Back to Journals » Nature and Science of Sleep » Volume 16
Tongue Biting Event in Patients with Sleep-Related Facial Mandibular Myoclonus: A Case Series Study
Authors Hu G, Pan Y , Yuan N, Yang Z, Shi X, Ma S, Li S, Hou X, Liu F, Li D, Bao J, Liu Y
Received 31 August 2023
Accepted for publication 28 December 2023
Published 22 February 2024 Volume 2024:16 Pages 207—215
DOI https://doi.org/10.2147/NSS.S433628
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Gengyao Hu,1,* Yuanhang Pan,1,* Na Yuan,1,* Zhixian Yang,2 Xiuyu Shi,3 Sha Ma,4 Shan Li,4 Xiaohua Hou,5 Fei Liu,5 Dongmei Li,6 Junxiang Bao,7 Yonghong Liu1
1Department of Neurology, Xijing Hospital, Fourth Military Medical University (Air Force Medical University), Xi’an, People’s Republic of China; 2Department of Pediatrics, Peking University First Hospital, Beijing, People’s Republic of China; 3Department of Pediatrics, Chinese People’s Liberation Army General Hospital, Beijing, People’s Republic of China; 4Department of Neurology, The First People’s Hospital of Yunnan Province, The Affiliated Hospital of Kunming University of Science and Technology, Kunming, Yunnan, People’s Republic of China; 5Department of Neurology, The First Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 6Department of Dentistry for Special Services, Fourth Military Medical University (Air Force Medical University), Xi’an, People’s Republic of China; 7Department of Aerospace Hygiene, Fourth Military Medical University (Air Force Medical University), Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yonghong Liu, Email [email protected]
Background: Sleep-related facial mandibular myoclonus (SRFMM) remains rare in clinical practice. The aim of this study was to provide a comprehensive understanding of the electroclinical manner, therapeutic regimen, and prognosis of SRFMM.
Methods: Twenty-three patients who were diagnosed with SRFMM by clinical manifestation, video-electroencephalography (EEG) and electromyography over bilateral masseter and temporalis muscles were enrolled. Clinical and electrophysiological evaluation as well as follow-up information were recorded and analyzed.
Results: The cohort involved 4 infants and 19 adults with a mean onset age of 43.5 years for SRFMM, among whom 19 were male. Twenty-one patients complained of tongue injuries and disturbed night-time sleep. SRFMM in 4 patients were ascribed to oral aripiprazole, brainstem ischemia and brain trauma. In 62 SRFMM episodes, 93.5% occurred in NREM sleep and 6.5% in REM sleep, and all events were associated with EEG arousals. In 13 patients with or without clonazepam, the motor events gradually disappeared, and the rest turned to be sporadic.
Conclusion: SRFMM is a characteristic parasomnia manifested by tongue biting and accompanying facial mandibular myoclonus, leading to disrupted sleep. Besides adults, infants can also experience SRFMM with spontaneous remission. Most patients respond well to clonazepam, eventually with favorable prognosis.
Keywords: sleep-related facial mandibular myoclonus, tongue biting, movement disorder, SRFMM
Sleep-related facial mandibular myoclonus (SRFMM) is manifested by sudden, brief and involuntary myoclonic jerks of masseter and temporalis muscles, mostly occurring in sleep, leading to frequent tongue biting events and accompanying bleeding, pain, scar and sleep disruption.1,2 Diagnosis and treatment for SRFMM are confused. It is often misdiagnosed as nocturnal epileptic seizure or sleep bruxism.2,3 The former is usually characterized by tongue biting along with tonic-clonic movements while very few epilepsy patients evince nocturnal tongue biting only without convulsion of limbs. Bruxism presented with rhythmic contractions of the masticatory muscles that, rarely resulted in tongue biting. Various treatments have been tried in SRFMM, such as antiseizure medications, benzodiazepine, interdental plate, which however showed different outcomes. In a previous report, partial tongue resection was performed in an adult patient with severe tongue biting injuries who responded poorly to medication, but still eventually failed to eliminate the attacks.4 Even with the involuntary self-injurious nature and sleep disruption, SRFMM has not been listed in the current version of International Classification of Sleep Disorders (ICSD-3).5
Since first reported in 1991, SRFMM has rarely been reported and the knowledge about the etiopathogenesis and clinical features are still largely unknown. Moreover, prior studies on SRFMM mainly focused on adults. In this study, we collected both adult and infant cases, to elucidate the electroclinical manner, therapeutic regimen, and prognosis of SRFMM in more detail, which would be helpful for the diagnosis and treatment in clinic.
