Back to Journals » Journal of Pain Research » Volume 14
Total Knee Arthroplasty Postsurgical Chronic Pain, Neuropathic Pain, and the Prevalence of Neuropathic Symptoms: A Prospective Observational Study in Turkey
Authors Şahin F , Beyaz SG, Karakuş N, İnanmaz ME
Received 25 November 2020
Accepted for publication 3 March 2021
Published 19 May 2021 Volume 2021:14 Pages 1315—1321
DOI https://doi.org/10.2147/JPR.S293856
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Fatih Şahin,1 Serbülent Gökhan Beyaz,2 Nazım Karakuş,3 Mustafa Erkan İnanmaz4
1Sakarya University Training and Research Hospital, Department of Anesthesiology, Sakarya, Turkey; 2Istinye University Medical School, Department of Anesthesiology and Reanimation, Pain Medicine, Istanbul, Turkey; 3Sakarya Yenikent State Hospital, Department of Orthopedics and Traumatology, Sakarya, Turkey; 4Sakarya University Faculty of Medicine, Department of Orthopedics and Traumatology, Sakarya, Turkey
Correspondence: Serbülent Gökhan Beyaz
Istinye University Medical School, Department of Anesthesiology and Reanimation, Pain Medicine, Istanbul, Turkey
Tel +90 5322879490
Fax +90 2642552105
Email [email protected]
Purpose: Chronic post-surgical pain (CPSP) is a detrimental condition that persists at least two months after surgical procedures and seriously affects patients’ quality of life. Although its incidence varies according to operation types and definitions, its prevalence is between 3% and 85%. The purpose of this study is to evaluate the prevalence of CPSP and neuropathic pain in patients undergoing TKA for osteoarthritis.
Patients and Methods: In this study, patients who had undergone total knee arthroplasty (TKA) were examined prospectively and observationally. 42 patients were included in the study. Numeric rate scale (NRS) for developing chronic pain, Douleur Neuropathique 4 (DN-4) questionnaire to evaluate neuropathic pain and symptoms, and von Frey filaments to evaluate mechanical hyperesthesia and alladony.
Results: NRS scores were 1 or higher for all patients. Twenty-seven patients constituted the mild pain group (NRS: 1– 4), and 15 patients constituted the moderate pain group (NRS: 4– 7). The number of patients defined as having “neuropathic pain,” according to DN-4 scores, was 17 (40.4%; DN-4 ≥ 4). The moderate pain group reported greater severity of neuropathic symptoms on average than the mild pain group (p = 0.039). When patients knees affected by TKA were divided into suprapatellar, patellar, and infrapatellar regions and evaluated with von Frey filaments, a significant difference was found between the three regions (p < 0.05).
Conclusion: In this study, we showed—unlike other studies—that the rate of neuropathic pain was higher among CPSP patients, and all patients had neuropathic symptoms. In evaluating patients knees with von Frey filaments, we showed that the neuropathic component of patients’ pain occurred mostly in the knee’s infrapatellar region. Although the incidence of CPSP and neuropathic pain in these patients was higher than expected, we think CPSP, its diagnosis, and its treatment present an important issue that requires further examination.
