Back to Journals » International Journal of General Medicine » Volume 16
Total T Cell Density and Expression of T Memory Stem Cell Markers are Associated with Better Prognosis in Colon Cancer
Authors Ding J, Wang H, Hou R, Shi Y, Fan H, Li Y, Mei J, Zhang Q, Ruan T, Xu J
Received 22 March 2023
Accepted for publication 29 May 2023
Published 5 June 2023 Volume 2023:16 Pages 2285—2294
DOI https://doi.org/10.2147/IJGM.S411122
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Junli Ding,1,* Hao Wang,1,* Rui Hou,1,* Yuxin Shi,1 Honghong Fan,1 Yuting Li,1 Jie Mei,1 Qinglin Zhang,2 Tingyan Ruan,1 Junying Xu1
1Department of Oncology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, 214023, People’s Republic of China; 2Department of Gastroenterology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, 214023, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junying Xu; Tingyan Ruan, Department of Oncology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, No. 299 Qingyang Road, Wuxi, Jiangsu, 214023, People’s Republic of China, Tel/Fax +86-510-82700775, Email [email protected]; [email protected]
Background: Immune checkpoint inhibitors have achieved limited clinical effectiveness in colon cancer. Stem memory T cells (TSCMs) and in-situ cytotoxic T cells are dominant contributors to host immunity. Currently, data on the correlation between TSCM and T cell abundance and clinicopathological characteristics in colon cancer are largely unavailable.
Methods: In-situ cytotoxic T cells are identified based on the quantification of CD3+ and CD8+ markers using immunohistochemistry (IHC) in the core of the tumor and the invasive margin of the tumor. The expression of representative markers of TSCMs, CD27 and CD95, was assayed using IHC in colon cancer tissues. Correlations between the levels of each marker and the clinicopathological characteristics as well as prognosis were evaluated.
Results: High densities of CD3+ and CD8+ T cells correlated with stage I-II tumors, whereas a lower infiltration of cytotoxic T cells correlated with advanced-stage tumors. CD27 and CD95 were both expressed in the membrane of T cells present in the tumor stroma and their levels showed a negative correlation with the TNM stage. CD3, CD8, and CD27 were expressed at the same locations simultaneously, indicating their coordinated action against cancer. In addition, cytotoxic T cell densities and CD27 and CD95 expression remained independent prognostic factors for overall survival.
Conclusion: In-situ cytotoxic T cells and TSCMs play important roles in colon cancer development. TSCMs marker CD27 and CD95 were both indicators of survival in patients with colon cancer. Thus, it is believed that TSCMs represent a desirable population for future use in combination immunotherapy.
Keywords: T cell, CD27, CD95, prognosis, colon cancer
Introduction
Colorectal cancer (CRC) is one of the most aggressive cancers in digestive system and incidence and mortality rank third and second, respectively.1 As the prognosis of CRC patients is largely heterogeneous, it has come a long way in terms of prognosis prediction accuracy for patients. Immune score was considered a better predictor than AJCC/UICC TNM classification in colorectal cancer.2 In-situ immune cell infiltration in tumors is associated with a favorable prognostic effect. Accordingly, immunotherapies, characterized primarily by the use of programmed cell death 1 (PD-1) signaling inhibitors, have made remarkable advances in many solid tumors;3,4 however, limited effect has been reported in CRC.5 Molecules that exist independent of the PD-1 signaling pathway but play important roles in the immune microenvironment could be present, which could help predict patient prognosis.
Stem memory T cells (TSCMs), a rare population of memory lymphocytes with stem cell-like abilities to self-renew and supply additional memory and effector subsets, have recently been identified. TSCMs were first discovered in human peripheral blood in 2011 by Gattnoni and were found to account for approximately 2–4% of CD4+ or CD8+ T cells.6 Later, these cells were identified in renal and bladder tumors. Patients with high TSCM infiltration exhibited a long disease-free survival after operation.7,8 Researchers studying immunotherapy have been interested in TSCMs because of these traits as well as new indications of their potential function in immunological reconstitution in immunodeficient hosts and their capacity to mediate improved anti-tumor immunity in humanized mice models.9,10 However, their role in CRC remains poorly understood.