Methods
The prospective research was conducted in epilepsy and sleep centers of Xijing Hospital, and proved by the Medical Ethical Committee of Xijing Hospital (KY20222053-C-1) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was acquired from all patients or their guardians.
Population
We enrolled 23 patients with SRFMM who were referred to Epilepsy and Sleep Disorders Unit between February 2016 and March 2022. Inclusion criterion was set according to previous literature that the patients report painful tongue biting during sleep, without convulsion of limbs and facial twitching. Epileptic seizures and nonepileptic events were excluded. Baseline data were collected by face-to-face interviews with patients or their families. Specifically, demographic data, age at disease onset, frequency of SRFMM, triggering factors, family history and medication were obtained. Neurological examination, brain MRI or CT scans were performed in all 23 patients. The diagnosis of sleep disorders was achieved according to the ICSD-3 criteria. SRFMM was diagnosed by two experienced sleep specialists. The differential diagnosis from any possible epileptic seizures was made by two epilepsy experts according to the patients’ symptom and video-EEG data. Follow-up was scheduled monthly by telephone, every six months by interviews in outpatient clinic or at the request of patients or their families. All the information including the onset SRFMM and side effects (if received clonazepam treatment) were recorded in a chart. At each visit, the patients or their relatives were asked to report the response to drug treatment. The identification of adverse events was mainly based on previous reports concerning clonazepam.
Electrophysiological Evaluation
All patients received a long-term continuous monitoring for video-electroencephalography (EEG). The 24-hour-EEG was performed by a 32-channel digital video-EEG system with scalp electrodes placed according to the international standard lead 10–20 system. The reference was placed at Cz′, a location 1 cm behind Cz. The sampling rate was 500 Hz with a band pass filter (high pass cutoff frequency at 1.6 Hz and low pass cutoff frequency at 70 Hz). Electromyography electrodes were placed over left and right deltoid muscles. The most pronounced activity of masticatory muscle was evaluated with additional surface EMG over bilateral anterior temporalis and masseters by the high-pass filter of 53 Hz, low-pass filter of 120 Hz, sampling rate of 500 Hz and impedance lower than 5 kΩ.
Results
Cohort Characteristics
We enrolled 23 patients (15 male adults, 4 female adults, 4 male infants, mean age 44.6 with the range of 0.75–89 years). The mean age of first onset of tongue biting for all the participants were 43.5 (range 0.5–84) years, for adults 52.5 years and for infants 0.9 years. Patients underwent an average 3.5 years (6–5 years) of follow-up.
Twenty-two patients reported SRFMM only in sleep, but one male adult found that in both sleep and wakefulness. All patients or their parents reported only tongue was affected except for two adult patients whose lips and tongue were both involved. Sleep disturbance associated with involuntary tongue or lips biting appeared in everyone, which even caused the fear of falling asleep. Four adult patients were initially misdiagnosed as epileptic seizure before being confirmed to have SRFMM.
The tongue biting event occurred to the four male infants at the age of 0.5–2 years along with sudden scream 1–20 times per night, crying, breast-feed difficulty and severe ulcers on the tongue (Figure 1). They showed a normal brain MRI and negative family history. Among them, a 10-month-old infant experienced tongue biting 5–20 times every night for four months after the head and facial trauma, and his brain MRI was normal. Clonazepam 0.5mg per night removed the symptom completely without any side effect being observed. The parent stopped medication 1 month later. During the follow-up, no tongue biting reoccurred. The other 3 male infants experienced tongue biting 1–5 times every night without clear cause. One of them received clonazepam 0.5mg per night for 2 months. The tongue biting disappeared and no side effect arose. This outcome lasted through the six months of follow-up. The other two baby’s parents refused clonazepam for fear of the side effects, so the tongue biting occurred every night for 3 months to 1 year and then disappeared spontaneously. Without any medication, all the four infants were free of SRFMM during the 0.5–6 years follow-up period, evincing a normal growth.
