Back to Journals » Journal of Inflammation Research » Volume 15
Topical Anti-Inflammatory Agents for Non-Infectious Uveitis: Current Treatment and Perspectives
Authors Balasubramaniam B, Chong YJ, Azzopardi M, Logeswaran A, Denniston AK
Received 16 May 2022
Accepted for publication 12 November 2022
Published 28 November 2022 Volume 2022:15 Pages 6439—6451
DOI https://doi.org/10.2147/JIR.S288294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Balini Balasubramaniam,1 Yu Jeat Chong,2 Matthew Azzopardi,3 Abison Logeswaran,4 Alastair K Denniston1
1Ophthalmology Department, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham, B15 2TH, UK; 2Birmingham & Midland Eye Centre, Birmingham, B18 7QH, UK; 3Ophthalmology Department, Royal London Hospital, Barts Health NHS Trust, London, E1 1BB, UK; 4Moorfields Eye Hospital NHS Foundation Trust, London, UK
Correspondence: Yu Jeat Chong, Birmingham & Midland Eye Centre, Dudley Road, Birmingham, B18 7QH, UK, Email [email protected]
Abstract: Non-infectious uveitis represents a heterogenous group of immune-mediated ocular diseases, which can be associated with underlying systemic disease. While the initial choice of treatment of non-infectious uveitis depends on a number of factors such as anatomical location and degree of inflammation, topical therapies often remain the initial choice of non-invasive therapy. In this narrative review, we aim to describe the literature on non-infectious uveitis, with specific focus on the current perspective on topical anti-inflammatory therapy.
Keywords: non-infectious uveitis, topical anti-inflammatory therapy, topical corticosteroids, topical non-steroid anti-inflammatory drugs, topical cycloplegics
Introduction and Historical Evolution of the Definition of Uveitis
Uvea originates from the Latin word “uva”, referring to the “black grape” appearance of the uveal tissue underneath the sclera.1 One of the earliest descriptions of uveitis was by Hippocrates (460–360 BC), where he observed the effects of inflammation on the iris, resulting in irregular adhesions of the pupil.2
In the 1930s, Sir Duke-Elder argued that most cases of uveitis should be accepted as being caused by “infective foci”, and that uveitis caused by an infectious agent was “not necessarily due to enlodgement of toxin in the eye but mostly represented as an allergic sensitization response”.3 Uveitis is, however, now understood to be a heterogenous group of inflammatory disease affecting both the uvea (iris, ciliary body, choroid) as well as its adjacent structures, such as the sclera, cornea, vitreous and optic nerve.
Over the years, a number of attempts to develop and further refine the classification of uveitis have been made. In 1987, the International Uveitis Study Group (IUSG) established an anatomical classification of uveitis which is still commonly used today. This includes anterior uveitis, intermediate uveitis (a term coined to replace previous definitions such as posterior cyclitis, vitritis, and basal uveoretinitis), posterior uveitis, and finally panuveitis, the latter referring to uveitis involving all three anatomical locations.4 This classification was further expanded on in 2005, through the Standardization of the Uveitis Nomenclature.5 Here, grading systems were created for anterior chamber cells, anterior chamber flare and vitreous haze, with the first two based on Hogan et al’s classification, and vitreous haze grading based on the work done by Nussenblatt et al6,7 Finally, this international consensus also included onset (sudden or insidious), duration (limited or persistent) and course (acute, recurrent, or chronic) in the classification of uveitis.
However, while these classifications were precise, they did not attempt to describe the cause of uveitis, which could ultimately be due to underlying systemic diseases or masquerade syndromes mimicking uveitis. To address this, in 2008, the IUSG proposed a simplified clinical classification of uveitis to be used in conjunction with the other classification systems.8 In this classification, uveitis is subdivided into infectious uveitis, owing its aetiology to bacteria, viruses, fungi, parasites or other organisms, non-infectious uveitis, which could either have systemic associations or no cause identified (idiopathic), and finally masquerade uveitis, further divided into neoplastic and non-neoplastic.
In this narrative review, we aim to describe the literature on non-infectious uveitis, with a specific focus on current topical anti-inflammatory therapy. For the purpose of our paper, we will refer to non-infectious uveitis as uveitis associated with non-infectious systemic disease, as well as idiopathic uveitis.
Epidemiology and Types of Non-Infectious Uveitis
Uveitis as a category of ocular disease causes up to 5–10% of visual impairment globally.9 It is a major cause of visual morbidity with prolonged visual loss occurring in two-thirds of patients, and up to 22% meeting the criteria for legal blindness at some point during the course of their disease.10
Non-infectious uveitis represents an important subset of uveitis, and is even more common than infectious uveitis. A large retrospective study of insurance claims database in the United States (US) of 4 million eligible patients estimated the prevalence of non-infectious uveitis at 121 cases per 100,000 adults, and 29 per 100,000 children.11 In the Asia-Pacific region, non-infectious uveitis is more common than infectious uveitis in all countries except for Myanmar and Nepal.12 Furthermore, non-infectious uveitis represents 41 to 55% of cases in China, 35 to 63% of cases in Japan and 25% to 45% of cases in India.12 It can, however, be difficult to generalize the prevalence of uveitis with population-based estimates, due to variations in disease definition, methodologies and geographical regions.13 For example, in the review by Hsu et al, the majority of uveitis cases are classed as idiopathic (30–60%), in a distinct category from infectious and non-infectious uveitis.12 However, on the other hand, others might consider idiopathic uveitis as part of non-infectious uveitis.14
The commonest form of uveitis overall is anterior uveitis, which can account for up to 90% of cases in primary care and 50 to 60% of cases in tertiary centres.15 Between 38% and 88% of these anterior uveitis cases would actually fall under the category of non-infectious uveitis, with no identifiable underlying cause. This further highlights that the majority burden of uveitis is non-infectious.15
Non-infectious uveitis can be also associated with systemic disease, with HLA-B27-associated anterior uveitis accounting for between 4% and 32% of cases.15 In contrast, in children the commonest systemic association of non-infectious uveitis is juvenile idiopathic arthritis (JIA).16
Other types of autoimmune disease associated with non-infectious uveitis include spondyloarthritis, psoriatic arthritis, multiple sclerosis, sarcoidosis, Behcet’s disease, and Vogt-Koyanagi-Harada disease.
