Back to Journals » Biologics: Targets and Therapy » Volume 17
Tocilizumab Vs Methotrexate in a Cohort of Patients Affected by Active GCA: A Comparative Clinical and Ultrasonographic Study
Authors Grazzini S, Conticini E , Falsetti P , D'Alessandro M, Sota J, Terribili R, Baldi C , Fabiani C, Bargagli E, Cantarini L, Frediani B
Received 21 July 2023
Accepted for publication 31 October 2023
Published 1 December 2023 Volume 2023:17 Pages 151—160
DOI https://doi.org/10.2147/BTT.S431818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Silvia Grazzini,1,* Edoardo Conticini,1,* Paolo Falsetti,1 Miriana D’Alessandro,2 Jurgen Sota,1 Riccardo Terribili,1 Caterina Baldi,1 Claudia Fabiani,3 Elena Bargagli,2 Luca Cantarini,1 Bruno Frediani1
1Rheumatology Unit, Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy; 2Respiratory Diseases and Lung Transplant Unit, Department of Medicine, Surgery and Neurosciences, University of Siena, Siena, Italy; 3Ophthalmology Unit, Department of Medicine, Surgery and Neuroscience, University of Siena, Siena, Italy
*These authors contributed equally to this work
Correspondence: Edoardo Conticini, Rheumatology Unit, Department of Medicine, Surgery and Neurosciences, AOUS Le Scotte, viale Mario Bracci 14, Siena, 53100, Italy, Email [email protected]
Introduction: No head-to-head study has assessed the superiority of tocilizumab versus methotrexate in giant cell arteritis (GCA), and few studies have demonstrated its effectiveness in terms of ultrasonographic findings, but without a control group. The primary endpoint was to assess whether tocilizumab was superior to methotrexate in inducing normalization of US findings, whereas the secondary endpoint was to assess the effectiveness of precocious withdrawal of glucocorticoids.
Methods: We prospectively enrolled all the patients with active GCA at our clinic. The inclusion criteria were clinical diagnosis of GCA; active disease; and clinical, laboratory, and US data, evaluated using the halo count (HC) and OMERACT GCA Ultrasonography Score (OGUS). Evaluations were repeated at 3, 6, and 12 months.
Results: Twenty patients were treated with Tocilizumab and 9 with Methotrexate. All but three tocilizumab-treated patients achieved remission at six months, whereas at 12 months, all patients were in glucocorticoid-free remission. Up to three of the nine methotrexate patients experienced a lack of efficacy or minor relapses. Tocilizumab-treated patients showed a statistically significant difference between baseline and all follow-ups in terms of OGUS and HC, whereas the difference in the Methotrexate group was significant after 1 year. The mean glucocorticoid dosage significantly decreased in both groups. No severe adverse events or major relapses were reported.
Conclusion: Our study demonstrates the superiority in terms of rapidity of a tocilizumab-based scheme over a methotrexate-based scheme in inducing clinical and US remission. Precocious withdrawal of glucocorticoids did not increase the risk of relapse.
Keywords: GCA, giant cell arteritis, methotrexate, tocilizumab, ultrasonography, vasculitis
Introduction
The approval of Tocilizumab (TCZ) has dramatically changed the therapeutic approach and prognosis of giant cell arteritis (GCA). Glucocorticoids (GCs), burdened by poor long-term efficacy, a high rate of relapse, and several side effects, appear to be surpassed by a molecule that has proven its superiority in a Phase III trial1 and in some real-life studies.2
At the same time, disease-modifying anti-rheumatic drugs (DMARDs), such as methotrexate (MTX), have never met the endpoint in several studies aimed at assessing their efficacy in GCA, and cannot be considered an effective tool in the management of this rare condition. Nevertheless, despite such evidence, GCs and MTX remain the most common first- and/or second-line treatments for GCA.1,3 Almost 5 years after the publication of the GIACTA trial results, TCZ is generally restricted to recalcitrant and/or relapsing cases,4 and even the most recently published papers aimed at assessing the role of ultrasonography (US) during follow-up5 do not include patients treated with anti-IL6 agents.