CD27 and CD95 are important molecular markers of TSCM.11 As a subset of T-cell co-stimulatory TRAF-interacting TNFRSF receptors, CD27 activates signaling pathways that, in turn, activate MAP kinases and members of the NF-κB family of transcription factors. CD27 increases cellular proliferation and triggers anti-apoptotic proteins by activating these pathways.12 A recent study showed that CD27 activation using an agonistic CD27 antibody works in conjunction with a blocking antibody that recognizes the ligand of the inhibitory programmed death (PD)-1 receptor13 (PD-L1) to restore the functionality of CD8+ T lymphocytes. The death receptor prototype, CD95 (also known as Fas), appears to primarily activate non-apoptotic signaling pathways including, NF-κB, MAPK, and PI3K, which are involved in cell migration, differentiation, survival, and cytokine production.14 In addition, naive T cell differentiation and activation can be altered by the CD95-mediated PI3K/Akt signaling pathway, regardless of whether it is activated in memory CD4+ or CD8+ T cells.15 Nevertheless, CD95 stimulates cell proliferation in neural progenitors and cancer stem cells by triggering TCF/β-catenin signaling in terminally differentiated neurons, where it induces apoptosis through the creation of a death-inducing complex.16 Taking into account the newly discovered function of TCF/β-catenin signaling in the development and maintenance of memory T cells,17 in addition to serving as a marker for memory T cells, CD95 may also be crucial for the durability and renewal potential of these cells.
In this study, we investigated the correlation between the extent of in-situ immune cell infiltration, considering factors such as the density of T cells and CD27+ and CD95+ TSCMs, and the overall survival (OS) of patients with colon cancer. To avoid the influence of preoperative radiotherapy and chemotherapy on the immune system, cases of rectal cancer were excluded. Early-stage (TNM stage I to III) patients who did not undergo neoadjuvant therapy were included.
Materials and Methods
Patients
The records of 155 patients with colon cancer who underwent primary tumor resection at the Affiliated Wuxi People’s Hospital of Nanjing Medical University between 2012 and 2014 were reviewed. These patients represented a continuous, unselected cohort of patients. Memory T cell infiltration in primary tumors isolated from patients with stage I–III colon cancer was evaluated. Histological diagnosis and staging were established according to TNM classification (stage I, n=10; II, n=73; III, n=72), according to standard criteria. Samples were discarded if the patient did not meet the inclusion criteria (clinical quality control; for example, if the patient had metastatic cancer, had received neoadjuvant chemotherapy, had a Tis/Tx tumor, or if their mortality status data were missing), if counting data were missing, if the staining intensity was too low, or if the staining intensity was insufficient (biomarker quality control). Table 1 lists the essential patient and tumor characteristics. During diagnosis, the median age of patients was 63 years (from 38 to 89 years). The majority of patients were diagnosed with Stage II disease, and the right colon was the most typical location for the initial tumor. The patients underwent routine follow-up care following surgery. The typical period of follow-up was 66.2 months (range 0.5–192.2 months). Patients with high-risk Stage II CRC were defined as having either T4 stage II disease or biomarkers for venous emboli, lymphatic invasion, or perineural invasion (VELIPI+). Meanwhile, low-risk stage II CRC was defined by either T1-3 stage II or VELIPI- biomarkers. An ethics review board provided approval for the legal, ethical, and social ramifications of the study. Written consent was provided by each patient, and our study complies with the Declaration of Helsinki.