General Approach to Treatment in Non-Infectious Uveitis
The initial choice of treatment for uveitis is dependent on a number of different factors, including the degree of inflammation, the laterality (unilateral versus bilateral), the anatomical location (anterior, intermediate or posterior uveitis) and the presence and extent of systemic disease. Other important factors to consider in treatment decisions would be the experiences and perspectives of patients and carers. A qualitative study by Tallouzi et al identified different core domains which are important to patients with non-infectious uveitis, including visual function, treatment burden, treatment side effects, and disease control.17
In non-infectious uveitis, therapy is aimed at suppressing the local immune response. It is often useful for the clinician to think of the concept of disease activity versus damage.18–20 Disease activity is the ongoing inflammation from the immune response, which might be acute or chronic, and is usually reversible. However, uncontrolled disease activity could potentially lead to permanent irreversible damage. Successful immunosuppression would theoretically prevent this from happening.
The primary utility of topical therapy in non-infectious uveitis is to control disease activity and limit damage from anterior uveitis. For cases of posterior segment uveitis which would include intermediate, posterior, or panuveitis, topical therapy alone is usually inadequate due to poor tissue penetration, although it remains crucial as adjunctive therapy.18 The only exception to this is topical non-steroidal anti-inflammatory drugs (NSAIDs) such as bromfenac, with evidence of therapeutic levels in the retina after topical application.21
In fact, the treatment of non-infectious uveitis would depend greatly on its anatomical type. For example, a general stepwise approach for therapy in patients with non-infectious anterior uveitis would usually begin with topical corticosteroids, followed by systemic corticosteroids, systemic immunomodulators and anti-metabolites such as azathioprine and mycophenolate mofetil, and finally biologics such as adalimumab and infliximab. Other local therapies include intravitreal injections such as triamcinolone acetate, and intraocular implants with dexamethasone or fluocinolone.14 On the other hand, the remaining anatomical types are more commonly treated with ocular or periocular injections, or systemic treatment with or without these topical agents. A summary of the indications and considerations of initiating therapy in non-infectious uveitis is demonstrated in Table 1.
 |
Table 1 Indications for Topical Treatments in Non-Infectious Uveitis and Important Considerations in Treatment Decisions |
Considerations in the Use of Topical Corticosteroids in Non-Infectious Uveitis
One of the main topical anti-inflammatory agents used is topical steroid. There are a variety of different steroid subtypes, but the main ones used in ophthalmology are the glucocorticoids, which have anti-inflammatory and immunosuppressive activity. One of the earliest advances in the ophthalmic use of topical corticosteroid eye drops was described by Woods et al in the 1950s, wherein they reported the successful treatment of uveitis patients with topical cortisone solution.22 Seventy years later, it is still impossible to imagine modern ophthalmic practice without topical steroid eye drops. They remain the most common method of administering steroids to the eye, with measurable concentrations in the human aqueous humour within 15–30 minutes.23–25
Glucocorticoids work by interacting with intracellular glucocorticoid receptors found in the cytosol.26 A number of complex and intricate molecular mechanisms have been identified through which glucocorticoids then affect ocular inflammation, as depicted in Figure 1. However, these have been largely grouped into four main glucocorticoid-mediated anti-inflammatory mechanisms. The first entails glucocorticoid receptors binding to glucocorticoid-responsive elements, leading to direct effects on gene expression, such as induction and activation of annexin I. This is an anti-inflammatory protein which inhibits cytosolic phospholipase A2α, blocking the release of arachidonic acid and by extension its subsequent conversion to eicosanoids such as prostaglandins, thromboxanes, prostacyclins, and leukotrienes.27 Another mechanism which has been described involves interaction of the glucocorticoid receptor with other transcription factors, such as nuclear factor-κB (NF-κB) and activator protein 1, leading to indirect effects on gene expression and prostaglandin synthesis.27 Glucocorticoids can also affect inflammation by decreasing the mRNA stability for gene coding for inflammatory proteins, such as vascular endothelial growth factor (VEGF) and cyclooxygenase 2.28,29 Finally, activated glucocorticoid receptors have also been shown to have anti-inflammatory effects which are not mediated by changes in gene expression, through influencing second-messenger cascades. An example of this is the phosphatidylinositol-3-hydroxykinase – Akt – endothelial nitric oxide synthetase pathway.30 All these mechanisms ultimately lead to decreased production of important cytokines and inflammatory mediators, which affects the blood-retina barrier, leading to a reduction in fibroblast proliferation, fibrin deposition, retinal oedema, capillary leakage, intraretinal migration of inflammatory cells and VEGF levels.
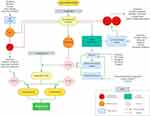 |
Figure 1 Simplified anti-inflammatory pathways of glucocorticoids. Abbreviations: NF-κB, Nuclear factor-κB; IκB, inhibitory protein of NF-κB; COX-2, cyclooxygenase-2; c-Jun/Fos, inflammation transcription factor polypeptide subunits; MAPK, mitogen-activated protein kinases; cPLA2α, cytosolic phospholipase A2α; 5-LOX, 5-lipoxygenase.27 |
There are a variety of routes through which glucocorticoids can be administered for uveitis. Regional administration is often preferred, as it provides higher levels of ocular delivery whilst minimizing systemic side effects, and includes topical, sub‐conjunctival, periocular (sub‐Tenon, orbital floor, peribulbar) and intravitreal routes. Furthermore, if required other systemic routes can also be used such as oral and intravenous administration. As mentioned above, topical steroid eye drops remain the first-line treatment choice for anterior uveitis. However, initial assessment of the severity of anterior uveitis is crucial to decide on the treatment regime, as well as for monitoring of the treatment response. The SUN workshop grading of anterior chamber flare and cells remains the gold standard for this.5 Topical steroid eye drops are often prescribed as a course of hourly or half-hourly intensive treatment for a week, followed by a slow taper over the following weeks, depending on the treatment response.31 Patients with chronic disease sometimes require long-term topical steroid eye drops to maintain remission. The exact regime of topical steroid eye drops therapy often remains at the discretion of individual clinicians.