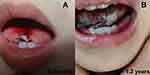 |
Figure 1 SRFMM tongue ulcer in patient 2. (A) shows a small ulcer at 8 months of age. (B) shows multiple ulcers at 1.2 year of age. |
Patient 7, diagnosed with bipolar disorder, suffered from sudden tongue biting at night, but began taking single oral dose of aripiprazole (2.5mg per night) 10 years after the diagnosis. With the dose of aripiprazole increased to 10mg per night, the tongue biting occurred more frequently 4–5 times per night. Conversely, the withdrawal of the medicine terminated the attacks. The patients reported no extrapyramidal symptoms. Patient 15 developed vertigo, hemiplegia and tongue biting almost simultaneously, who was diagnosed as acute brainstem infarction based on the MRI T2 image. The tongue biting event arose during sleep, lasting for 1 month and then disappeared spontaneously and gradually. Patient 16 showed critical anxiety before tongue biting, both of which disappeared without any medication. Different from most of the subjects, patient 17 experienced tongue biting during both sleep and wakefulness 2–3 times per day, with occasional unilateral hand jerk in wakefulness. Initially, the motor events mainly involved tongue frenulum stimulated by activities. After taking clonazepam (1 mg/night) for 1 month, the tongue movement improved substantially. However, the drug withdrawal stirred the symptoms again which mainly affected tip of the tongue. Patient 20 experienced tongue biting 20 days after the hit on the forehead, although the brain CT showed no abnormal signals. The intense tongue biting emerged in every daytime nap and night sleep, disturbing the rest of the patient substantially. Clonazepam (0.5mg/night) relieved the condition, which recurred along with the drug withdrawal at a relative low frequency.
Six patients with severe symptoms responded to clonazepam, half of whom were free of the tongue movement and the rest still have sporadic symptom during follow-up period. In 14 patients without clonazepam treatment, 9 were attack-free and the other 5 suffered from occasional attacks. Clinical characteristics of the patients are listed in Table 1.
 |
Table 1 Clinical and Electrophysiological Characteristics of Patients with SRFMM |
Assessment of SRFMM
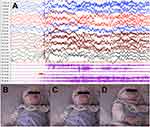
A total of 62 episodes of SRFMM were recorded during the whole video-EEG, with 58 (93.5%) presented in non-rapid eye movement (NREM) sleep (35 in NREM 1, 21 in NREM 2 sleep, 2 in NREM 3 sleep) and 4 (6.5%) in tonic rapid eye movement (REM) sleep. Synchronized video-EEG disclosed a mouth movement, or sudden mouth closing which occasionally were triggered by sound or touch and always followed by limbs lifting and crying in infants (Figure 2). The mean EMG duration of masseter/ temporalis muscles was 100±55.2ms. None had epileptiform discharge during ictal and inter-ictal period. The first tongue biting event appeared abruptly 5–46 mins after falling asleep, and recurred 0–22 times during one night monitoring, which evenly distributed in whole sleep. All of the motor events were associated with EEG arousals, with 8 (12.9%) transitioned to NREM 1 stage and 24 (38.7%) to wakefulness. The mean time length of alpha rhythm background following SRFMM was 47.1 min. Obstructive sleep apnea was recorded in ten patients and periodic limb movement syndrome in five patients. In patient 20, the second video-EEG monitoring after clonazepam treatment confirmed the apparent improvement of SRFMM episodes and sleep efficiency (Table 2).
 |
Table 2 Comparisons of Electrophysiological Features of the Patient 20 Before and After Clonazepam Treatment |
Discussion
In the present study, we investigated electroclinical features of 23 SRFMM patients and found a strong association between SRFMM and EEG arousals, suggesting the disturbance of sleep by SRFMM. Moreover, the self-injurious nature of involuntary movement especially with high frequency caused great harm to patients, which when in infants hurt their parents as well. To the best of our knowledge, this work includes the most cases among researches on SRFMM.
Interestingly, in our study, there were 4 male infants whose age at SRFMM onset were 0.5–2 year, which has never been reported in other case series. The tongue movement in one infant are secondary to head and facial trauma, strongly indicated the cause. A small dose of 0.5mg clonazepam was effective to this manifestation with no obvious adverse side effect. For all the infant patients, though with severe tongue biting, the symptom disappeared completely before the age of 3 years, some of whom even were not on any medication, suggesting this is a transient sleep disorder with benign prognosis.
