Back to Journals » Journal of Inflammation Research » Volume 16
Toxic Epidermal Necrolysis and Stevens - Johnson Syndrome Following Sintilimab Administration in a Non-Small Cell Lung Cancer Patient: A Case Report
Authors Jiang Z, Chen X, Sun Z, Shen X, Huang Y, Liu J
Received 23 June 2023
Accepted for publication 25 October 2023
Published 2 November 2023 Volume 2023:16 Pages 5061—5067
DOI https://doi.org/10.2147/JIR.S427336
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ziyu Jiang,1,2,* Xiaoli Chen,2,3,* Zhaoshen Sun,2,4 Xiaowei Shen,2,3 Yaju Huang,2,3 Jingbing Liu1,2
1Department of Oncology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210028, People’s Republic of China; 2Jiangsu Province Academy of Traditional Chinese Medicine, Nanjing, 210028, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210028, People’s Republic of China; 4Department of Dermatology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210028, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingbing Liu; Yaju Huang, Department of Oncology, Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine, Nanjing, 210028, People’s Republic of China, Tel +86 025 85637121 ; +86 025 85639241, Email [email protected]; [email protected]
Abstract: Immune checkpoint inhibitors such as monoclonal antibodies have been used recently with greater effect for the management of non-small cell lung cancer (NSCLC). Sintilimab, a fully human IgG4 monoclonal antibody is specific for the immune checkpoint protein programmed cell death receptor-1 (PD-1). It is a common medication adopted for treating Hodgkin’s lymphoma and NSCLC. The adverse effects associated with the use of monoclonal antibodies should be closely monitored and in the current report, the use of sintilimab for treating NSCLC led to skin-associated adverse effects such as Stevens-Johnson syndrome and toxic epidermal necrolysis. Genetic testing showed that genes such as KRAS, CREBBP, NTRK1, RAF1, and TP53 were mutated. Initial visible symptom included the formation of a vesicular rash on the skin that had spread to the upper limbs, chest, and dorsum 1 week after the administration of sintilimab. The patient received anti-inflammatory agents to prevent worsening of the rashes and further infections. When the vesicles in back and limbs enlarged and the neck skin began to desquamate, the patient was diagnosed with Stevens-Johnson syndrome and sintilimab-induced toxic epidermal necrolysis. Toxic epidermal necrolysis was diagnosed via clinical symptoms and physical examination. The patient also reported the symptoms of oral mucositis. As soon as the dose of sintilimab was reduced to 20 mg/day, the skin-associated condition of the patient began to improve. Although the lump in the lungs decreased considerably 45 days after initial administration of sintilimab, the medication was stopped from use as soon as the skin-related symptoms improved after its withdrawal. This report suggests that close monitoring, personal care, and proper use of medications such as sintilimab should be implemented to avoid such rare skin-associated toxicities as an adverse effect.
Keywords: sintilimab, checkpoint inhibitor, monoclonal antibody, NSCLC, adverse effects
Introduction
Adverse drug reactions (ADRs) are usually caused by errors in medications and their doses, leading to undesirable side effects or allergies that may occur as a result of hospital admission.1 It can arise due to diverse reasons including age, gender, route of administration, combinatorial interventions, genetic predispositions, comorbid conditions, and drug sensitivity of a patient.2,3 Information on the epidemiology of ADRs and primary care is scarce in published literature considering the life-threatening effects of ADRs associated with immunotherapy. Hence, creating an awareness and empowering a wide range of patients by educating them about the treatment-related ADRs and reporting them at the right time can help improve the treatment outcomes.4,5 Personal medicine can reduce the mortality associated with hospitalization. Adverse effects can be classified into two types: augmented, predictable, and preventable dose-related type A and bizarre and unpredictable non-dose-related type B pharmacological effects.6,7