Because glucocorticoids are potent drugs with a plethora of molecular effects, side-effects can occur with their use. Elevation in intraocular pressure (IOP) is one of the most documented side effects of topical steroid eye drops. Although there are several theories and the exact mechanism of how this happens remains unclear, topical steroid eye drops are thought to increase aqueous outflow resistance by affecting the extracellular matrix of the trabecular meshwork.32 In fact, following the use of topical dexamethasone, between 34% and 42% of individuals can develop an IOP rise from a baseline of 6 mmHg to 15mmHg, to a final pressure of 20mmHg to 31mmHg.33 This degree of pressure rise is found in nearly all patients with primary open-angle glaucoma. Unfortunately, since many of the early generation topical steroid eye drops were introduced prior to modern regulatory requirements, placebo-controlled trials and comparative studies looking at their effects on IOP are limited.34
While the effects of IOP elevation with topical steroid eye drops are well known, it is important to consider other risk factors for ocular hypertension (OHT) in non-infectious uveitis. It has been shown that risk factors for increased incidence of OHT ≥30mmHg include systemic hypertension, prior history of ocular hypertension in the fellow eye, anterior chamber cells and peripheral anterior synechiae.35 In contrast, a history of bilateral uveitis and previous hypotony were associated with a reduced risk of OHT.35 This highlights the importance of a comprehensive history and clinical examination in patients presenting with raised IOP, including gonioscopy to identify synechial closure in uveitis.36
Cantrill et al looked at the IOP effects of different topical corticosteroids, including dexamethasone 0.1%, prednisolone 1.0%, dexamethasone 0.005%, fluorometholone 0.1%, hydrocortisone 0.5%, tetrahydrotriamcinolone 0.25%, and medrysone 1.0%.37 Topical steroids were given four times a day for an average of 4.6 weeks, and whilst the study sample was small (six men and four women), they confirmed that the more potent the topical steroid, the higher the IOP response. To our knowledge, the only prospective randomized controlled trial comparing the safety and efficacy of different topical steroid eye drops in uveitis was by the Loteprednol Etabonate US Uveitis Study Group in 1998.38 In this study, the authors looked at the use of loteprednol etabonate 0.5% versus prednisolone acetate 1% in the treatment of uncomplicated anterior uveitis. They found that while loteprednol was less effective in reducing signs and symptoms, only one patient in the loteprednol group (out of 118) developed a pressure rise of more than 10mmHg, compared to seven patients in the prednisolone acetate group (out of 103). The treatment regime of the topical steroids was eight times a day from day 0 to day 7, six times a day from days 8 to 14, four times a day from days 15 to 21, finally followed by a 14-day tapering regime from four times a day to none.
Interestingly, in the majority of cases with topical steroid-induced IOP elevation, the IOP tends to lower spontaneously back to baseline levels after the cessation of topical steroid therapy.37,39 The different preparations of available steroid eye drops are outlined in Table 2.
 |
Table 2 A Breakdown of the Characteristic of the Various Topical Steroid Eye Drops, Arranged in Order of Relative Potency |
The other well-known side effect of topical corticosteroids is the development of cataracts, particularly posterior subcapsular cataracts.40–42 However, non-infectious uveitis in itself is a risk factor for the development of cataracts, with the 5-year risk of developing cataracts being 3-times higher in patients with uveitis compared to controls.43 In fact, in a paediatric cohort of patients with juvenile idiopathic arthritis, the prevalence of cataract was 44% (out of 140 children), affecting 77% of panuveitis patients, 48% of anterior uveitis patients and 48% of intermediate uveitis patients.44 Other risk factors identified for the development of cataracts included the number of uveitis flares per year, the presence of cystoid macular oedema, and posterior synechiae. Remarkably, while the use of local corticosteroid injections is a risk factor, treatment with topical corticosteroids was not found to be a significant risk factor. This highlights the importance of an appropriate long-term regimen for patients with chronic uveitis who require long-term corticosteroids, to reduce the risk of cataract formation. A retrospective review of JIA patients by Thorne et al over 4 years demonstrated that there was an 87% reduction in the risk of cataract formation in patients who were using topical steroid eye drops less than three times a day, compared to patients using these more than three times a day.45 This is an important consideration when counselling patients requiring long-term topical steroid eye drop therapy, as uncontrolled disease activity with frequent flares probably constitutes a greater risk of developing cataracts than simply as a side-effect of topical steroid eye drops.
Topical corticosteroids are also used to treat macular oedema associated with uveitis. In a retrospective case series of 58 patients (72 eyes) with non-infectious uveitic macular oedema treated with topical 0.05% difluprednate, central macular subfield thickness decreased by an average of 17%. At 30 days, 76% of eyes had improved, with 48% of eyes achieving resolution of macular oedema.46 However, due to the higher rate of complications from glaucoma and cataracts, it has not yet been approved by European Medical Agency.47
Considerations in the Use of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
Another class of anti-inflammatory agents used in uveitis is the nonsteroidal anti-inflammatory drugs (NSAIDs). In ophthalmic practice, topical NSAIDs are commonly used for the treatment of postoperative inflammation and macular oedema following cataract surgery. Several types of topical NSAIDs have been approved by the US Food and Drug Agency (FDA) for this purpose, including bromfenac 0.09% (Bromday®; ISTA Pharmaceuticals Inc), and bromfenac 0.07% (Prolensa®; Bausch and Lomb Incorporated, Bridgewater, NJ, US).48 NSAIDs exert their anti-inflammatory action by inhibiting the cyclooxygenase enzymes (COX 1 and COX 2), therefore inhibiting the conversion of arachidonic acid into inflammatory mediators such as prostaglandins and thromboxanes, as illustrated in Figure 2.49
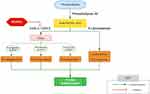 |
Figure 2 The anti-inflammatory effect of NSAIDs via the arachidonic acid pathway.97,98 |
Similar to glucocorticoids, the main route used for the application of NSAIDs in uveitis is topical administration. Although the evidence of their efficacy is limited, topical NSAIDs are frequently used-off label by clinicians for the treatment of uveitic macular oedema,47 as they are considered to be safe and effective alternatives in the topical management of ocular inflammation, due to the serious adverse effects associated with steroid use, as mentioned above.50 A comparative case series of 67 eyes evaluated the efficacy of bromfenac drops alone, versus bromfenac with intravitreal bevacizumab (IVB), versus bromfenac with intravitreal triamcinolone (IVTA) in the treatment of uveitic macular oedema. It was shown that while both combinations with IVB and IVTA were effective at improving visual acuity and decreasing central macular thickness, bromfenac alone was ineffective with no statistically significant change in visual acuity.51 Similarly, a retrospective review of 281 patients treated with three times a day topical nepafenac 0.1% for uveitic macular oedema failed to demonstrate statistically significant changes in visual acuity and central macular thickness.47
Consideration of Topical Therapies in Uveitic Macular Oedema in Non-Infectious Uveitis
Modern treatment of uveitic macular oedema usually requires the use of systemic immunosuppression, intravitreal steroids, or intravitreal implants.14 While the level of published evidence on the use of topical therapies for uveitic macular oedema is limited, clinicians can sometimes encounter patients who are unsuitable for anything other than topical therapies. This would include patients with significant co-morbidities unsuitable for systemic steroids, or who are unable to tolerate intravitreal steroids and implants. For these group of patients, it is prudent to consider off-label use of topical medications such as dorzolamide, NSAIDs, and topical steroids as summarized in Table 3.