Keywords: chronic postsurgical pain, neuropathic pain, neuropathic symptom, von Frey filament, total knee arthroplasty
Introduction
Total knee arthroplasty (TKA) is an effective surgical method to improve physical function and reduce pain among patients with end-stage knee osteoarthritis.1 Chronic post-surgical pain (CPSP) develops after surgical procedures, and at least two months should have passed after the operation to constitute CPSP.2 CPSP’s incidence varies according to the study methodology, type, and description of operations, but it ranges between 3% and 85%.3 Intraoperative nerve damage, operative time, pre-existing pain, young age, female gender, genetic predisposition, psychological variables, and the severity of acute postoperative pain are risk factors for CPSP.3
Although CPSP is multifactorial, it usually involves a neuropathic component associated with peripheral nerve injury during surgery or pain modulation impairment with central sensitization.4 It is characterized by a combination of neuropathic pain, sensory loss, and paradoxical hypersensitivity. Symptoms of neuropathic pain include spontaneous or triggered chronic pain as well as characteristic burning, stabbing, and electric shock-like warnings, which can present as sharp, sometimes dull, painful, pressurized, deep neuropathic itching.5 It has been shown that one of the most common symptoms in neuropathic pain due to peripheral nerve damage is mechanical hyperalgesia.6 These mechanical sensory changes can be evaluated with the algometer or von Frey filaments.7 The approximate prevalence of neuropathic pain in the general population is around 7%.8 Wylde et al9 demonstrated in their study that 44% of total knee replacement patients developed CPSP while 6% experienced neuropathic pain. Razmjou et al showed that 14% patients with TDR developed neuropathic pain.10 However, Beyaz et al7 identified the rate of neuropathic pain as 33.3% in their study investigating neuropathic pain and neuropathic symptoms after hysterectomy, and they reported observing neuropathic symptoms in almost all the patients participating in their study (96.8%). While Douleur Neuropathique 4 (DN-4) questionnaire ≥ 4 was considered as neuropathic pain, neuropathic symptom was defined as the presence of one of 10 items. It is argued that studies different rates of CPSP, neuropathic pain, and neuropathic symptoms have arisen from differences in study methodologies.
While the primary purpose of this study was to evaluate the prevalence of CPSP and neuropathic pain in patients undergoing TKA due to osteoarthritis, the secondary aim was to evaluate the presence of neuropathic symptoms, the localization of hyperesthesia, and the role of anesthesia methods in the development of chronic pain.
Patients and Methods
Patients
This prospective observational study was conducted with approval from the local ethics committee of Sakarya University Faculty of Medicine. Patients who had undergone a TKA operation at Sakarya Yenikent State Hospital at least six months previously were included in the study, and their data were obtained from the Yenikent State Hospital electronic data program Karmed, using Kardelen Software (Kardelen Software, Mersin, Republic of Turkey). The identified patients were contacted by phone. Patients who were contacted were invited to the hospital and informed about the study. Written consent was obtained from all patients that they would participate in the study. This study was conducted in accordance with the Declaration of Helsinki. Data (age, weight, height, surgical indication, and previous knee operations) were recorded for patients who agreed to participate in the study and came to the hospital. Patients were questioned about type of surgery, incision technique, analgesic use (opioid or non-opioid drug), and other drug. The patients anesthesia type (general anesthesia or regional anesthesia) and postoperative analgesia technique (intravenous or epidural patient controlled analgesia) were obtained from the hospital data system. Patients whose TKA procedures had occurred at least six months and at most 36 months previously were included in the study. Patients who had previous TKA and revision surgery were not included.
Pain Assessment
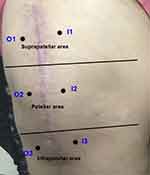
The numeric rating scale (NRS; 0: “no pain”; 10: “the most severe pain imaginable”) was used to calculate pain intensity.11 According to NRS scores, 0 signified “no pain,” 1–3 signified “mild pain,” 4–7 signified “moderate pain,” and 8–10 signified “severe pain.” The NRS scores of the patients were evaluated according to their pain at rest and only TKA laterality and side. Patients were divided into the mild pain group (NRS 1–3) and the moderate pain group (NRS 4–7) to compare nonparametric numerical data. Chronic neuropathic pain was assessed using the DN-4 questionnaire.5 The total score is calculated as the total of the 10 items and the cut-off value for the diagnosis of neuropathic pain is a total score of 4.8 The presence of at least 1 of 10 items was considered as a neuropathic symptom. Surgical incision types were determined during physical examination of all patients. During vertical incisions in the midline, TKA laterality and side was divided first into two regions, the right and left sides, and then into three regions (suprapatellar, patellar, infrapatellar regions) on both sides (Figure 1). Von Frey filaments grading 0.04 g, 0.07 g, 0.16 g, 0.4 g, 0.6 g, 1 g, 1.4 g, 2 g, 4 g, 6 g, 8 g, 10 g, 15 g, 26 g, 60 g, 100 g, 180 g and 300 g it has bending forces. A number-5.88 von Frey filament (Touch Test Sensory Evaluator Kit; North Coast, Inc., Gilroy, CA, USA) was used to assess mechanical allodynia and hyperesthesia along the knee incision scar. Bending forces were applied sequentially from the smallest filament to the filament where the patient felt real touch. Three separate measurements were made for each of the six areas, and the average of the last two values was recorded after the first measurement value was ignored. Von Frey filament measurements were made at rest.