Monoclonal antibodies are a new class of antitumor agents used for targeted therapy of cancer by enhancing the effects of the immune system in clinic.8 These agents were expected to be less toxic than conventional chemotherapy by specifically targeting the cancer cells.9 In this connection, Sintilimab is an anti-PD-1 monoclonal antibody elucidated recently to possess antitumor effects and is widely used to treat NSCLC in China. It has been reported to be safe for use in targeted therapy of NSCLC compared to nivolumab and pembrolizumab.10 The antibody was studied for the very first time with regard to its clinical effects on lymphoma in March 2017 and on NSCLC in December 2017. Hence, it is relatively a new class of antibody used to treat NSCLC as a neoadjuvant and in monotherapy. The major pathological response and pathologic complete response for sintilimab in treating NSCLC were more than 40% and 16%, respectively, with a greater 2-year disease-free survival rate. According to their pharmacokinetic behaviour, the epitope of sintilimab towards the PD-1 complex is situated at the FG loop of PD-1. It is efficient in binding (owing to the hydrophobic and aromatic amino acid residues in the complementarity-determining region and a low dissociation constant of 74 pM for better activation of T-cells) with slower off-rate compared to several other monoclonal antibodies. Hence, they can stay at the targeted site for longer durations producing enhanced drug effect. Sintilimab at 1 mg/kg showed high levels of occupying PD-1 receptor in both tissues and blood. The monoclonal antibody showed no anti-drug antibody-positive reactions (0.52% of clinical patients) and did not critically raise neutralizing antibodies (0.26% of patients) after therapeutic administration. Yet, it is effective in producing antitumor effects at limited doses (range of 1 to 10 mg/kg).11,12
Although Sintilimab as a neoadjuvant has shown positive outcomes in NSCLC treatment, Stevens-Johnson syndrome (SJS) is a rare life-threatening type IV hypersensitivity reaction to immune checkpoint inhibitors (ICIs) that target programmed cell death protein 1 (PD-1).13,14 SJS and toxic epidermal necrolysis (TEN) are life-threatening hypersensitivity reactions intermediated by cytotoxic T lymphocytes displaying blistering autoimmune reactions of the mucocutaneous regions with detachment of the epidermis and pervasive necrosis. The skin reaction of more than 30% is referred to as TEN, whereas the reactions existing to less than 10% of the body are referred to as SJS.15 To the best of our knowledge, this is one of the very few case reports published till date on TEN and SJS as an ADR of Sintilimab therapy in a patient with NSCLC. Therefore, the objectives were to present the clinical indices of ADR to Sintilimab, to prevent it, and to understand the pathophysiology of this ADR.
Case Presentation
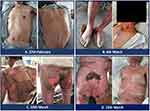
A 68-year-old man was diagnosed with stage IV right lung adenocarcinoma with liver and mediastinal lymph node metastasis in January 2020. Genetic testing was performed on the patient’s blood and tumor tissues. The result showed that genes such as KRAS, CREBBP, NTRK1, RAF1, and TP53 were mutated. The tumor mutation burden (TMB) was 5.2 per MB, microsatellite stable (MSS), and the expression of PD-L1 was 90% (TPS, tumor proportion score). On 31st January, the patient received first-line chemotherapy with an AP formulation: pemetrexed 0.75 g d1, cisplatin (40 mg d1-3). To improve the effect and refer to the gene test results, the patient received sintilimab (200 mg) on 20 February 2020, followed by chemotherapy with the AP formulation at the same dose as before. On 27 February 2020, the patient was diagnosed with a vesicular rash on the skin that had spread to the upper limbs, chest, and dorsum (Figure 1A). The patient was orally administered 8 mg methylprednisolone and 8.8 mg loratadine and intravenously administered 5 mg dexamethasone sodium phosphate starting 27th February. A clobetasol propionate cream was used to embrocate the rash. As soon as the patient began to show symptoms of fever (39.3 ◦C) on 1st March, we administered methylprednisolone 40 mg Q8h together with cefoperazone sodium, sulbactam sodium (sulperazone), and vancomycin to prevent further infection. Bacteriological cultures of the patient’s blood, urine, and skin were negative on 4th March.
Although the body temperature decreased to 38 ◦C on 3rd March, the vesicles in back and limbs enlarged, and the neck skin began to desquamate (Figure 1B). Based on these findings and the patient’s history, SJS and TEN induced by Sintilimab were clinically diagnosed. Since skin biopsy was not performed, these two conditions were diagnosed predominantly by means of physical examination.