 |
Table 3 Published Evidence About Topical Therapies for Uveitic Macular Oedema |
Complications and Consequences of Non-Infectious Uveitis
Chronic inflammation in non-infectious uveitis can increase the risk of many complications, specifically posterior synechiae formation, anterior capsule pigment deposition, glaucoma, cataracts, loss of vision, visual disturbance due to epiretinal membrane formation or macula oedema, retinal disorders and retinal detachment.43
Loss of vision in anterior uveitis alone is rare. A study of 2526 eyes with a mean follow up of 6.8 years showed that the incidence of permanent moderate vision loss (≤20/50) in anterior uveitis was 3.8%.52 The most common cause of this was found to be uveitic glaucoma, accounting for up to 31.3% of cases of moderate vision loss. Other causes of permanent vision loss in anterior uveitis include cornea scarring, cornea oedema, pupillary membrane, epiretinal membrane, macular hole and hypotony. Posterior synechiae can also lead to loss of vision, and have been shown to be associated with worse visual outcomes.53,54 Another study reported that for patients with uveitis who do develop vision loss, the primary reason was macular oedema, causing reduction of vision to less than 20/40 in up to 30% of patients with posterior uveitis.55
In the next section, we will discuss the specific indications of topical therapy to reduce the long-term complications and consequences of chronic non-infectious uveitis.
Topical Cycloplegics to Treat Posterior Synechiae and Anterior Lens Capsule Pigment Deposition
Topical cycloplegic drugs such as atropine and cyclopentolate are used in anterior uveitis to break existing posterior synechiae, and prophylactically to prevent the formation of synechiae in chronic uveitis. Severe posterior synechiae could limit the movement of aqueous from the posterior chamber into the anterior chamber, resulting in iris bombe and secondary angle closure.
Of the cycloplegic agents, atropine, which is an organic compound derived from tropic acid and tropine, is the most potent. It has a slow onset of effect with duration of action lasting up to 2 weeks. On the other hand, cyclopentolate, a synthetic compound, has a faster onset of action (30–45 min) and duration of action (24 hours). Their mechanism of action is very similar, wherein they are both anticholinergic agents which inhibit contraction of the circular pupillary sphincter muscle by blocking muscarinic receptors. This inhibition along with the counteracting radial pupillary dilator muscle contraction results in pupillary dilation.56,57
One of the considerations with topical cycloplegics is whether or not they affect aqueous flare measurements in the assessment of anterior chamber activity. Studies using laser flare meter as a quantitative method of measuring cells and protein in the aqueous have shown that mydriatic agents could reduce laser flare values by 10–20% following dilation in normal eyes.58 However, in a cohort of 25 patients with chronic anterior uveitis, it was shown that the measurement of flare was not affected by pupillary dilation.59 Similarly, a larger and more recent study of 148 eyes from 83 patients with inactive uveitis showed no significant differences in flare after pupillary dilation.60 This is a potentially important consideration when monitoring disease activity in patients on long-term cycloplegics.
Both cyclopentolate and atropine are available in preservative-free minims preparations such as cyclopentolate 0.5%, cyclopentolate 1% and atropine sulphate 1%.
Adverse effects of cycloplegic agents range from localised ocular effects to generalised systemic effects due to absorption. For atropine, the ocular adverse effects include allergic contact dermatitis of the lids, allergic conjunctivitis, keratitis, and increased intraocular pressure, and the systemic side effects include dryness of secretions, fever, irritability, tachycardia and convulsions. Similarly, for cyclopentolate, the ocular side effects may include irritation, lacrimation, allergic blepharoconjunctivitis, conjunctival hyperemia, and increase in intraocular pressure. Systemic side effects include drowsiness, ataxia, disorientation, incoherent speech, restlessness, and visual hallucinations.56,57
Topical Glaucoma Agents for Management of Uveitic Glaucoma
As previously mentioned, uveitic glaucoma is one of the most common causes of visual loss in patient with uveitis. As mentioned previously, the mechanism of increased IOP in uveitic glaucoma is often complex and multifactorial, occurring either due to the inflammatory process itself, or secondary to long-term topical steroid use.61,62
While there are an increasing variety of topical glaucoma drops available, it is useful to be aware of certain glaucoma agents which have been associated with drug-induced uveitis.
Brimonidine tartrate, a selective alpha2-adrergic receptor agonist, is a glaucoma agent which in a number of case reports link to granulomatous anterior uveitis and increased IOP.63–65 In one of the largest case series of brimonidine-associated anterior uveitis (16 patients and 26 eyes), the key clinical features were conjunctival ciliary injection and mutton fat keratic precipitates.66 This group of patients had different types of glaucoma, with none of them having a prior diagnosis of uveitis. The time between initiation of brimonidine treatment and presentation varied widely between 1 week to 49 months, with complete resolution of uveitis within 4 weeks of stopping treatment.