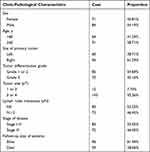 |
Table 1 Patients’ Clinic-Pathological Characteristics |
IHC Staining
Formalin-fixed and paraffin-embedded tissue samples from 155 primary colon tumors were available. For the CD3 and CD8 assays, expert pathologists selected one tumor block containing the colon tumor and an invasive margin (IM) from each patient. Two paraffinized tissue sections of 4 µm were processed for IHC staining. IHC staining was performed using a rabbit Ultraview Universal DAB IHC Detection Kit (Ventana, Tucson, AZ, USA). IHC staining was performed using rabbit monoclonal IgG anti-CD3 (Cat. ab135372) or CD8 (Cat. ab237709) antibody (Abcam, UK). For CD27 and CD95 detection, conventional tumor blocks from each patient were selected. Rabbit monoclonal IgG anti-CD27 (Cat. ab131254) or anti-CD95 (Cat. ab133619) antibodies were also selected from Abcam.
For each case, the number of CD3+ and CD8+ cells in the core of the tumor (CT) and in its IM is determined based on the sum of numbers in the high-power fields (100×) selected at random. Two pathologists evaluated the results independently. High and low immunoscores were plotted according to the cutoff value of cell numbers defined at the median of the cohort (50% of patients with high cell number and 50% of patients with low cell number).18
For CD27 and CD95 expression assessment, typical whole tissue samples, whole tumor samples, and the peritumoral region were examined under a light microscope. The abundance of cells expressing the markers and the dye intensity were used to grade expression. The marker-expressing cell populations were graded as 0 (5%), 1 (5–25%), 2 (25–50%), 3 (51–75%), or 4 (>75%), depending on the intensity of the dye color and the number of cells expressing the molecules. The two grades were combined, and specimens were graded on a scale of 0 to 2 (negative) and >2 (positive).
Statistical Analysis
SPSS 11.5 was used to analyze the data (SPSS Inc, IL, USA). The χ2 test was used to assess data from a frequency table. To ascertain the correlation between two variables, Spearman analysis was performed. For single variant analysis, both Kaplan–Meier technique and Log rank test were used. The Cox’s proportional hazards regression model was used to conduct a multivariate survival study. Parameters for the Cox regression included sex, age, site of tumor, differentiation grade, tumor size, lymph node metastasis, stage of disease, T cell density, CD27 expression, and CD95 expression. The multivariate model included explanatory factors that were substantially linked with the OS in the univariate study. P values less than 0.05 were considered significant.
Results
Associations Among T Cell Density, CD27 and CD95 Expression, and Clinicopathological Factors
Based on the measurement of cytotoxic CD3+ and CD8+ T cell abundance in the CT and IM, local immune infiltrates were identified. According to the median of the cohort’s threshold value for cell numbers (ie, 50% of patients had high cell numbers and 50% had low cell numbers), high and low immunoscores were provided. CD3 and CD8 were expressed primarily in the plasma membrane of T cells (Figure 1). The number of CD3+ cells was 1.5 times that of the number of CD8+ cells. Most CD3+ and CD8+ T cells infiltrated at the edge of invasive region, indicating their resistance to tumor invasion. Based on the cutoff value of 427, the cohort was divided into two groups: 77 patients received high immunoscores and 78 patients received low immunoscores. Furthermore, the immunoscore was inversely correlated with the TNM stage (P=0.002); an advanced disease stage was correlated with a lower immunoscore (Table 2).
 |
Table 2 Associations Between Patients’ Characteristics and T Cell Density, CD27 and CD95 Expression |
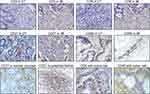 |
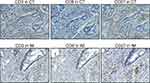
Figure 1 Representative IHC staining of CD3, CD8, CD27, and CD95 in the core of the tumor (CT) and in the tumor’s invasive margin (IM) in colon tumor tissue and in normal colon tissue (100×). |
Naive and memory T-cells, as well as certain immune cells, express CD27, which is of major importance for T cell co-stimulation. In our results, CD27 was expressed in the cell membrane of T cells in the stroma of tumors, in lymphoid follicles, and in normal intestinal mucosa (Figure 1). Among 155 cases, CD27 expression was observed in 71 (46%) patients. The pattern of CD27 expression did not correlate with the following variables: age, sex, pN, differentiation, tumor location. Notably, CD27 expression exhibited a significant negative correlation with the TNM stage (P=0.035, P=0.004, respectively) (Table 2). Furthermore, CD27+, CD3+, and CD8+ T cells appeared simultaneously in the tumor center and IMs, indicating their synchronized resistance to cancer spread (Figure 2).