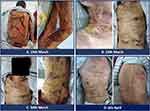
The immunoglobulin was intravenously administered at a dose of 20 mg/day from 5th to 13th March. The desquamation had spread to multiple regions of the head, neck, back, buttocks, and limbs with pain since 10th March, and this condition took 1 week to improve (Figure 1C and D). Hence, methylprednisolone was reduced to 80 mg Q12h from 19th March (Figure 2A). Exudation decreased significantly starting 23rd March (Figure 2B and C), and skin-associated symptoms returned to near-normal levels (Figure 2D). On 19th February, the patient underwent CT scan (before sintilimab infusion). On 6th April, the lump in the right lower lung slightly reduced in dimension from 2.7 cm to 2.4 cm (11%) compared to the former CT scan (Figure 3). The patient did not receive treatment with PD1 antibody again.
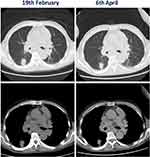 |
Figure 3 CT scans of the tumor before and after treatment with Sintilimab. |
Adverse events arose after sintilimab administration, as confirmed by objective evidence. These effects were alleviated after discontinuation of the medication or administration of antagonist gamma globulin. Reasons other than drugs were not identified as the basis for these after-effects, which did not recur after the placebo administration. Toxic levels were not detected in blood circulation. The severity of the after-effects was neutral. Similar effects were observed in patients who were administered with similar drugs. The modified Naranjo adverse drug reaction questionnaire score was 6, suggesting that the drug was a probable cause of the after-effects.16
As an ADR related to the ulcerations of the oral mucosa, the patient reported pain while swallowing or drinking water, which was an outcome of oral mucositis. Although oral mucositis is a very rare ADR (occurrence rate of less than 10%) arising due to immunotherapy, it was observed in this case.17,18 This ADR is more commonly seen with the use of anti-PD-1 inhibitors than with the use of CTLA-4 inhibitors.19
Informed consent was obtained from the patient before the submission of this case report for publication including the use of images. Ethical approval for this report was obtained from the Affiliated Hospital of Integrated Traditional Chinese and Western Medicine, Nanjing University of Chinese Medicine.
Discussion
Tumor cells often escape the immune response by involving checkpoint proteins, such as PD-1, which may elude the activity elicited by monoclonal antibodies as immune checkpoint inhibitors.20 In the last decade, checkpoint inhibitors have been used to improve the sensitivity of NSCLC cells to such inhibitors by tailored therapy. However, ADRs can develop with constant use of monoclonal antibodies.21 Previous studies have suggested that targeted cancer therapy using monoclonal antibodies can lead to SJS. Drug-reactive T-cells are primarily responsible for ADRs associated with the use of sintilimab, resulting in SJS.22,23 SJS is a lethal or fatal ADR associated with the extensive use of drugs, particularly anticancer agents.24,25
Mutations in carcinogenic KRAS and TP53 genes are widely detected in patients with NSCLC, where conventional chemotherapy may require the assistance of adjuvants. These genes remain drivers of metastasis in patients who are critically-ill.26,27 These mutations could lead to intensified expression of other gene cascades, making the cancerous forms more aggressive. This may lead to a shortening of survival in such patients, even after the administration of therapeutic drugs. This can assist in the upregulation of PD-L1 in NSCLC cases in an ERK-phosphorylation-dependent manner by causing failure in PD-1/PD-L1-targeted therapy. The co-mutated expression of TP53 along with KRAS can be advantageous as the survival value is higher in this case rather than “KRAS-only” mutations.28 Concomitant mutations in CREBBP, NTRK1, and RAF1 have also been observed previously in patients with, NSCLC which can decrease mean progression-free survival.29