This is presumably due to the limitations of the use of biological agents in some countries, as well as to the substantial paucity of real-life studies confirming the safety and efficacy of TCZ, particularly its superiority to DMARDs.
Indeed, it is regrettable that, to date, no head-to-head study has been carried out to assess the superiority or even non-inferiority of TCZ vs MTX or other synthetic immunosuppressants. At the same time, the few studies displaying the effectiveness of TCZ in terms of imaging findings4,6 did not include a control group; thus, we were not able to confirm whether the normalization of PET ad/or US is a peculiarity of anti-IL6 agents and whether such an aspect occurs earlier or even later in patients precociously treated with biologic drugs.
Therefore, we aimed to explore the landscape of therapeutic agents commonly used for GCA through serial US evaluation, graded with two semiquantitative scores.
Materials and Methods
Patients
In this single-center, prospective study, we included all consecutive patients affected by GCA and evaluated them for the first time at the Vasculitis Center of our Rheumatology Unit, Siena University Hospital, from January 2021 to June 2022.
The inclusion criteria were a clinical diagnosis of GCA and the All patients fulfillment of 1990 American College of Rheumatology classification criteria7 and/or GIACTA trial inclusion criteria;8 active disease; availability of a defined set of routine clinical, laboratory,9 and imaging data; and pathological US findings.
The exclusion criteria were diagnosis of large-vessel vasculitis other than GCA, such as Takayasu arteritis or isolated aortitis, and negative US findings.
Patients were diagnosed with GCA by a single rheumatologist who was diagnosed with vasculitis and were classified as being affected by cranial GCA, LV GCA, or cranial plus LV GCA. Disease remission and relapse were defined according to the European League Against Rheumatism (EULAR definitions.10
Clinical, serological, and US findings as well as concomitant treatments at 3 (T1), 6 (T2), and 12 (T3) months were also included.
All patients signed a “Patient Consent Form” in full knowledge of how their data would be used.
US
US examination was carried out by three rheumatologists experienced in the field of ultrasonography.
An Esaote MyLab X8 Xp Twice machine (Genoa, Italy) equipped with 4–15 MHz and 8–24 MHz probes and an Esaote MyLab Twice equipped with a 6–18 linear probe were employed. The following parameters were used for AxA and TA CDUS: pulse repetition frequency (PFR) 1–3 kHz, color frequency mode (CFM) 11.1–14.3 MHz, and gain just under the artifact level. IMT measurements were performed in millimeters on the wall distal to the probe on the longitudinal images during systole. AxA and TA CDUS findings were graded according to “halo count”11 and “OMERACT GCA ultrasonography scores (OGUS)”.12
Treatment
All patients diagnosed with GCA were treated, according to our “Vasculitis Center” treatment scheme established in January 2021, with GCs at a dose of 50 mg of oral prednisone (PDN), preceded, in case of cranial GCA, by three boluses of 1000 mg intravenous methylprednisolone (MPDN). In all cases, oral PDN was administered in association with an immunosuppressive agent: TCZ 162 mg once a week was the treatment of choice, except in patients in whom anti-IL6 agents were contraindicated (eg, neutropenia or thrombocytopenia), risky (eg, elderly patients with high infectious hazard), or considered unnecessary (eg, patients with bilateral blindness).
In this subset of patients, the immunosuppressive treatment MTX was administered subcutaneously at a dose of 0.3 mg/Kg once a week (max 20 mg) followed by folate 5 mg.
PDN was maintained at the highest dosage for one month and then tapered at a pace of 6.25 mg every 2 weeks. Once a dosage of 6.25 mg was achieved, tapering was slowed to 1.25 mg every 2 weeks for those treated with TCZ, with a complete withdrawal at 6 months from T0. Conversely, in the case of MTX, 1.25 mg was tapered every six weeks, thereby achieving GC-free treatment at 12 months (Table 1).