Another class of widely used glaucoma agents are prostaglandin analogues, such as latanoprost, travoprost and bimatoprost. These are associated with a number of well-known side effects such as eyelash growth, iris and periocular skin pigmentation, iris cysts, and conjunctival injections.67,68 In a case series of 94 patients and 163 eyes on latanoprost, 6% of patients developed anterior uveitis, while 2% developed cystoid macular oedema.69 Furthermore, in a small case series of four patients with anterior uveitis associated with latanoprost use, the anterior uveitis recurred after a latanoprost re-challenge.70 A recent systematic review and meta-analysis of 214 studies looked at uveitis and cystoid macular oedema associated with uveitis in glaucoma patients. Out of 28,232 patients, the incidence of uveitis and cystoid macular oedema were 0.22% and 0.09%, respectively, with higher frequency of association with latanoprost compared to bimatoprost.71 The cases of uveitis or cystoid macular oedema are often confounded by other effects such as ocular surgery, subluxed introcular lens, and aphakia. This makes it difficult to conclude a cause-effect relationship.
Ocular Surface Disease Considerations in the Use of Topical Therapy
An important consideration in the use of topical eye drops is the presence of preservatives. Benzalkonium chloride (BAK) is the most commonly used preservative in eye drops.72 It has been associated with ocular side effects such as dry eyes and ocular surface inflammation.73,74 Ocular surface irritants can impede the recovery of the ocular surface in patients with pre-existing ocular surface disease.75 It is important for clinicians to conduct a baseline assessment of the patient’s ocular surface before starting treatment, especially when prolonged use of topical therapy is expected.68 Ideally, preservative-free formulations of eye drops should be used when possible. Patients with ocular surface disease could have exacerbation of their symptoms of uveitis with symptoms such as redness, discharge, fluctuation of vision, gritty sensation, and excess tearing. If necessary, the ocular surface should be optimized with the management of blepharitis, and support of the tear film with ocular lubrication.
Conclusions
The modern management of non-infectious uveitis often requires systemic corticosteroids, intravitreal injections, intraocular implants and systemic immunosuppression therapy. However, topical therapies remain an important non-invasive treatment modality for the management of non-infectious uveitis, especially in patients who are unsuitable for systemic and invasive therapies. It is important for clinicians to consider the different aspects of topical therapies in non-infectious uveitis for the optimisation of treatment response while minimizing side effects.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Williams GS, Westcott M. Practical Uveitis: Understanding the Grape. CRC Press; 2017.
2. Mackenzie W. A Practical Treatise on the Diseases of the Eye. Forgotten Books; 1855.
3. Forrester JV. New concepts on the role of autoimmunity in the pathogenesis of uveitis. Eye. 1992;6(5):433–446. doi:10.1038/eye.1992.93
4. Bloch-Michel E. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–235. doi:10.1016/S0002-9394(14)74235-7
5. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140(3):509–516.
6. Hogan MJ, Kimura SJ, Thygeson P. Signs and symptoms of uveitis: i. Anterior uveitis. Am J Ophthalmol. 1959;47(5):155–170. doi:10.1016/S0002-9394(14)78239-X
7. Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi:10.1016/S0161-6420(85)34001-0
8. Deschenes J, Murray PI, Rao NA, Nussenblatt RB. International uveitis study group (IUSG) clinical classification of uveitis. Ocul Immunol Inflamm. 2008;16(1–2):1–2. doi:10.1080/09273940801899822
9. Miserocchi E, Fogliato G, Modorati G, Bandello F. Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol. 2013;23(5):705–717. doi:10.5301/ejo.5000278
10. Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88(9):1159–1162. doi:10.1136/bjo.2003.037226
11. Thorne JE, Suhler E, Skup M, et al. Prevalence of noninfectious uveitis in the United States: a claims-based analysis. JAMA Ophthalmol. 2016;134(11):1237–1245. doi:10.1001/jamaophthalmol.2016.3229
12. Hsu YR, Huang JCC, Tao Y, et al. Noninfectious uveitis in the Asia–Pacific region. Eye. 2019;33(1):66–77. doi:10.1038/s41433-018-0223-z
13. García-Aparicio Á, García de Yébenes MJ, Otón T, Muñoz-Fernández S. Prevalence and incidence of uveitis: a systematic review and meta-analysis. Ophthalmic Epidemiol. 2021;28(6):461–468. doi:10.1080/09286586.2021.1882506
14. Rosenbaum JT, Bodaghi B, Couto C, et al. New observations and emerging ideas in diagnosis and management of non-infectious uveitis: a review. Semin Arthritis Rheum. 2019;49(3):438–445. doi:10.1016/j.semarthrit.2019.06.004
15. Tsirouki T, Dastiridou A, Symeonidis C, et al. A focus on the epidemiology of uveitis. Ocul Immunol Inflamm. 2018;26(1):2–16. doi:10.1080/09273948.2016.1196713
16. Nalcacioglu-Yuksekkaya P, Ozdal PC, Yazici A, Tirhis H. Clinical and demographic characteristics of patients with uveitis starting later in life. Ocul Immunol Inflamm. 2015;23(4):304–310. doi:10.3109/09273948.2014.938761
17. Tallouzi MO, Moore DJ, Bucknall N, et al. Outcomes important to patients with non-infectious posterior segment-involving uveitis: a qualitative study. BMJ Open Ophthalmol. 2020;5(1):e000481. doi:10.1136/bmjophth-2020-000481
18. Barry RJ, Nguyen QD, Lee RW, Murray PI, Denniston AK. Pharmacotherapy for uveitis: current management and emerging therapy. Clin Ophthalmol Auckl NZ. 2014;22(8):1891–1911.