An observed 5.2 per MB indicated intermediate TMB, and SJS was observed at this moderate level of TMB in our report.30 The PD-L1 score of 90% TPS was predominantly male sex-associated, accounted for 9% of TDS for all ages regardless of their smoking behavior and was located as a central tumor, as reported previously.31 The cancer cells were capable of efficient mismatch repair, and the tumor cells possessed similar repeats to those of normal cells, as they were microsatellite-stable (MSS). There is a great challenge in treating such ‘cold tumors’ as immune response is hard to exhibit as they are tough to home into tumor environment and are resistant to ICIs. Cold tumors can be transformed into hot tumors for effective therapy.32,33
Sintilimab is a fully human IgG4 monoclonal antibody and an inhibitor that interacts with PD-1 and reinstates antitumor response. It has reached Phase III trials for the treatment of NSCLC with several ongoing clinical trials. Grade 3 or 4 pyrexia is the most common sintilimab-associated ADR. Early symptoms of SJS include fever, malaise, anorexia, pharyngitis, and headache, which can persist for approximately 11 days. Oral and skin lesions were the major types of lesions observed. Pulmonary symptoms can also accompany adults rather than young children. Skin biopsy and immunofluorescence are used for the clinical diagnosis of SJS, whereas SCORTEN is associated with prognosis. The early withdrawal of drugs is critical for prognosis. In addition, elevated levels of granulysin were observed in the serum of patients with SJS before the appearance of skin lesions. In addition, the levels of high-mobility group B protein (HMGB1) and interleukin 15 (IL-15) are correlated with the pathogenesis and progression of SJS. However, reports of systemic therapy are inconclusive. Therefore, calorie and fluid requirements should be monitored, along with supportive care, to decrease mortality.34–36
A vesicular rash of the skin that had spread to the upper limbs, chest, and dorsum was commonly observed in patients with SJS after 4 days to 2 weeks, which was 7 days in the present case.37,38 Methylprednisolone, Dexamethasone, and Loratadine are usually administered to manage and treat SJS symptoms; therefore, they were used in this case.39–41 As supportive care is the primary mode of pain management or therapy in SJS, topical application of clobetasol once daily was recommended and used.42,43
With regard to previous literature that relates to this report, there is a case report that connects the use of sintilimab and TEN as an adverse effect of use in treating a 59-year-old NSCLC patient. Sintilimab was administered as a neoadjuvant along with chemotherapy, which resulted in more than 50% of skin-associated effects referred to as TEN. TEN was diagnosed by the patient’s history of sintilimab administration, medical symptoms, and physical examination. Interestingly, the skin-associated symptoms were observed only after three cycles of neoadjuvant therapy, which resulted in a partial response after lobectomy. The condition of the patient worsened to be diagnosed with TEN after the continuation of neoadjuvant therapy ten days post-surgery. Fever (>40 °C) and maculopapular rashes of the chest and abdomen were observed. To treat the symptoms of sintilimab-induced TEN, anti-inflammatory agents were used, similar to that of our report.44 Similar to sintilimab, anti-PD-1 antibodies such as nivolumab and pembrolizumab resulted in TEN and SJS with fewer occurrences (approximately 0.4% of the adverse effects reported with the use of immune checkpoint inhibitors). The median onset age was 66 and the adverse effects were predominantly observed among male lung cancer patients and almost all patients were withdrawn from monoclonal antibody use as soon as SJS/TEN was observed. SJS/TEN was observed after a median duration of 25.5 days.45 Similar reports suggesting the occurrence of SJS/TEN after the administration of sintilimab or other anti PD-1 antibodies like nivolumab and pembrolizumab for treating other types of malignancies have also been published.46–51