Statistical Analysis
The Chi-square test and Fischer's exact test was used to compare categorical data, which are shown as counts (n) and percentages (%). The Kolmogorov–Smirnov normality test was used to check the normality of continuous data. The Mann–Whitney U-test was used to compare nonparametric numerical data and student t-test was used to compare parametric numerical data between 2 groups. Student t-test was used to compare knee skar pain pressure thresholds among 6 areas. Paired samples t-test was used to compare between 3 regions [suprapatellar region (I1-O1), patellar region (I2-O2) and infrapatellar region (I3-O3) areas of the knee incision scar]. Numerical data are presented as means±standard deviation. In all analyses, p<0.05 was taken to indicate statistical significance. Analyses were performed using commercial software (IBM SPSS Statistics Version 22.0; IBM Corp., Armonk, NY).
Results
A total of 52 patients were evaluated. 7 of these patients were excluded because of revision knee surgery. Three patients were excluded from the study because insufficient communication was established and correct evaluation of their pain could not be made. In total, 42 patients were included in the study. According to their NRS scores, two groups were formed: the mild pain group (NRS 1–3), the moderate pain group (NRS 4–7). NRS scores were 1 and higher for all patients. The mild pain group comprised 27 patients while the moderate pain group comprised 15 patients. No patients were in the severe pain group.
Demographic Characteristics
While two patients (4.8%) were male, 40 patients (95.2%) were female. The mean age of all patients was 64.8 ± 8.2. Time elapsed since surgery was 17.7±6.1 month. No differences were observed in demographic data, patients TKA laterality and side, or time elapsed since surgery between the groups (p > 0.05; Table 1).
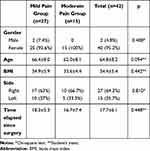 |
Table 1 Demographic Data of Chronic Postsurgical Pain Patients Undergoing Total Knee Arthroplasty |
Intraoperative Features
In patients anesthetic management, three patients (7%) received general anesthesia and 39 patients (93%) received regional anesthesia (of whom 5 had spinal and 34 had combined spinal epidural anesthesia). In postoperative pain management, 34 patients (81%) had used epidural patient-controlled analgesia (PCA), and eight patients (19%) had used intravenous PCA. No significant differences were observed between the groups in terms of patients’ anesthesia and analgesia techniques (p > 0.05; Table 2).
 |
Table 2 Distribution of Anesthesia and Postoperative Analgesia Methods Applied for Total Knee Arthroplasty by Groups |
Localization and Characteristics of Pain
A significant difference was observed between the mild pain group and the moderate pain group in terms of patients’ postoperative NRS scores (p < 0.001). The moderate pain group reported greater severity of neuropathic symptoms on average than the mild pain group (p = 0.039; Table 3). The number of patients defined as having “neuropathic pain,” according to DN-4 scores, was 17 (40.4%; DN-4 4). Those who reported greater pain at the study visit also had higher postoperative pain ratings. The moderate pain group reported greater severity of neuropathic symptoms on average than the mild pain group. While 20 patients (47.6%) were using regular nonsteroidal anti-inflammatory drugs, three patients (7.1%) were using antidepressants (tricyclic antidepressants) and four patients (9.5%) were using pregabalin due to their neuropathic complaints.
 |
Table 3 Postoperative NRS Scores and DN-4 Capital Symptom Number of Patients Applied for Total Knee Arthroplasty |
When the six points of the operated knee were examined one by one, no significant difference was found between the groups (p > 0.05; Table 4). A significant difference was found between the three regions when TKA was performed on the knee with the help of the lines drawn perpendicular to the incision scar, separated into suprapatellar (I1-O1), patellar (I2-O2), and infrapatellar (I3-O3) regions, and evaluated with von Frey filaments (p < 0.05; Table 5). While a significant difference was found between the paired comparisons of the suprapatellar, patellar, and infrapatellar regions, the highest scores were observed in the lower region (Table 5).
