J, et al. A novel cross-linked haemoglobin-based oxygen carrier is beneficial to sepsis in rats. Artif Cells Nanomed Biotechnol. 2019;47(1):1496–1504. doi:10.1080/21691401.2019.1602049
109. Taverne Y J, de Wijs-Meijler D, te Lintel Hekkert M, et al. Normalization of hemoglobin-based oxygen carrier-201 induced vasoconstriction: targeting nitric oxide and endothelin. Journal of Applied Physiology. 2017;122(5):1227–1237. doi:10.1152/japplphysiol.00677.2016
110. Silverman TA, Weiskopf RB, Committee P. Hemoglobin-based oxygen carriers: current status and future directions. J Am Soc Anesthesiol. 2009;111(5):946–963.
111. Butt OI, Buehler PW, D'Agnillo F. Differential Induction of Renal Heme Oxygenase and Ferritin in Ascorbate and Nonascorbate Producing Species Transfused with Modified Cell-Free Hemoglobin. Antioxidants & Redox Signaling. 2010;12(2):199–208. doi:10.1089/ars.2009.2798
112. Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. HBOC-201 as an Alternative to Blood Transfusion: Efficacy and Safety Evaluation in a Multicenter Phase III Trial in Elective Orthopedic Surgery. Journal of Trauma: Injury, Infection & Critical Care. 2008;64(6):1484–1497. doi:10.1097/TA.0b013e318173a93f
113. Gomez MF, Aljure O, Ciancio G, Lynn M. Hemoglobin-Based Oxygen Carrier Rescues Double-Transplant Patient From Life-Threatening Anemia. Am J Transplant. 2017;17(7):1941–1944.
114. Korte EA, Pozzi N, Wardrip N, Ayyoubi M, Jortani SA. Analytical interference of HBOC-201 (Hemopure, a synthetic hemoglobin-based oxygen carrier) on four common clinical chemistry platforms. Clinica Chimica Acta. 2018;482:33–39. doi:10.1016/j.cca.2018.03.017
115. Buehler PW, Alayash AI. Oxidation of hemoglobin: mechanisms of control in vitro and in vivo. Transfus Alternat Transfus Med. 2007;9(4):204–212. doi:10.1111/j.1778-428X.2007.00081.x
116. Schaäfer A, Wiesmann F, Neubauer S, Eigenthaler M, Bauersachs J, Channon KM. Rapid Regulation of Platelet Activation In Vivo by Nitric Oxide. Circulation. 2004;109(15):1819–1822. doi:10.1161/01.CIR.0000126837.88743.DD
117. Griffiths E, Cortes A, Gilbert N, Stevenson P, MacDonald S, Pepper D. Haemoglobin-based blood substitutes and sepsis. The Lancet. 1995;345(8943):158–160. doi: 10.1016/S0140-6736(95)90168-X
118. Jahr JS, Nesargi SB, Lewis K, Johnson C. Blood substitutes and oxygen therapeutics: an overview and current status. Am J Ther. 2002;9(5):437–443. doi:10.1097/00045391-200209000-00012
119. Bernard AC, Moore EE, Moore FA, et al. Postinjury resuscitation with human polymerized hemoglobin prolongs early survival: a post hoc analysis. J Trauma. 2011;70(5 Suppl):S34–7. doi:10.1097/TA.0b013e31821a586e
120. Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol. 2006;26(4):697–705. doi:10.1161/01.ATV.0000204350.44226.9a
121. Marrazzo F, Larson G, Sherpa Lama TT, et al. Inhaled nitric oxide prevents systemic and pulmonary vasoconstriction due to hemoglobin-based oxygen carrier infusion: a case report. J Crit Care. 2019;51:213–216. doi:10.1016/j.jcrc.2018.04.008
122. Gould SA, Moore EE, Hoyt DB, et al. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195(4):445–452. doi:10.1016/S1072-7515(02)01335-2
123. Hsia CJ, Ma L. A hemoglobin-based multifunctional therapeutic: polynitroxylated pegylated hemoglobin. Artif Organs. 2012;36(2):215–220. doi:10.1111/j.1525-1594.2011.01307.x
124. Moallempour M, Jahr JS, Lim JC, Weeks D, Butch A, Driessen B. Methemoglobin effects on coagulation: a dose-response study with HBOC-200 (Oxyglobin) in a thrombelastogram model. J Cardiothorac Vasc Anesth. 2009;23(1):41–47. doi:10.1053/j.jvca.2008.06.006
125. Murphy MF, Wallington TB, Kelsey P, et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113(1):24–31.
126. Kozek-Langenecker SA. The effects of drugs used in anaesthesia on platelet membrane receptors and on platelet function. Curr Drug Targets. 2002;3(3):247–258. doi:10.2174/1389450023347759
127. Posluszny JA, Napolitano LM. Hemoglobin-based oxygen carrier for traumatic hemorrhagic shock treatment in a Jehovah’s witness. Arch Trauma Res. 2016;5(2). doi:10.5812/atr.30610
128. Sarani B, Gracias V. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. JAMA. 2008;300(11):1295–1299. doi:10.1001/jama.300.11.1297-b
129. Kim HW, Jahr JS, Mozzarelli A, Sakai H. International consortium for development of hemoglobin-based oxygen carriers, oxygen therapeutics and multifunctional resuscitation fluids–a white paper. Hemoglobin Based Oxygen Carriers Red Cell Substit Oxygen Therapeut. 2013;2013:737–746.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