Conclusions
SJS/TEN as an ADR of the PD-1 inhibitor Sintilimab use was reported in this case study. Initially, mutations of oncogenic genes such as KRAS, CREBBP, NTRK1, RAF1, and TP53 indicating the onset of NSCLC was witnessed. Vesicular rashes were observed across the chest, upper limbs, and dorsum regions after the administration of sintilimab. The patient was diagnosed with SJS/TEN after the vesicles in back and limbs enlarged and the neck skin began to shed off. Anti-inflammatory agents were administered to prevent rashes from getting worse and sintilimab administration was withdrawn, which resulted in improvement of patient’s condition from SJS/TEN. This case report concludes that proper use of adjuvant therapy at proper doses and assessing the duration of administration based on the patient characteristics such as age can minimize the ADRs of antibodies intended for NSCLC treatment. Therefore, close monitoring of the patients is a preventive measure for eluding unintended adverse effects. Specific care by communicating with the patient about the ADR recorded should be properly executed. Physician input, proper nursing care, and patient compliance, therefore, remain critical for better management and rapid recovery in such cases. These measures can result in better treatment and help in avoiding undesired severe and life-threatening skin-associated toxicities such as SJS/TEN as an adverse effect of the use of immune checkpoint inhibitors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Coleman JJ, Pontefract SK. Adverse drug reactions. Clin med. 2016;16(5):481–485. doi:10.7861/clinmedicine.16-5-481
2. Alomar MJ. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm j. 2014;22(2):83–94. doi:10.1016/j.jsps.2013.02.003
3. Malki MA, Pearson ER. Drug–drug–gene interactions and adverse drug reactions. Pharmacogenomics J. 2020;20(3):355–366. doi:10.1038/s41397-019-0122-0
4. Costa C, Abeijon P, Rodrigues DA, Figueiras A, Herdeiro MT, Torre C. Factors associated with underreporting of adverse drug reactions by patients: a systematic review. Int J Clin Pharm. 2023;1–10.
5. Sabaté Gallego M, Pérez Esquirol E, Garcia Doladé N, et al. Incidence and characteristics of adverse drug reactions in a cohort of patients treated with PD-1/PD-L1 inhibitors in real-world practice. Front med. 2022;9:891179. doi:10.3389/fmed.2022.891179
6. Khalil H, Huang C. Adverse drug reactions in primary care: a scoping review. BMC Health Serv Res. 2020;20(1):5. doi:10.1186/s12913-019-4651-7
7. Phillips EJ. Classifying ADRs--does dose matter? Br J Clin Pharmacol. 2016;81:10–12. doi:10.1111/bcp.12749
8. Jin S, Sun Y, Liang X, et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduction Targeted Therapy. 2022;7:39.
9. Zahavi D, Weiner L. Monoclonal Antibodies in Cancer Therapy. Antibodies (Basel, Switzerland). 2020;9(3). doi:10.3390/antib9030034
10. Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: a promising anti-tumor PD-1 antibody. Front Oncol. 2020;10:594558. doi:10.3389/fonc.2020.594558
11. Zhang L, Lin W, Tan F, et al. Sintilimab for the treatment of non-small cell lung cancer. Biomarker Res. 2022;10(1):23. doi:10.1186/s40364-022-00363-7
12. Wang J, Fei K, Jing H et al. Durable blockade of PD-1 signaling links preclinical efficacy of sintilimab to its clinical benefit. mAbs. 2019;11(8):1443–1451.