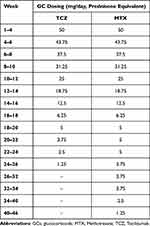 |
Table 1 Glucocorticoids Tapering Scheme for Patients with a Novel Diagnosis of GCA |
In the case of major relapse (8), PDN increased at 1 mg/kg, whereas in the case of minor relapse (8) at 0.5 mg/kg. Relapses under MTX led to a switch to TCZ. Oral bisphosphonate and vitamin D therapy were prescribed to all patients, as well as vaccinations against the Zoster virus, influenza virus, and pneumococcus.
Statistical Analysis
Parametric data are presented as mean ± standard deviation (SD) and non-parametric data as medians with interquartile ranges (IQR). Categorical variables are presented as either a percentage of the total, or numerically, as appropriate. Multiple comparisons were assessed by non-parametric one-way ANOVA (Kruskal–Wallis test) and the Dunn test while paired t-test was performed to compare baseline to different time points. Chi-squared or Fischer’s exact tests were assessed for categorical variables.
The level of statistical significance was set at a p-level < 0.05. Statistical analyses were performed using the Jamovi 2.3.1 software.
Results
Study Population
We enrolled a total of 29 patients (mean age 76.6 years ± 7.86 SD, 16 females, 13 males). The clinical, imaging, and serological findings are summarized in Table 2.
 |
Table 2 Demographic, Clinical and Laboratory Data of Patients |
All nine patients treated with MTX (median age 80.6 years, four female) started DMARD as first-line treatment, except for one patient who discontinued TCZ for AE. Conversely, in the TCZ-treated cohort (median age 74.85 years, 11 females), 16 subjects started IL-6R inhibitors at diagnosis, 2 after disease relapse while in treatment with GC and MTX, and another 2 requested a second evaluation after diagnosis.
Patients differed, although not significantly (p=0.05), in terms of age at diagnosis, whereas no difference was observed in laboratory values and US findings. The mean HC and OGUS values were 3 ± 1.9 and 0.99 ± 0.25, respectively. (Figure 1).
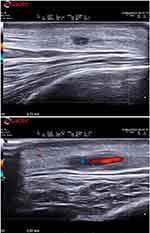 |
Figure 1 Transverse and longitudinal scans of frontal and common branch of right temporal artery, displaying halo sign, poor compressibility and intima media thickening. |
Three Months Follow-Up
At 3 months follow-up visit, three patients treated with TCZ IMT were still pathological, although improveddue to the onset of AE related to steroid treatment (decompensated diabetes mellitus, tachyarrhythmia). TCZ was discontinued in two patients because of the onset of severe thrombocytopenia.
One subject treated with MTX displayed a slight worsening of US findings and was therefore switched to TCZ.
In terms of US findings, both OGUS and HC displayed a strong, statistically significant difference from baseline in TCZ-treated patients (p=0.005 and p=0.0108, respectively), but not in MTX-treated patients (p=0.28). Conversely, a statistically significant improvement was observed in both groups for all the serum biomarkers.
Six Months Follow-Up
At 6 months follow-up visit, all patients treated with TCZ achieved full clinical and laboratory remission, and all but 3 – three same who underwent faster GCs tapering also displayed complete normalization of all US findings.
Among the MTX-treated patients, one experienced a minor relapse (Figure 2), therefore requiring an increase in the GCs dosage.
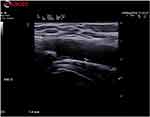 |
Figure 2 Axillary artery intima media thickening of a patient treated with MTX and suffering from minor relapse. |
Both the OGUS and HC groups displayed a strong, statistically significant difference from baseline to T2 (p=0.0011 and p=0.0003, respectively) in the TCZ group; however, no difference was observed between T1 and T2 (p=0.704 and p=0.908, respectively). No US differences were assessed, either between baseline and T2 (p=0.184) or between T1 and T2 (p=0.933), in MTX-treated subjects.
Twelve Months Follow-Up
At 12 months, all patients in the TCZ group achieved full clinical, US, and laboratory remission, except for 2 subjects who had experienced a minor relapse (headache) at 10 and 12 months, both of which were successfully treated with a temporary increase in steroid dose. Only four patients were still on GCs therapy (mean dosage 4.3 mg): two experienced relapse, one displayed a slower response to treatment, and the other displayed adrenal failure.