In addition to serving as a marker for memory T cells, CD95 may also be crucial for the durability and self-renewal of these cells. It was expressed in the membrane of T cells present in the tumor stroma and also in tumor cells. Interestingly, the fusion of CD95+ T cells and tumor cells was observed, indicating the phagocytosis of tumor cells by T cells (Figure 1). We observed CD95 expression in 60 (39%) patients. A negative correlation was observed between CD95 expression and the TNM stage (P=0.009, Table 2).
Associations Among T Cell Density, CD27 and CD95 Expression, and OS
In view of the close correlation between T cell density, CD27 and CD95 expression, and clinicopathological factors, we conducted survival analysis using follow-up data. The survival rate and densities of T cells were found to be significantly correlated. Patients with a higher T cell density had a longer survival. The 3-year OS was recorded for 77 (96%) of 80 patients with a higher T cell density and 40 (53%) of 75 patients with a lower T cell density. The 5-year OS was recorded for 71 (89%) patients with a higher T cell density and 36 (48%) patients with a lower T cell density (HR = 0.38, 95% CI: 0.22–0.63, P < 0.001, Figure 3A).
CD27 expression in both the tumor stroma and lymphoid follicle was positively correlated with prognosis. Patients with CD27 expression showed longer survival. The 3-year OS was recorded for 60 (98%) of 62 patients with CD27 expression and 59 (63%) of 93 patients without CD27 expression. The 5-year OS was recorded for 50 (82%) patients with CD27 expression and 49 (53%) patients without CD27 expression (HR = 0.38, 95% CI: 0.25–0.69, P < 0.001, Figure 3B).
CD95 expression in lymphocytes present in the tumor stroma was positively correlated with prognosis. Patients with positive CD95 expression had longer survival. The 3-year OS was recorded for 54 (91%) of 60 patients with CD27 positive expression and 67 (71%) of 95 patients without CD27 expression. The 5-year OS was recorded for 46 (76%) patients with CD27 expression and 60 (63%) patients without CD27 expression (HR = 0.57, 95% CI: 0.34–0.96; P=0.035, Figure 3C).
Univariate and Multivariate Analyses of Prognostic Factors for OS
To further assess the prognostic factors of these patients, the Cox regression model was used for univariate and multivariate survival analyses. Univariate analysis showed stage of disease, T cell density, CD27 expression, and CD95 expression were prognostic factor for OS in these patients (Table 3). Moreover, multivariate Cox regression analysis results indicated that, apart from advanced cancer stage (HR = 0.324, P = 0.001), T cell density, CD27 expression, and CD95 expression were independent prognostic factors for OS (HR = 0.423, P = 0.021; HR = 0.325, P = 0.025; HR = 0.451, P = 0.036) in patients with colon tumor. Collectively, the data implied that T cell density and CD27 and CD95 expression were valuable prognostic biomarkers in colon cancer.
 |
Table 3 Univariate and Multivariate Cox Regression Analysis of Survival Factors in Colon Cancer |
Discussion
CRC is the third most prevalent cancer type and a major contributor to cancer-related fatalities in the US. According to the latest statistics, the incidence and mortality rate of cancer in China is three times that in the United States.19 CRC ranks second among new patients, with lung cancer ranking first. This is closely related to the increasing obesity and aging in the population. The 5-year survival rate for metastatic CRC is poor, at approximately 14%, despite significant scientific and clinical advancements in early identification and surgery that have resulted in five-year survival rates of 90% and 71% for localized and regionalized CRCs, respectively.19 Therefore, CRC has a heavy disease burden in China. Methods for the accurate prediction of prognosis and improvement of survival are of great importance in research.