13. Salati M, Pifferi M, Baldessari C, et al. Stevens-Johnson syndrome during nivolumab treatment of NSCLC. Ann Oncol. 2018;29:283–284. doi:10.1093/annonc/mdx640
14. Gao S, Li N, Gao S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thoracic Oncol. 2020;15(5):816–826. doi:10.1016/j.jtho.2020.01.017
15. Zimmerman D, Dang NH. Stevens–Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN): immunologic Reactions. Oncologic Critical Care. 2019;267–280. doi:10.1007/978-3-319-74588-6_195
16. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. doi:10.1038/clpt.1981.154
17. Sheth H, Pragya R, Kovale S, et al. Oral mucositis—case series of a rare adverse effect associated with immunotherapy. Supportive Care Cancer. 2021;29(8):4705–4709. doi:10.1007/s00520-021-05993-5
18. Peña-Cardelles JF, Salgado-Peralvo AO, Garrido-Martínez P, Cebrián-Carretero JL, Pozo-Kreilinger JJ, Moro-Rodríguez JE. Oral mucositis. Is it present in the immunotherapy of the immune checkpoint pd1/pd-l1 against oral cancer? A systematic review. Med Oral Patol Oral Cir Bucal. 2021;26:e494–e501. doi:10.4317/medoral.24353
19. Fazer C, Price KA. Management of immune-related dermatitis and mucositis associated with pembrolizumab in metastatic human papillomavirus–associated squamous cell carcinoma of the oropharynx. J Oncol Practice. 2020;16:20s–24s. doi:10.1200/JOP.19.00648
20. Kim SK, Cho SW. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Front Pharmacol. 2022;13:868695. doi:10.3389/fphar.2022.868695
21. Antoun J, Titah C, Cochereau I. Ocular and orbital side-effects of checkpoint inhibitors: a review article. Curr Opin Oncol. 2016;28(4):288–294. doi:10.1097/CCO.0000000000000296
22. Ishida T, Ito A, Sato F, et al. Stevens-Johnson Syndrome associated with mogamulizumab treatment of adult T-cell leukemia / lymphoma. Cancer Sci. 2013;104(5):647–650. doi:10.1111/cas.12116
23. Venkateswaran N, Khianey R, Generoso A. Stevens Johnson Syndrome in a Patient with Giant Cell Arteritis During Short Term Tocilizumab Therapy. Cureus. 2020;12(4):e7662. doi:10.7759/cureus.7662
24. Rosen AC, Balagula Y, Raisch DW, et al. Life-threatening dermatologic adverse events in oncology. Anticancer Drugs. 2014;25(2):225–234. doi:10.1097/CAD.0000000000000032
25. Smelik M. Stevens-Johnson Syndrome: a Case Study. Perm J. 2002;6(1):29–31. doi:10.7812/TPP/02.997
26. Tomasini P, Mascaux C, Jao K, et al. Effect of Coexisting KRAS and TP53 Mutations in Patients Treated with Chemotherapy for Non–Small-Cell Lung Cancer. Clin Lung Cancer. 2019;20(3):e338–e345. doi:10.1016/j.cllc.2018.12.009
27. Pandey R, Johnson N, Cooke L, et al. TP53 Mutations as a Driver of Metastasis Signaling in Advanced Cancer Patients. Cancers. 2021;13(4):597. doi:10.3390/cancers13040597
28. Gu M, Xu T, Chang P. KRAS/LKB1 and KRAS/TP53 co-mutations create divergent immune signatures in lung adenocarcinomas. Ther Adv Med Oncol. 2021;13:17588359211006950. doi:10.1177/17588359211006950
29. Zeng L, Li Y, Xiao L, et al. Crizotinib presented with promising efficacy but for concomitant mutation in next-generation sequencing-identified ROS1-rearranged non-small-cell lung cancer. Onco Targets Ther. 2018;11:6937–6945. doi:10.2147/OTT.S176273
30. Riviere P, Goodman AM, Okamura R, et al. High Tumor Mutational Burden Correlates with Longer Survival in Immunotherapy-Naïve Patients with Diverse CancersTMB and Overall Survival. Mol Cancer Ther. 2020;19(10):2139–2145. doi:10.1158/1535-7163.MCT-20-0161
31. Lin G, Fan X, Zhu W, et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget. 2017;8(48):83986–83994. doi:10.18632/oncotarget.20233
32. Bonaventura P, Shekarian T, Alcazer V, et al. Cold Tumors: a Therapeutic Challenge for Immunotherapy. Front Immunol. 2019;10:168. doi:10.3389/fimmu.2019.00168