In one patient, TCZ was withdrawn after a diagnosis of rectal adenocarcinoma.
Among MTX-treated patients, one suffered from a minor relapse, as evidenced by an increase in inflammatory markers, occurrence of B-symptoms and headache, and worsening of US findings, in which the PDN dosage was re-increased and TCZ was started. The same approach was adopted for another patient who had developed an aortic aneurysm and could not bear any increase in PDN dosage because of severe osteoporosis. A third patient discontinued MTX because of nausea and diarrhea. In the MTX group, four of 7 were still tapering PDN, and the mean dose was 5 mg.
In both subgroups, a statistically significant difference was observed for both OGUS and HC between baseline and T3, but not between T1 and T3 or T2 and T3 ((Figure 3)).
Glucocorticoids
No statistically significant difference was observed between the two groups in terms of mean GCs dose (T0 p=0.1002, T1 p=0.5536, T2 p=0.2001, T3 p=0.3009), despite faster tapering in the TCZ group, leading to a statistically significant reduction in PDN dosage at 3 months (p=0.0007); conversely, in the MTX cohort, a statistically significant difference from baseline was assessed only at T2 (p=0.0069).
No GC-related severe AEs were reported, but one patient presented with adrenal failure and two others, who already suffered from diabetes mellitus, temporarily increased metformin dosage. In one patient, hyperglycemia was not adequately controlled and the PDN dosage was tapered. Finally, the pacemaker wearer experienced tachyarrhythmia.
Adverse Events
Two TCZ-treated patients developed mild AE (zoster reactivation and injection site reaction) but did not discontinue therapy, while two subjects stopped the treatment due to severe thrombocytopenia and one due to the onset of rectal adenocarcinoma. Finally, one patient discontinued MTX owing to gastrointestinal AE, while two presented with a mild increase in liver enzyme levels.
Discussion
Our study demonstrated the superiority of a TCZ-based scheme over an MTX-based scheme in inducing both laboratory and US remission in patients with GCA.
Despite the robust amount of evidence, supported by both clinical trials13 and real-life studies,2 displaying the efficacy and substantial safety of TCZ in GCA, as well as its superiority over sole GCs, no study has directly compared TCZ with any other synthetic or biological immunosuppressant.
Moreover, despite its overall availability, TCZ is seldom used as a first-line treatment in GCA. In a nationwide, retrospective study1 including 306 patients affected by GCA, only 29% were taking synthetic or biologic immunosuppressants, while up to 89% of them were taking GCs, despite a median disease duration superior to one year. More importantly, 40% of them had at least one disease flare and 37% presented comorbidities directly attributable to prolonged steroid treatment. A similar, although slightly higher, ratio was reported in another study,14 in which only of 114/165 subjects were treated with immunosuppressants within three months of diagnosis, in addition to GCs, within 3 months from diagnosis.
This highlights a regrettable gap between real-life and scientific literature, the latter demonstrating that the precocious administration of steroid-sparing agents reduces the incidence of treatment-related AE as well as the number of relapses.14
Moreover, in all the studies considered, the rate of patients treated with TCZ was low, ranging from 15%1 to 16%.14
In our cohort, no patient was treated with GCs alone, and in the majority of patients TCZ was administered immediately after diagnosis and after appropriate screening tests. The normalization of CRP and ESR and the disappearance of clinical signs or symptoms attributable to active vasculitis occurred in the majority of cases at 3 months from diagnosis, except for a few cases in which PDN was rapidly tapered due to uncontrolled side effects.
The relapse was 13.79%, which was significantly lower than that previously reported, particularly in the TCZ cohort (10%) compared to the MTX one (22.2%). Moreover, none of the patients suffered from major relapses or severe complications directly related to vasculitis or GC treatment. Relapsing patients taking MTX were switched to TCZ, thereby achieving full remission, while in those already receiving treatment with anti-IL6 agents the dosage of GCs was temporarily increased. Among the latter, both suffered from mild frontal headaches and displayed normal CRP values; thus, the diagnosis was made using US.