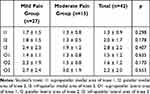 |
Table 4 Knee Incision Scar Pain Von Frey Filament of Patients |
 |
Table 5 Von Frey Filament Scores of the Suprapatellar, Patellar, Infrapatellar Regions Separated on the Knee with Total Knee Arthroplasty |
Discussion
TKA is a frequently performed procedure to improve pain and disability in patients with end-stage knee arthritis when non-surgical methods are ineffective.12 Despite all the diagnostic tests and physical examinations performed on such patients, CPSP can occur after operation. The International Association of the Study of Pain defines CPSP as pain that occurs at least two months after surgical intervention.2 An NRS score of 0 is classified as “no pain,” an NRS score of 1–3 is classified as “mild CPSP,” and an NRS score of 4 is classified as “moderate” and “severe” CPSP.13–15 A review examining 14 cohort studies showed that discomfort and long-term pain developed among 7%–23% of patients after a hip operation and among 10%–34% of patients after a TKA operation.16 Hasegawa et al17 reported that the rate of moderate and severe pain was 28% in their study conducted on 154 patients for the prevalence of pain after TKA. Güngör et al18 examined the NRS scores of 578 TKA patients at least three months postoperatively, and they showed that the incidence of developing CPSP was 31.3%. All 42 patients (100%) included in this study were observed to have an NRS of 1 or higher, and 15 patients (35.8%) had an NRS of four or higher and developed moderate and severe CPSP at a rate similar to other studies.16–18 Also notably, unlike other studies, no patients were found to have an NRS score of zero and no pain.
Regional anesthesia is frequently used—especially in orthopedic surgeries—and both intravenous analgesia and epidural analgesia can be used in analgesia management, although with variations according to application centers. A 2006 study showed that patients who underwent epidural and spinal anesthesia in lower-extremity amputation surgery experienced better analgesia in the first weeks after amputation than patients who received general anesthesia, but epidural and spinal anesthesia had no effect on pain after 14 months.19 In a study examining 563 TKA patients, Bugada et al20 compared CPSP development between patients who received long-term analgesia (continued epidural and peripheral block), patients who received a single dose of regional anesthesia, and patients who did not receive regional anesthesia. While lower NRS scores were observed in patients who continued analgesia for the first month after surgery, no difference was found between the two groups afterwards. In our study, most patients used the regional anesthesia and analgesia method, and despite continuous and aggressive analgesia with epidural PCA for three days, no difference was observed in CPSP development and the relationship between anesthesia and analgesia type.
Neuropathic pain is defined as pain affecting the somatosensory system that occurs as a direct result of a lesion or disease in the sympathetic nervous system or the parasympathetic nervous system.21 A study conducted in 2008 reported that the prevalence of neuropathic pain in the general population was around 7%.8 Recent studies have found that the rates of neuropathic symptoms and pain development after surgery in different tissues have increased.7,22 Neuropathic pain symptoms may appear in the form of chronic burning, stabbing, electric-like shocks, and sharp, sometimes dull, painful, pressurized, deep neuropathic itching, and they may present as a sensory deficiency or hypersensitivity in the painful area or a gradual increase in pain after repetitive stimulation.5,23 Many studies have reported hyperesthesia and hypoesthesia around the incision after TKA.24–26 Retrospective studies with different follow-up periods showed that 60% of TKA patients using the midline skin incision technique had hyperesthesia.26 Black et al25 found that 27% of patients had a symptom of numbness in the incision area after TKA.