19. Barry RJ, Sutcliffe N, Isenberg DA, et al. The Sjögren’s Syndrome Damage Index--a damage index for use in clinical trials and observational studies in primary Sjögren’s syndrome. Rheumatol Oxf Engl. 2008;47(8):1193–1198. doi:10.1093/rheumatology/ken164
20. Skopouli FN, Dafni U, Ioannidis JP, Moutsopoulos HM. Clinical evolution, and morbidity and mortality of primary Sjögren’s syndrome. Semin Arthritis Rheum. 2000;29(5):296–304. doi:10.1016/S0049-0172(00)80016-5
21. Jones J, Francis P. Ophthalmic utility of topical bromfenac, a twice-daily nonsteroidal anti-inflammatory agent. Expert Opin Pharmacother. 2009;10(14):2379–2385. doi:10.1517/14656560903188425
22. Woods AC. Clinical and experimental observation on the use of ACTH and cortisone in ocular inflammatory disease. Am J Ophthalmol. 1950;33(9):1325–1351. doi:10.1016/0002-9394(50)91827-7
23. Watson D, Noble MJ, Dutton GN, Midgley JM, Healey TM. Penetration of topically applied dexamethasone alcohol into human aqueous humor. Arch Ophthalmol. 1988;106(5):686–687. doi:10.1001/archopht.1988.01060130748037
24. Watson DG, McGhee CNJ, Midgley JM, Dutton GN, Noble MJ. Penetration of topically applied betamethasone sodium phosphate into human aqueous humour. Eye. 1990;4(4):603–606. doi:10.1038/eye.1990.84
25. McGhee CNJ, Noble MJ, Watson DG, et al. Penetration of topically applied prednisolone sodium phosphate into human aqueous humour. Eye. 1989;3(4):463–467. doi:10.1038/eye.1989.69
26. Sulaiman RS, Kadmiel M, Cidlowski JA. Glucocorticoid receptor signaling in the eye. Steroids. 2018;133:60–66. doi:10.1016/j.steroids.2017.11.002
27. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353(16):1711–1723. doi:10.1056/NEJMra050541
28. Saklatvala J, Nagase H, Salvesen G, Dean J, Clark A. Control of the expression of inflammatory response genes. In: Biochemical Society Symposia. Portland Press; 2003:95–106.
29. Gille J, Reisinger K, Westphal-Varghese B, Kaufmann R. Decreased mRNA stability as a mechanism of glucocorticoid-mediated inhibition of vascular endothelial growth factor gene expression by cultured keratinocytes. J Invest Dermatol. 2001;117(6):1581–1587. doi:10.1046/j.0022-202x.2001.01573.x
30. Hafezi-Moghadam A, Simoncini T, Yang Z, et al. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med. 2002;8(5):473–479. doi:10.1038/nm0502-473
31. Airody A, Heath G, Lightman S, Gale R. Non-infectious uveitis: optimising the therapeutic response. Drugs. 2016;76(1):27–39. doi:10.1007/s40265-015-0502-y
32. Phulke S, Kaushik S, Kaur S, Pandav SS. Steroid-induced glaucoma: an avoidable irreversible blindness. J Curr Glaucoma Pract. 2017;11(2):67. doi:10.5005/jp-journals-10028-1226
33. Raizman M. Corticosteroid therapy of eye disease: fifty years later. Arch Ophthalmol. 1996;114(8):1000–1001. doi:10.1001/archopht.1996.01100140208016
34. Pleyer U, Ursell PG, Rama P. Intraocular pressure effects of common topical steroids for post-cataract inflammation: are they all the same? Ophthalmol Ther. 2013;2(2):55–72. doi:10.1007/s40123-013-0020-5
35. Daniel E, Pistilli M, Kothari S, et al. Risk of ocular hypertension in adults with noninfectious uveitis. Ophthalmology. 2017;124(8):1196–1208. doi:10.1016/j.ophtha.2017.03.041
36. Din NM, Isa H, Taylor SR, Barton K, Lightman SL. Intraocular pressure elevation in uveitis. Expert Rev Ophthalmol. 2012;7(1):45–59. doi:10.1586/eop.11.75
37. Cantrill HL, Palmberg PF, Zink HA, Waltman SR, Podos SM, Becker B. Comparison of in vitro potency of corticosteroids with ability to raise intraocular pressure. Am J Ophthalmol. 1975;79(6):1012–1017. doi:10.1016/0002-9394(75)90687-X
38. Loteprednol Etabonate US Uveitis Study Group. Controlled evaluation of loteprednol etabonate and prednisolone acetate in the treatment of acute anterior uveitis. Am J Ophthalmol. 1999;127(5):537–544. doi:10.1016/S0002-9394(99)00034-3
39. Sallam A, Sheth HG, Habot-Wilner Z, Lightman S. Outcome of raised intraocular pressure in uveitic eyes with and without a corticosteroid-induced hypertensive response. Am J Ophthalmol. 2009;148(2):207–213.e1. doi:10.1016/j.ajo.2009.02.032
40. Basilious A, Lloyd JC. Posterior subcapsular cataracts and hypotony secondary to severe pembrolizumab induced uveitis: case report. Can J Ophthalmol J Can Ophtalmol. 2016;51(1):e4–e6. doi:10.1016/j.jcjo.2015.09.008
41. Dikshit SK, Avasthi PN. Posterior lenticular opacities in children on corticosteroid therapy. Indian J Pediatr. 1965;32(3):93–96. doi:10.1007/BF02756568
42. Jobling AI, Augusteyn RC. What causes steroid cataracts? A review of steroid-induced posterior subcapsular cataracts. Clin Exp Optom. 2002;85(2):61–75. doi:10.1111/j.1444-0938.2002.tb03011.x
43. Dick AD, Tundia N, Sorg R, et al. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. 2016;123(3):655–662. doi:10.1016/j.ophtha.2015.10.028
44. Blum-Hareuveni T, Seguin-Greenstein S, Kramer M, et al. Risk factors for the development of cataract in children with uveitis. Am J Ophthalmol. 2017;177:139–143. doi:10.1016/j.ajo.2017.02.023
45. Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2010;117(7):1436–1441. doi:10.1016/j.ophtha.2009.12.003