The use of US in the diagnosis of GCA is often overlooked, particularly in the monitoring of GCA. Nevertheless, a routine axillary and temporal artery US assessment should be considered during follow-up: GCA relapses, even cranial relapses, may be fully asymptomatic or present with non-specific features,15 while the usefulness of markers of inflammation can be impaired in patients treated with anti-IL6 agents.16
In this regard, in line with the growing number of papers displaying a precocious modification of US findings during treatment,5,6,15,17–19 we observed normalization of all pathologic vessels in our patients. However, the direct comparison between TCZ- and MTX-treated patients showed a statistically significant difference; in our cohort, we demonstrated a precocious normalization of all US findings in TCZ-treated patients, and a statistically significant difference was achieved as soon as 3 months after the start of the treatment, in terms of HC and OGUS.
To the best of our knowledge, this is the first study to clearly demonstrate, through quantitative scoring, the imaging-based superiority of TCZ over MTX in the precocious achievement of full remission in GCA. Despite a slower response, MTX also led to full normalization of US findings; nevertheless, such a result was achieved later than with TCZ and was burdened by a higher rate of relapse.
There is no clear evidence regarding the optimal starting and tapering doses of GCs. Most real-life retrospective cohorts report a high persistence of GCs even one year after diagnosis,1,20,21 while only a few studies have assessed the efficacy and safety of regimens with low cumulative dosages of PDN22 or TCZ as monotherapy.23
Given the fact that major relapses seem to occur when patients are taking a low dosage of GCs, we decided to treat our patients with a dose of 50 mg of PDN, which is in line with most published studies;16 tapering in TCZ-treated patients was made according to the GiACTA trial (modified to make it feasible for our patients, as PDN is generally available in Italy at dosages of 5, 20, and 25 mg); conversely, in MTX-treated patients, tapering was slower and full discontinuation was set at 12 months.
In our study, all patients achieved a low dosage of PDN at 6 months in both the TCZ and MTX groups, and most discontinued GCs within 12 months. In the majority of studies, patients were treated with steroids for up to 3 years from diagnosis.20,21
In terms of GC-related AE, only a few effects have been reported (eg, uncontrolled hyperglycemia and tachyarrhythmia), which are lower than previously reported.20,21 Fragility fractures did not occur at all, probably because of both prophylactic therapy with bisphosphonate and the reduced dosage of GCs.
Our study has some limitations: first, the monocentric design of the study, which limited the generalizability of our results. To this end, multicenter international studies with more heterogeneous cohorts are warranted to reach solid conclusions second, we included a reduced number of patients treated with MTX, representing less than half of the cohort, due to the fact that according to our protocol anti-IL6 agents represent the treatment of choice; third, the lack of patients treated with both TCZ and conventional immunosuppressants, which did not allow evaluation of the efficacy and safety of the combination treatment;16,24 fourth, the unavailability of imaging procedures other than US (eg, MRA and PET), which were performed only in a minority of patients;6,25–27 and finally, the limited observational period, which was not sufficient to assess the onset of long-term complications, such as aneurysms and dissections, nor AE related to prolonged immunosuppression: in this regard, due to the paucity of studies about long-term safety and efficacy of TCZ in GCA, we lack clear-cut data about the optimal duration of the treatment, which should balance the risk of relapse, the direct and indirect costs of such medications and the occurrence of late-onset complications.
In conclusion, TCZ appears to be an effective and potentially safe treatment option for GCA: TCZ appears to be faster than MTX, allowing full normalization of US findings after six months of treatment. The precocious administration of a steroid-sparing agent should be performed as soon as possible to minimize the risk of relapse and rapidly achieve discontinuation of GCs.
Data Sharing Statement
The data underlying this article will be shared upon reasonable request by the corresponding author.
Ethics Statement
The study was approved by the local Ethical Committee (Rhelabus, 22271) and was conducted in accordance with the Declaration of Helsinki and further amendments.
Patient Consent Form
Written informed consent for the publication of the patients’ clinical data was obtained, and a copy of the consent form was available for review by the editor.