The von Frey filament set provides an approximate logarithmic scale of true force and a linear measurement of perceived density. It has a long history of use, particularly in animal experiments and clinical applications, and it can also be used to diagnose hyperesthesia or hypoesthesia. In this study, we divided the TKA laterality and side into six regions (Figure 1) and examined it with von Frey filaments, and we could not find a significant difference between the values. When we divided the array into three areas—suprapatellar, patellar, and infrapatellar (Figure 2)—higher values were found in the lower part of the knee while lower values were observed in the upper part of the knee. Therefore, a significant difference was found when the upper and lower regions of the knee were compared using von Frey filaments. This finding led us to conclude that sensory damage was greater under the knee. Allodynia and hyperesthesia are just two of the well-known symptoms or signs of neuropathy. Notably, the DN-4 questionnaire evaluates multiple neuropathic symptoms. In this study, we evaluated neuropathic symptoms and neuropathic pain separately because even a single neuropathic symptom causes a feeling of pain and a decrease in patients’ quality of daily life.7 While the neuropathic pain cut-off value is 4 and above in the DN-4 score, each finding value is considered as a neuropathic symptom. In this study, neuropathic pain was diagnosed in 17 patients (40.4%; DN 4 ≥ 4) followed for 17.7 ± 6.1 months (6–36 months), and neuropathic pain was found to be significantly higher than in other studies. In addition, the number of patients with a DN-4 score of 1 and above was 42 (100%). This finding is very important for all patients to have neuropathic symptoms after TKA. Previous studies showed that the rate of neuropathic pain in patients followed for three months to one year after TKA was between 3% and 11%.27–29 Phillips et al29 reported that 35% of TKA patients have neuropathic pain symptoms in their sixth postoperative week. The basis of neuropathic pain management is pharmacological, and antidepressants, antiepileptics, topical anesthetics, and opioid agents are often used. Non-pharmacological treatments include psychological approaches, spinal cord stimulation, and physical and invasive treatments.30 Our study found that the rate of neuropathic pain was 40.4% higher than other studies, and the use of tricyclic antidepressants was 18% among the patients in our study while the use of pregabalin was 22%. When we examined these rates, we saw that most of the patients with neuropathic pain in clinical practice did not receive treatment for this pain.
This study involved limitations. First, the sample comprised a small patient group that included all risk factors. Second, patients’ neuropathic pain and pain in the preoperative period could not be evaluated. Differences in time since surgery was another important limitation. However, this study was planned as a prospective observational investigation, the patient group was homogeneous and patients eligible to participate in the study were included.
Conclusion
In conclusion, unlike other studies, this study observed that the rate of neuropathic pain and CPSP were higher, all patients had neuropathic symptoms, and these neuropathic symptoms mostly occurred in the infrapatellar region of the knee. The incidence of CPSP and neuropathic pain after TKA were observed to be considerably higher than the rates in the literature, which is an important issue that requires further effort in diagnosing and treating CPSP and neuropathic pain. Therefore, new studies are needed for CPSP and neuropathic pain.
Disclosure
The authors report no conflicts of interest in this work. This article was not supported or funded by any drug company.
References
1. Lau RL, Gandhi R, Mahomed S, et al. Patient satisfaction after total knee and hip arthroplasty. Clin Geriatr Med. 2012;28(3):349e65. doi:10.1016/j.cger.2012.05.001
2. Macrae WA, Davies HTO. Chronic postsurgical pain. In: Crombie IK, Croft PR, Linton SJ, LeResche L, Von Korff M, editors. Epidemiology of Pain. Seattle, WA: IASP Press, International Association for the Study of Pain; 1999:125e142.
3. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi:10.1016/S0140-6736(19)30352-6
4. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. doi:10.1016/S0140-6736(06)68700-X
5. Unal-Cevik I, Sarioglu-Ay S, Evcik DA. Comparison of the DN4 and LANSS questionnaires in the assessment of neuropathic pain: validity and reliability of the Turkish version of DN4. J Pain. 2010;11(11):1129–1135. doi:10.1016/j.jpain.2010.02.003
6. Maier C, Baron R, Tölle TR, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–450. doi:10.1016/j.pain.2010.05.002
7. Beyaz SG, Özocak H, Ergönenç T, et al. Chronic postsurgical pain and neuropathic symptoms after abdominal hysterectomy: a silent epidemic. Medicine (Baltimore). 2016;95(33):e4484. doi:10.1097/MD.0000000000004484
8. Bouhassira D, Lanteri-Minet M, Attal N, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi:10.1016/j.pain.2007.08.013
9. Wylde V, Hewlett S, Learmonth ID, et al. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. doi:10.1016/j.pain.2010.11.023
10. Razmjou H, Boljanovic D, Wright S, et al. Association between neuropathic pain and reported disability after total knee arthroplasty. Physiother Can. 2015;67(4):311–318. doi:10.3138/ptc.2014-46
11. Correl DJ. Chapter 18 - The measurement of pain: objectifying the subjective. In: Waldman SD, Bloch IB, editors. Pain Medicine. Vol. 1. 2007:197–211.