Immune score, which has emerged as a useful tool with time, has garnered significant attention in academia, especially in Europe, wherein the combined density of CD3+ and CD8+ T cell effectors are scored within the tumor and its IM.20 Immunoscore, assayed using IHC, can be used to segregate patients with CRC into subcategories with significant differences in time to recurrence, disease-free survival, and OS, independent of the MSI status and TNM staging results.21 The reliability and consistency of consensus immunoscores have significant potential in therapeutic applications. An investigation of immunoscore assays, led by Galon, involved the analysis of data from 14 expert centers in 13 countries across North America, Europe, and Asia.2 Most patients included were from Europe. Patients in China were recruited from the First Affiliated Hospital of Xi’an Jiaotong University. The incidence rate of CRC is the highest in South and East China and Wuxi is more representative in East China. The heterogeneity of the population prompted us to study the prognostic role of the immunoscore in patients from Wuxi People’s Hospital.
As expected, the densities of CD3+ and cytotoxic CD8+ T cells in the tumor and IM were significantly correlated with OS. Through Cox multivariate stratified analysis, the immunoscore was found to be independent of other factors, such as patient age, gender, T stage, N stage, and existing prognostic factors. The results imply that immunoscore is a simple and reliable estimate of the risk of death, even in patients from China. Immunoscore not only predicted the prognosis but also indicated the therapeutic effect, which could help avoid unnecessary chemotherapy. An analysis of 481 patients with stage III colon cancer who received adjuvant chemotherapy with an mFOLFOX6 regimen for 3 months and 6 months was conducted. Compared with adjuvant chemotherapy for 3 months, adjuvant chemotherapy for 6 months was more beneficial for patients with medium to high immunoscores, whereas it was not beneficial for patients with low immunoscores.22 The results suggested that the immunoscore may predict the efficacy of adjuvant chemotherapy. Validating the results of the standardized immunoscore assay will now be crucial for assessing prognostic significance and predicting chemotherapy response in clinical trials.
The strong prognostic potential of the immunoscore may be used for predicting immunotherapy efficacy in CRC. However, PD-1 inhibitors have not achieved significant efficacy in neoadjuvant, adjuvant, and palliative treatment, except in patients with MSI-H. MSS accounts for the majority of patients with CRC; therefore, identifying novel targets and potential combination treatment strategies is an urgent need.
Memory T cells are heterogeneous and can be categorized into four subgroups based on their biological characteristics and antitumor efficacy. These subgroups are central, effector, stem-like, and tissue-resident memory T cells.23 TSCMs have been demonstrated to exhibit characteristics similar to naive T cells. These cells mediate superior antitumor responses and exhibit a strong proliferative capacity, self-renewal potential, and long-term survival during immune responses. The cells are also more efficiently reconstituted in immunodeficient hosts.24 These cells, which are specific for many viral and self-tumor antigens, were identified in a subpopulation of T cells known as naive T cells, which had the following characteristics: CD27+, CD45RO−, CCR7+, CD45RA+, CD62L+, CD28+, and IL-7R+. It has been revealed that high TSCM infiltration was associated with good prognosis in advanced NSCLC patients receiving anti-PD-1 immunotherapy,25 indicating the significant prognostic value of TSCM in human cancer. However, their role in CRC remains poorly understood.
CD27 belongs to the TNF receptor family, which is of major importance for T-cell co-stimulation. CD27 is expressed on the surface of naïve and memory T cells, and its expression on these cells further increases after T-cell activation. So, CD27+ T cells are a driving force for immune responses. Our results show that the expression of CD27 decreased with the advancement of the TNM stage, resulting in a shorter survival time. CD27 is considered a potential prognostic biomarker in addition to the immunoscore. CD27 has been investigated as a potential therapeutic target for tumor treatment. Research in an insufficiently immunogenic B16 melanoma model demonstrated that the effectiveness of mouse CD27-specific rat mAb AT124-1 therapy is attributable to the increased activity of NK cells and CD8+ T-cells infiltrating the tumor.26 It has been shown that 1F5 is an effective anti-human CD27 antibody (CDX1127, Varlilumab), which is currently investigated in clinical trials; combination with PD-1 or CTLA4 inhibitor, with anti-angiogenic therapy, has also potent T-cell co-stimulatory activity.27 Because CD27 activation can limit T-cell tolerance, even in cases of malignancies with low immunogenicity, current trials seem promising for combination therapy using CD27 agonists and immune checkpoint inhibitors.