Although both previous studies6–8 and ours demonstrated an association of SRFMM and NREM sleep, a 79-year-old man exhibited forceful myoclonic jerks mainly present in REM sleep.4 The distribution of movements in phasic and tonic REM sleeps was different from physiological phasic myoclonic activities in REM sleep, which substitutes a unique SRFMM preferring REM sleep. Additionally, in the present study, one patient reported involuntary tongue biting in both sleep and wakefulness which was similar to the report of Wehrle et al.4
SRFMM should be differentiated from sleep bruxism. Myoclonus in SRFMM was mostly isolated, but also can be clustered containing 2–6 consecutive bursts, just like that in two cases with familial nocturnal facio-mandibular myoclonus and four cases with sleep bruxism.7,8 The EMG duration of masseter and temporalis muscles in SRFMM was about 50–250 ms,6–8 distinct from sleep bruxism manifested by repetitive jaw-muscle activity with phasic (EMG duration from 0.25–2.0 sec), tonic (sustained EMG bursts lasting more than 2.0 sec) or mixed type.9 Meanwhile, the two parasomnias showed different consequences, with the former inducing tongue injury, while the latter tooth wear. In the literature, some patients were initially diagnosed as sleep bruxism for tooth sounds, and then as SRFMM after reviewing the video-PSG and EMG features. Actually, sleep bruxism co-existed with SRFMM in approximate 10% of patients with sleep bruxism. So far, the differences and relations between the two motor events as well as the underlying mechanism remains unclear.8
Involuntary tongue biting and bleeding related to both SRFMM and epileptic seizure. The former lasts for few seconds, usually followed by painful facial expression and awakening. But, diagnosis of epileptic seizure relies mainly on accurate history and manifestation of convulsions, tong biting and urinary incontinence for few minutes. In addition, epileptic discharges in synchronous EEG provided solid proof for seizure. In clinical practice, most patients with nocturnal tongue biting are diagnosed as epilepsy at first even without epileptiform discharge recorded in EEG monitoring. For these patients, anti-seizure medications always failed to reverse the situation, as treatment options, duration and prognosis differ significantly between SRFMM and epilepsy. So for the patients with confused clinical features such as epilepsy, SRFMM, bruxism or other mouth and face movements, video-PSG-EEG investigations with mandibular and facial EMG are recommended.
Myoclonus displays as a hyperkinetic movement characterized by sudden, brief, involuntary jerks of a single muscle or a group of muscles with four subtypes according to the different anatomy origins, namely cortical, subcortical, spinal and peripheral forms.10 In line with previous reports, there was no evidence of cortical epileptic discharge in all recorded SRFMM in the present study, precluding the involvement of cortical activation. Though the etiology of SRFMM remains poorly understood, it has been hypothesized that activation of V and VII cranial nerves may play a critical role.11 SRFMM mostly manifests itself as an idiopathic myoclonus for the lack of definite neurologic deficits or EEG abnormalities.8,12 However, in our work, there were one infant and three adults whose tongue biting was associated with acute brain trauma, drug therapy or brainstem ischemia, suggesting a symptomatic nature of SRFMM. The SRFMM in patient 7 came out immediately after taking aripiprazole and disappeared with the drug withdrawal. This tongue movement was not accompanied by extrapyramidal symptoms which was a relatively common adverse effect of aripiprazole. Because of lack of rechallenge of dosage increase, it should be a probable adverse reaction of drug according to World Health Organization-Uppsala Monitoring Center (WHO-UMC) causality categories.13 Moreover, patient 14 in the present study exhibited clear stress and anxiety, and the emotional control contributed to the remission of myoclonus, indicating the possible link of the two disorders.
The etiopathogenesis of SRFMM are still unclear and multiple pathways may be involved. As disruption of GABAA receptor in neural axis produces clear myoclonus, recovery of which mitigates the condition substantially.14 So the SRFMM may be ascribed to brainstem GABAergic dysfunction. In this regard, both previous studies and ours revealed clonazepam, a long GABA receptor agonist, effective in suppressing SRFMM and improving sleep quality. Previous study had ever reported in 9 of 10 SRFMM patients, the benzodiazepine (clonazepam or diazepam) alleviated myoclonus substantially;2 however, the lack of EEG recording discounted the reliability of the evidence. The present study makes up for the deficiency providing a more powerful clue for the efficacy of clonazepam in treating SRFMM. For some special cases such as obstructive sleep apnea, which was recorded in 10/23 patients in the present work having no clear association with tongue movement, the clonazepam therapy should be used in caution for the possible aggravation of hypoxia by the drug. When necessary, a ventilator should be available.
Based on our case series study, criteria for diagnosing SRFMM may be proposed, which includes: 1) a sudden, brief, involuntary tongue or lips biting mainly in sleep; 2) definite EMG burst of masseter or temporalis muscles along with the tongue biting; 3) absence of epileptiform discharges when the tongue biting emerges; 4) clonazepam being effective in most cases: 5) being idiopathic or secondary to acute brain damage or adverse drug reaction; and 6) excluding other sleep disorders.