12. Hawker G, Wright J, Coyte P, et al. Health-related quality of life after knee replacement. J Bone Joint Surg Am. 1998;80(2):163–173. doi:10.2106/00004623-199802000-00003
13. Oldenmenger WH, de Raaf PJ, de Klerk C, et al. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton symptom assessment scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45(6):1083–1093. doi:10.1016/j.jpainsymman.2012.06.007
14. Pinto PR, McIntyre T, Ferrero R, et al. Risk factors for moderate and severe persistent pain in patients undergoing total knee and hip arthroplasty: a prospective predictive study. PLoS One. 2013;8(9):e73917. doi:10.1371/journal.pone.0073917
15. Elson DW, Brenkel IJ. Predicting pain after total knee arthroplasty. J Arthroplasty. 2006;21(7):1047e1053. doi:10.1016/j.arth.2005.12.010
16. Beswick AD, Wylde V, Gooberman-Hill R, et al. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi:10.1136/bmjopen-2011-000435
17. Hasegawa M, Tone S, Naito Y, et al. Prevalence of persistent pain after total knee arthroplasty and the impact of neuropathic pain. J Knee Surg. 2019;32(10):1020–1023. doi:10.1055/s-0038-1675415
18. Gungor S, Fields K, Aiyer R, et al. Incidence and risk factors for development of persistent postsurgical pain following total knee arthroplasty A retrospective cohort study. Medicine (Baltimore). 2019;98(28):e16450. doi:10.1097/MD.0000000000016450
19. Ong BY, Arneja A, Ong EW. Effects of anesthesia on pain after lower-limb amputation. J Clin Anesth. 2006;18(8):600–604. doi:10.1016/j.jclinane.2006.03.021
20. Bugada D, Allegri M, Gemma M, et al. Effects of anaesthesia and analgesia on long-term outcome after total knee replacement. Eur J Anaesthesiol. 2017;34(10):665–672. doi:10.1097/EJA.0000000000000656
21. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
22. Leonard G, Perrouin-Verbe M, Levesque A, et al. Place of surgery in the management of post-operative chronic pain after placement of prosthetic material based on a series of 107 cases. Neurourol Urodyn. 2018;37(7):2177–2183. doi:10.1002/nau.23544
23. Jensen TS. Neuropathic pain; definition and screening. Eur J Pain. 2007;11(S1):S7. doi:10.1016/j.ejpain.2007.03.030
24. Hopton BP, Tommichan MC, Howell FR. Reducing lateral skin flap numbness after total knee arthroplasty. Knee. 2004;11(4):289–291. doi:10.1016/j.knee.2003.09.004
25. Black R, Green C, Sochart D. Postoperative numbness of the knee following total knee arthroplasty. Ann R Coll Surg Engl. 2013;95(8):565–568. doi:10.1308/rcsann.2013.95.8.565
26. Sundaram RO, Ramakrishnan M, Harvey RA, et al. Comparison of scars and resulting hypoaesthesia between the medial parapatellar and midline skin incisions in total knee arthroplasty. Knee. 2007;14(5):375–378. doi:10.1016/j.knee.2007.06.002
27. Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg. 2010;110(1):199–207. doi:10.1213/ANE.0b013e3181c4273a
28. Lavand’homme PM, Grosu I, France MN, et al. Pain trajectories identify patients at risk of persistent pain after knee arthroplasty: an observational study. Clin Orthop Relat Res. 2014;472(5):1409–1415. doi:10.1007/s11999-013-3389-5
29. Phillips JR, Hopwood B, Arthur C, et al. The natural history of pain and neuropathic pain after knee replacement: a prospective cohort study of the point prevalence of pain and neuropathic pain to a minimum three-year follow-up. Bone Joint J. 2014;96B(9):1227–1233. doi:10.1302/0301-620X.96B9.33756
30. Kerstman E, Ahn S, Battu S, et al. Neuropathic pain. Handb Clin Neurol. 2013;110:175–187.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.