CD95 is also a member of the TNFR superfamily. Although CD95 is regarded as a death receptor prototype, this view has been contested. CD95 is now known to promote non-apoptotic signaling pathways important in numerous physiological functions, including cell proliferation, survival, differentiation, and migration, similar to tumor necrosis factor receptor (TNFR)-1. Human TSCMs are an appealing population to utilize in adoptive transfer-based immunotherapy for cancer because they have better persistence and effector functions and are emerging as key participants in the preservation of long-lived T-cell memory. Methods for the generation of CD45RO−CD45RA+CCR7+CD27+CD95+ cells, resembling naturally occurring human TSCMs, have been studied extensively.28 In this study, CD95 expression was negatively correlated with the primary tumor size and tumor stage. In addition, decreased cytoplasm expression of CD95 was associated with poor grade in NSCLC,29 implying that CD95+ T cell infiltration has anti-tumor efficacy in not only CRC but at least NSCLC. Notably, CD95 is an independent prognostic factor, and its expression is positively correlated with OS. This implies that the maintenance of long-lived T-cell memory mediates superior anti-tumor immunity.
However, this study still has many shortcomings. First, a major limitation is that the current cohort is single cohort analysis. Thus, multi-center validation is a must before this study can move to clinical use. In addition, the absence of sufficient molecular markers for TSCMs and the single study methodology are main limiting factors for this study. In our next prospective study, we intend to use fresh tumor tissue and peripheral blood samples to detect the abundance of TSCMs using flow cytometry, with the simultaneous application of multiple molecular markers.
Conclusion
To our knowledge, this is the first study to explore the role of TSCMs in the prognosis of colon cancer. In this study, we conclude that in-situ cytotoxic T cells and markers for TSCMs are important prognostic indicators in colon cancer, which may contribute to accurate prognosis prediction and treatment choice for colon cancer.
Data Sharing Statement
The original contributions presented in this study are included in the article. Further inquiries are available upon request. Please contact Dr Junying Xu ([email protected]) for further inquiries.
Ethics Approval
Ethical approval (NJMU-2020-352) was granted by the Clinical Research Ethics Committee of Nanjing Medical University. This is a retrospective study, and a trial registration number is not needed.
Author Contributions
Junying Xu and Tingyan Ruan designed the research and the experiments. Junli Ding, Hao Wang, Rui Hou, Yuxin Shi, Honghong Fan, Yuting Li, and Jie Mei conducted data analysis and data interpretation; Junli Ding, Hao Wang, and Rui Hou wrote the manuscript and completed statistical analysis. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Wuxi Science and Technology Bureau Foundation (No. N20202018). Wuxi Municipal Health Commission Foundation (No. M202108), Beijing Hengji Health Management and Development Foundation (HJ-HX-ZLXD-202209-002), and Project of Wuxi Medical Center of Nanjing Medical University (WMCC202319).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139.