46. Schallhorn JM, Niemeyer KM, Browne EN, Chhetri P, Acharya NR. Difluprednate for the treatment of uveitic cystoid macular edema. Am J Ophthalmol. 2018;191:14–22. doi:10.1016/j.ajo.2018.03.027
47. Petrushkin H, Rogers D, Pavesio C. The use of topical non-steroidal anti-inflammatory drugs for uveitic cystoid macular edema. Ocul Immunol Inflamm. 2018;26(5):795–797. doi:10.1080/09273948.2016.1269931
48. Sheppard JD. Topical bromfenac for prevention and treatment of cystoid macular edema following cataract surgery: a review. Clin Ophthalmol Auckl NZ. 2016;10:2099. doi:10.2147/OPTH.S86971
49. Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10(2):229–241. doi:10.1208/s12248-008-9024-9
50. Waterbury L, Kunysz EA, Beuerman R. Effects of steroidal and non-steroidal anti-inflammatory agents on corneal wound healing. J Ocul Pharmacol. 1987;3(1):43–54. doi:10.1089/jop.1987.3.43
51. Radwan AE, Arcinue CA, Yang P, Artornsombudh P, Abu Al-Fadl EM, Foster CS. Bromfenac alone or with single intravitreal injection of bevacizumab or triamcinolone acetonide for treatment of uveitic macular edema. Graefes Arch Clin Exp Ophthalmol. 2013;251(7):1801–1806. doi:10.1007/s00417-013-2309-4
52. Al-Ani HH, Sims JL, Tomkins-Netzer O, Lightman S, Niederer RL. Vision loss in anterior uveitis. Br J Ophthalmol. 2020;104(12):1652–1657. doi:10.1136/bjophthalmol-2019-315551
53. Loh AR, Acharya NR. Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol. 2010;150(4):534–542. doi:10.1016/j.ajo.2010.04.031
54. Gregory AC, Kempen JH, Daniel E, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the SITE study. Ophthalmology. 2013;120(1):186–192. doi:10.1016/j.ophtha.2012.07.052
55. Rothova A, Suttorp-van Schulten MS, Treffers WF, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80(4):332–336. doi:10.1136/bjo.80.4.332
56. Eperjesi F, Jones K. Cycloplegic refraction in optometric practice. Optom Pract. 2005;6(3):107.
57. Ihekaire DE. The comparative efficacy of Cycloplegic drugs–Tropicamide and Cyclopentolate on school children. Int J Sci Res Educ. 2012;5(3):223–246.
58. Shah SM, Spalton DJ, Smith SE. Measurement of aqueous cells and flare in normal eyes. Br J Ophthalmol. 1991;75(6):348–352. doi:10.1136/bjo.75.6.348
59. Ikeji F, Pavesio C, Bunce C, White E. Quantitative assessment of the effects of pupillary dilation on aqueous flare in eyes with chronic anterior uveitis using laser flare photometry. Int Ophthalmol. 2010;30(5):491–494. doi:10.1007/s10792-010-9373-0
60. Yilmaz M, Guven Yilmaz S, Palamar M, Ates H, Yagci A. The effects of tropicamide and cyclopentolate hydrochloride on laser flare meter measurements in uveitis patients: a comparative study. Int Ophthalmol. 2021;41(3):853–857. doi:10.1007/s10792-020-01639-3
61. Kalogeropoulos D, Sung VC. Pathogenesis of uveitic glaucoma. J Curr Glaucoma Pract. 2018;12(3):125. doi:10.5005/jp-journals-10078-1236
62. Sng CC, Ang M, Barton K. Uveitis and glaucoma: new insights in the pathogenesis and treatment. Prog Brain Res. 2015;221:243–269.
63. Becker HI, Walton RC, Diamant JI, Zegans ME. Anterior uveitis and concurrent allergic conjunctivitis associated with long-term use of topical 0.2% brimonidine tartrate. Arch Ophthalmol. 2004;122(7):1063–1066. doi:10.1001/archopht.122.7.1063
64. Byles DB, Frith P, Salmon JF. Anterior uveitis as a side effect of topical brimonidine. Am J Ophthalmol. 2000;130(3):287–291. doi:10.1016/S0002-9394(00)00491-8
65. Cates CA, Jeffrey MN. Granulomatous anterior uveitis associated with 0.2% topical brimonidine. Eye. 2003;17(5):670–671. doi:10.1038/sj.eye.6700392
66. Hopf S, Mercieca K, Pfeiffer N, Prokosch-Willing V. Brimonidine-associated uveitis–a descriptive case series. BMC Ophthalmol. 2020;20(1):1–7. doi:10.1186/s12886-020-01762-w
67. Bito LZ. Prostaglandins: a new approach to glaucoma management with a new, intriguing side effect. Surv Ophthalmol. 1997;41:S1–S14. doi:10.1016/S0039-6257(97)80002-1
68. Holló G. The side effects of the prostaglandin analogues. Expert Opin Drug Saf. 2007;6(1):45–52. doi:10.1517/14740338.6.1.45
69. Warwar RE, Bullock JD, Ballal D. Cystoid macular edema and anterior uveitis associated with latanoprost use: experience and incidence in a retrospective review of 94 patients. Ophthalmology. 1998;105(2):263–268. doi:10.1016/S0161-6420(98)92977-3
70. Fechtner RD, Khouri AS, Zimmerman TJ, et al. Anterior uveitis associated with latanoprost. Am J Ophthalmol. 1998;126(1):37–41. doi:10.1016/S0002-9394(98)00071-3
71. Hu J, Vu JT, Hong B, Gottlieb C. Uveitis and cystoid macular oedema secondary to topical prostaglandin analogue use in ocular hypertension and open angle glaucoma. Br J Ophthalmol. 2020;104(8):1040–1044. doi:10.1136/bjophthalmol-2019-315280
72. Baudouin C, Labbé A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
73. Aguayo Bonniard A, Yeung JY, Chan CC, Birt CM. Ocular surface toxicity from glaucoma topical medications and associated preservatives such as benzalkonium chloride (BAK). Expert Opin Drug Metab Toxicol. 2016;12(11):1279–1289. doi:10.1080/17425255.2016.1209481
74. Datta S, Baudouin C, Brignole-Baudouin F, Denoyer A, Cortopassi GA. The eye drop preservative benzalkonium chloride potently induces mitochondrial dysfunction and preferentially affects LHON mutant cells. Invest Ophthalmol Vis Sci. 2017;58(4):2406–2412. doi:10.1167/iovs.16-20903