33. Wang D-R, Wu X-L, Sun Y-L. Therapeutic targets and biomarkers of tumor immunotherapy: response versus non-response. Signal Transduction Targeted Therapy. 2022;7(1):331. doi:10.1038/s41392-022-01136-2
34. Das S, Ramamoorthy R. Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Indian J Paediatric Dermatol. 2018;19(1):9–14. doi:10.4103/ijpd.IJPD_120_17
35. Hoy SM. Sintilimab: first global approval. Drugs. 2019;79(3):341–346. doi:10.1007/s40265-019-1066-z
36. Shi Y, Su H, Song Y, et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): a multicentre, single-arm, Phase 2 trial. Lancet Haematol. 2019;6(1):e12–e19. doi:10.1016/S2352-3026(18)30192-3
37. Mawson AR, Eriator I, Karre S. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN): could retinoids play a causative role? Med Sci Monitor. 2015;21:133–143. doi:10.12659/MSM.891043
38. Venkateshwarlu M, Radhika B. Diagnosis and management of drug-induced Stevens-Johnson syndrome: report of two cases. J Indian Acad Oral Med Radiol. 2011;23:S429–S433. doi:10.5005/jp-journals-10011-1189
39. Auyeung J, Lee M. Successful Treatment of Stevens-Johnson Syndrome with Cyclosporine and Corticosteroid. Can J Hosp Pharm. 2018;71(4):272–275. doi:10.4212/cjhp.v71i4.2829
40. Gupta LK, Martin AM, Agarwal N, et al. Guidelines for the management of Stevens–Johnson syndrome/toxic epidermal necrolysis: an Indian perspective. Indian J Dermatol Venereol Leprol. 2016;82:603. doi:10.4103/0378-6323.191134
41. Czajkowski R, Weiss-Rostkowska V, Wankiewicz A, et al. Stevens-Johnson syndrome induced by carbamazepine. Acta Pol Pharm. 2007;64:89–92.
42. Wilken R, Li CS, Sharon VR, et al. Topical clobetasol for the treatment of toxic epidermal necrolysis: study protocol for a randomized controlled trial. Trials. 2015;16(1):374. doi:10.1186/s13063-015-0879-7
43. Hollingsworth J, Park SU, Bhagavathi V, Green A, Philips N. Stevens-Johnson syndrome with vulvar involvement: a case report and literature review. Case Rep Women’s Health. 2022;34:e00404. doi:10.1016/j.crwh.2022.e00404
44. Li G, Gong S, Wang N, Yao X. Toxic epidermal necrolysis induced by sintilimab in a patient with advanced non-small cell lung cancer and comorbid pulmonary tuberculosis: a case report. Front Immunol. 2022;13:989966. doi:10.3389/fimmu.2022.989966
45. Zhu J, Chen G, He Z, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis in patients treated with immune checkpoint inhibitors: a safety analysis of clinical trials and FDA pharmacovigilance database. EClinicalMedicine. 2021;37:100951. doi:10.1016/j.eclinm.2021.100951
46. Zhao Y, Cao Y, Wang X, Qian T. Treatment of PD-1 Inhibitor-Associated Toxic Epidermal Necrolysis: a Case Report and Brief Review. OncoTargets and Therapy. 2022;15:345–351. doi:10.2147/OTT.S353743
47. Lee O, Masood M, Nutan F. Case Series of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis With Nivolumab and Nivolumab/Ipilimumab Combination Therapy in Metastatic Melanoma. J Drugs Dermatol. 2022;21(5):529–530. doi:10.36849/JDD.6559
48. Kubicki SL, Welborn ME, Patel AB. Toxic epidermal necrolysis during cotherapy with ipilimumab and nivolumab. J Immunother Precision Oncol. 2018;1(2):78–81. doi:10.4103/JIPO.JIPO_7_18
49. Borg L, Buhagiar M, La Ferla E, Pisani D, Said J, Boffa MJ. Pembrolizumab-Induced Toxic Epidermal Necrolysis. Case Rep Oncol. 2022;15(3):887–893. doi:10.1159/000526931
50. Neema S, Sathu S, Vasudevan B, Shreshta S, Bhatt S, Lekshmipriya K. Pembrolizumab-induced toxic epidermal necrolysis: a rare cause of severe adverse drug reaction. Indian J Dermatol Venereol Leprol. 2023;1–4. doi:10.25259/IJDVL_7_2023
51. Cao J, Li Q, Zhi X, et al. Pembrolizumab-induced autoimmune Stevens-Johnson syndrome/toxic epidermal necrolysis with myositis and myocarditis in a patient with esophagogastric junction carcinoma: a case report. Transl Cancer Res. 2021;10(8):3870. doi:10.21037/tcr-21-470
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.