3. Mao W, Cai Y, Chen D, et al. Statin shapes inflamed tumor microenvironment and enhances immune checkpoint blockade in non-small cell lung cancer. JCI Insight. 2022. doi:10.1172/jci.insight.161940
4. Hamilton PT, Anholt BR, Nelson BH. Tumour immunotherapy: lessons from predator-prey theory. Nat Rev Immunol. 2022. doi:10.1038/s41577-022-00719-y
5. Ephraim R, Feehan J, Fraser S, Nurgali K, Apostolopoulos V. Cancer immunotherapy: the checkpoint between chronic colitis and colorectal cancer. Cancers. 2022;14(24):6131. doi:10.3390/cancers14246131
6. Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17(10):1290–1297. doi:10.1038/nm.2446
7. Yan C, Chang J, Song X, et al. Memory stem T cells generated by Wnt signaling from blood of human renal clear cell carcinoma patients. Cancer Biol Med. 2019;16(1):109–124. doi:10.20892/j.issn.2095-3941.2018.0118
8. Jin K, Yu Y, Zeng H, et al. CD103(+)CD8(+) tissue-resident memory T cell infiltration predicts clinical outcome and adjuvant therapeutic benefit in muscle-invasive bladder cancer. Br J Cancer. 2022;126(11):1581–1588. doi:10.1038/s41416-022-01725-6
9. Guan L, Li X, Wei J, et al. Antigen-specific CD8+ memory stem T cells generated from human peripheral blood effectively eradicate allogeneic targets in mice. Stem Cell Res Ther. 2018;9(1):337. doi:10.1186/s13287-018-1080-1
10. Siddiqui I, Schaeuble K, Chennupati V, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T cells with stem-like properties promote tumor control in response to vaccination and checkpoint blockade immunotherapy. Immunity. 2019;50(1):195–211 e10. doi:10.1016/j.immuni.2018.12.021
11. Zanon V, Pilipow K, Scamardella E, et al. Curtailed T-cell activation curbs effector differentiation and generates CD8(+) T cells with a naturally-occurring memory stem cell phenotype. Eur J Immunol. 2017;47(9):1468–1476. doi:10.1002/eji.201646732
12. Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin Ther Targets. 2016;20(8):959–973. doi:10.1517/14728222.2016.1158812
13. Buchan S, Manzo T, Flutter B, et al. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J Immunol. 2015;194(1):125–133. doi:10.4049/jimmunol.1401644
14. Guégan JP, Ginestier C, Charafe-Jauffret E, et al. CD95/Fas and metastatic disease: what does not kill you makes you stronger. Semin Cancer Biol. 2020;60:121–131. doi:10.1016/j.semcancer.2019.06.004
15. Rana MK, Aloisio FM, Choi C, Barber DL. Formin-dependent TGF-beta signaling for epithelial to mesenchymal transition. Mol Biol Cell. 2018;29(12):1465–1475. doi:10.1091/mbc.E17-05-0325
16. Beier CP, Schulz JB. CD95/Fas in the brain--not just a killer. Cell Stem Cell. 2009;5(2):128–130. doi:10.1016/j.stem.2009.07.008
17. Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107(21):9777–9782. doi:10.1073/pnas.0914127107
18. Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi:10.1200/JCO.2008.19.6147
19. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
20. Mlecnik B, Tosolini M, Kirilovsky A, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi:10.1200/JCO.2010.30.5425
21. Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44(3):698–711. doi:10.1016/j.immuni.2016.02.025
22. Li Y, Yao Q, Zhang L, et al. Immunohistochemistry-based consensus molecular subtypes as a prognostic and predictive biomarker for adjuvant chemotherapy in patients with stage II colorectal cancer. Oncologist. 2020;25(12):e1968–e1979. doi:10.1002/ONCO.13521
23. Lugli E, Galletti G, Boi SK, Youngblood BA. Stem, effector, and hybrid states of memory CD8(+) T cells. Trends Immunol. 2020;41(1):17–28. doi:10.1016/j.it.2019.11.004
24. Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med. 2017;23(1):18–27. doi:10.1038/nm.4241
25. Zhang G, Liu A, Yang Y, et al. Clinical predictive value of naive and memory T cells in advanced NSCLC. Front Immunol. 2022;13:996348. doi:10.3389/fimmu.2022.996348
26. Roberts DJ, Franklin NA, Kingeter LM, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33(8):769–779. doi:10.1097/CJI.0b013e3181ee238f
27. He LZ, Prostak N, Thomas LJ, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191(8):4174–4183. doi:10.4049/jimmunol.1300409
28. Cieri N, Camisa B, Cocchiarella F, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–584. doi:10.1182/blood-2012-05-431718
29. Fan CF, Xu HT, Lin XY, Yu JH, Wang EH. A multiple marker analysis of apoptosis-associated protein expression in non-small cell lung cancer in a Chinese population. Folia Histochem Cytobiol. 2011;49(2):231–239. doi:10.5603/FHC.2011.0032
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.