75. Dart J. Corneal toxicity: the epithelium and stroma in iatrogenic and factitious disease. Eye. 2003;17(8):886–892. doi:10.1038/sj.eye.6700576
76. Korenfeld MS, Silverstein SM, Cooke DL, Vogel R, Crockett RS, Emulsion DO. Difluprednate ophthalmic emulsion 0.05% for postoperative inflammation and pain. J Cataract Refract Surg. 2009;35(1):26–34. doi:10.1016/j.jcrs.2008.09.024
77. Smith S, Lorenz D, Peace J, McLeod K, Crockett RS, Vogel R. Difluprednate ophthalmic emulsion 0.05%(Durezol®) administered two times daily for managing ocular inflammation and pain following cataract surgery. Clin Ophthalmol Auckl NZ. 2010;4:983. doi:10.2147/OPTH.S10696
78. Slabaugh MA, Herlihy E, Ongchin S, Van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. 2012;153(5):932–938. doi:10.1016/j.ajo.2011.10.008
79. Kakimoto H, Takamura Y, Arimura S, et al. Effect of 0.05% difluprednate ophthalmic emulsion on proinflammatory cytokine levels after retinal laser photocoagulation in rabbits. J Ocul Pharmacol Ther. 2018;34(5):410–415. doi:10.1089/jop.2017.0109
80. Kersey JP, Broadway DC. Corticosteroid-induced glaucoma: a review of the literature. Eye. 2006;20(4):407–416. doi:10.1038/sj.eye.6701895
81. Armaly MF. Effect of corticosteroids on intraocular pressure and fluid dynamics: i. The effect of dexamethasone* in the normal eye. Arch Ophthalmol. 1963;70(4):482–491. doi:10.1001/archopht.1963.00960050484010
82. Kawamura M, Zako M. Long-term stability of uveitis with faint anterior chamber flare treated with once-daily topical ophthalmic betamethasone. Inflammation. 2014;37(2):417–425. doi:10.1007/s10753-013-9754-4
83. Fairbairn WD, Thorson JC. Fluorometholone: anti-inflammatory and intraocular pressure effects. Arch Ophthalmol. 1971;86(2):138–141. doi:10.1001/archopht.1971.01000010140004
84. Shokoohi-Rad S, Daneshvar R, Jafarian-Shahri M, Rajaee P. Comparison between Betamethasone, Fluorometholone and Loteprednol Etabonate on intraocular pressure in patients after keratorefractive surgery. J Curr Ophthalmol. 2018;30(2):130–135. doi:10.1016/j.joco.2017.11.008
85. Stewart R, Horwitz B, Howes J, Novack GD, Hart K. Double-masked, placebo-controlled evaluation of loteprednol etabonate 0.5 for postoperative inflammation. J Cataract Refract Surg. 1998;24(11):1480–1489. doi:10.1016/S0886-3350(98)80170-3
86. Lane SS, Holland EJ. Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery. J Cataract Refract Surg. 2013;39(2):168–173. doi:10.1016/j.jcrs.2012.10.039
87. Filippelli M, Gelso A, Rinaldi M, et al. Effects of topical low-dose preservative-free hydrocortisone on intraocular pressure in patients affected by ocular surface disease with and without glaucoma. Graefes Arch Clin Exp Ophthalmol. 2022;260(1):247–253. doi:10.1007/s00417-021-05345-3
88. Feiler DL, Srivastava SK, Pichi F, Bena J, Lowder CY. Resolution of noninfectious uveitic cystoid macular edema with topical difluprednate. Retina. 2017;37(5):844–850. doi:10.1097/IAE.0000000000001243
89. Symes RJ, Forooghian F. Topical difluprednate monotherapy for uveitic macular edema. Can J Ophthalmol. 2016;51(1):47–49. doi:10.1016/j.jcjo.2015.10.007
90. Onishi SM, Asahi MG, Chou C, Gallemore RP. Topical difluprednate for the treatment of Harada’s disease. Clin Ophthalmol Auckl NZ. 2015;9:157.
91. Hariprasad SM, Callanan D. Topical nepafenac 0.1% for treatment of chronic uveitic cystoid macular edema. Retin Cases Brief Rep. 2008;2(4):304–308. doi:10.1097/ICB.0b013e31809ed9db
92. Saade JS, Istambouli R, AbdulAal M, Antonios R, Hamam RN. Bromfenac 0.09% for the treatment of macular edema secondary to noninfectious uveitis. Middle East Afr J Ophthalmol. 2021;28(2):98. doi:10.4103/meajo.meajo_134_21
93. Gallenga PE, Mastropasqua L, Lobefolo L, et al. Efficacy of diclofenac eyedrops in preventing postoperative inflammation and long-term cystoid macular edema. J Cataract Refract Surg. 1997;23(8):1183–1189. doi:10.1016/S0886-3350(97)80313-6
94. Heier J, Cheetham JK, DeGryse R, et al. Ketorolac tromethamine 0.5% ophthalmic solution in the treatment of moderate to severe ocular inflammation after cataract surgery: a randomized, vehicle-controlled clinical trial. Am J Ophthalmol. 1999;127(3):253–259. doi:10.1016/S0002-9394(98)00413-9
95. Suzuki T, Hayakawa K, Nakagawa Y, Onouchi H, Ogata M, Kawai K. Topical dorzolamide for macular edema in the early phase after vitrectomy and epiretinal membrane removal. Clin Ophthalmol Auckl NZ. 2013;7:549. doi:10.2147/OPTH.S42188
96. Grover S, Apushkin MA, Fishman GA. Topical dorzolamide for the treatment of cystoid macular edema in patients with retinitis pigmentosa. Am J Ophthalmol. 2006;141(5):850–858. doi:10.1016/j.ajo.2005.12.030
97. Mazet R, Yaméogo JBG, Wouessidjewe D, Choisnard L, Gèze A. Recent advances in the design of topical ophthalmic delivery systems in the treatment of ocular surface inflammation and their biopharmaceutical evaluation. Pharmaceutics. 2020;12(6). doi:10.3390/pharmaceutics12060570
98. Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55(2):108–133. doi:10.1016/j.survophthal.2009.07.005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