There were some limitations about this study. Firstly, though we have tried to collect as many cases as we can, the sample size was still small which may prevent the full understanding on the disease. Secondly, the present study did not employ polysomnography which may prevent us understanding more sleep architecture and other co-occurring sleep disorders. Future studies with larger sample size are warranted to identify more sleep architectural features by video polysomnography assessment.
Conclusions
In conclusion, as a characteristic parasomnia, SRFMM is quite distinct from epileptic seizure and bruxism, mainly manifested by tongue biting and facial mandibular myoclonus. Besides adults, infants can also experience the tongue biting attack with spontaneous remission. Most patients respond well to clonazepam, eventually with favorable prognosis. The pathophysiological mechanism of SRFMM remains to be clarified.
Ethical Compliance Statement
Xijing Hospital approved the research, and patients or their guardians signed informed consent. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Acknowledgment
We thank all patients for participation in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Dr Yonghong Liu reports grants (No. 2020JSTS21) from Air Force Medical University. This study was also funded by National Key R&D Program of China (2022YFC2503806).
Disclosure
None of the authors has any conflict of interest to disclose for this work.
References
1. Hupalo M, Jaskolski DJ. Tongue biting resulting from sleep-related facio-mandibular myoclonus as a cause for misdiagnosed epilepsy. Acta Neurol Belg. 2017;117(3):787–788. doi:10.1007/s13760-017-0753-3
2. Zhang S, Aung T, Lv Z, et al. Epilepsy imitator: tongue biting caused by sleep-related facio-mandibular myoclonus. Seizure. 2020;81:186–191. doi:10.1016/j.seizure.2020.08.018
3. Dylgjeri S, Pincherle A, Ciano C, Binelli S, Villani F. Sleep-related tongue biting may not be a sign of epilepsy: a case of sleep-related faciomandibular myoclonus. Epilepsia. 2009;50(1):157–159. doi:10.1111/j.1528-1167.2008.01760.x
4. Wehrle R, Bartels A, Wetter TC. Facio-mandibular myoclonus specific during REM sleep. Sleep Med. 2009;10(1):149–151. doi:10.1016/j.sleep.2008.01.006
5. Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi:10.1378/chest.14-0970
6. Wang X, Yuan N, Wang B, Liu Y. Characteristics of nocturnal facio-mandibular myoclonus in four middle-aged patients. Sleep Med. 2020;68:24–26. doi:10.1016/j.sleep.2019.09.002
7. Vetrugno R, Provini F, Plazzi G, et al. Familial nocturnal facio-mandibular myoclonus mimicking sleep bruxism. Neurology. 2002;58(4):644–647. doi:10.1212/WNL.58.4.644
8. Kato T, Montplaisir JY, Blanchet PJ, Lund JP, Lavigne GJ. Idiopathic myoclonus in the oromandibular region during sleep: a possible source of confusion in sleep bruxism diagnosis. Mov Disord. 1999;14(5):865–871. doi:10.1002/1531-8257(199909)14:5<865::AID-MDS1025>3.0.CO;2-0
9. Hirsch LJ, Crispin D. EEG checkerboard pattern of bruxism. Neurology. 1999;53:669.
10. Zutt R, van Egmond ME, Elting JW, et al. A novel diagnostic approach to patients with myoclonus. Nat Rev Neurol. 2015;11(12):687–697. doi:10.1038/nrneurol.2015.198
11. Loi D, Provini F, Vetrugno R, D’Angelo R, Zaniboni A, Montagna P. Sleep-related faciomandibular myoclonus: a sleep-related movement disorder different from bruxism. Mov Disord. 2007;22(12):1819–1822. doi:10.1002/mds.21661
12. Aguglia U, Gambardella A, Quattrone A. Sleep-induced masticatory myoclonus: a rare parasomnia associated with insomnia. Sleep. 1991;14(1):80–82. doi:10.1093/sleep/14.1.80
13. Shukla AK, Jhaj R, Misra S, Ahmed SN, Nanda M, Chaudhary D. Agreement between WHO-UMC causality scale and the Naranjo algorithm for causality assessment of adverse drug reactions. J Family Med Prim Care. 2021;10(9):3303–3308. doi:10.4103/jfmpc.jfmpc_831_21
14. Matsumoto RR, Truong DD, Nguyen KD, et al. Involvement of GABA(A) receptors in myoclonus. Mov Disord. 2000;15(Suppl 1):47–52. doi:10.1002/mds.870150709
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.