Funding
AbbVie provided funding for medical writing (protocol number 0068880).
Disclosure
All authors have no conflicts of financial interest, direct or indirect, that may be perceived as affecting the conduct or reporting of the work submitted.
References
1. Mandl P, Limaye V, Adelaide Hospital R, et al. Presentation and Real-World Management of Giant Cell Arteritis (Artemis Study). Frontiers in Medicine. 2021;8.
2. Loricera J, Blanco R, Hernández JL, et al. Tocilizumab in giant cell arteritis: multicenter open-label study of 22 patients. Semin Arthritis Rheum. 2015;44(6):717–723. doi:10.1016/j.semarthrit.2014.12.005
3. Régent A, Mouthon L. Treatment of Giant Cell Arteritis (GCA). J Clin Med. 2022;12(1):11. doi:10.3390/jcm12010011
4. Sebastian A, Kayani A, Prieto-Pena D, et al. Clinical case: efficacy and safety of tocilizumab in giant cell arteritis: a single centre NHS experience using imaging (ultrasound and PET-CT) as a diagnostic and monitoring tool. RMD Open. 2020;6(3):e001417. doi:10.1136/rmdopen-2020-001417
5. Nielsen BD, Therkildsen P, Keller KK, Gormsen LC, Hansen IT, Hauge E-M. Ultrasonography in the assessment of disease activity in cranial and large-vessel giant cell arteritis: a prospective follow-up study. Rheumatology. 2023;62(9):3084–3094. doi:10.1093/rheumatology/kead028
6. Conticini E, Sota J, Falsetti P, et al. The Role of Multimodality Imaging in Monitoring Disease Activity and Therapeutic Response to Tocilizumab in Giant Cell Arteritis. Mediators Inflamm. 2020;2020:1–9. doi:10.1155/2020/3203241
7. Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33(8):1122–1128. doi:10.1002/art.1780330810
8. Unizony SH, Dasgupta B, Fisheleva E, et al. Clinical Study Design of the Tocilizumab in Giant Cell Arteritis Trial. Int J Rheumatol. 2013;2013:1–10. doi:10.1155/2013/912562
9. Ehlers L, Askling J, Bijlsma HWJ, et al. 2018 EULAR recommendations for a core data set to support observational research and clinical care in giant cell arteritis. Ann Rheum Dis. 2019;78(9):1160–1166. doi:10.1136/annrheumdis-2018-214755
10. Hellmich B, Agueda A, Monti S, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2020;79(1):19–30. doi:10.1136/annrheumdis-2019-215672
11. Van Der Geest KSM, Borg F, Kayani A, et al. Novel ultrasonographic Halo Score for giant cell arteritis: assessment of diagnostic accuracy and association with ocular ischaemia. Ann Rheum Dis. 2020;79.
12. Dejaco C, Ponte C, Monti S, et al. The provisional OMERACT ultrasonography score for giant cell arteritis. Ann Rheum Dis. 2022.
13. Stone JH, Tuckwell K, Dimonaco S, et al. Trial of Tocilizumab in Giant-Cell Arteritis. N Engl J Med. 2017;377(4):317–328. doi:10.1056/NEJMoa1613849
14. Quartuccio L, Isola M, Bruno D, et al. Treatment strategy introducing immunosuppressive drugs with glucocorticoids ab initio or very early in giant cell arteritis: a multicenter retrospective controlled study. J Transl Autoimmun. 2020;3:100072. doi:10.1016/j.jtauto.2020.100072
15. Conticini E, Falsetti P, Baldi C, Fabiani C, Cantarini L, Frediani B. Routine color Doppler ultrasonography for the early diagnosis of cranial giant cell arteritis relapses. Intern Emerg Med. 2022;17(8):2431–2435. doi:10.1007/s11739-022-03110-w
16. Castañeda S, Prieto-Peña D, Vicente-Rabaneda EF, et al. Advances in the Treatment of Giant Cell Arteritis. J Clin Med. 2022;11(6):1588. doi:10.3390/jcm11061588
17. Sebastian A, Coath F, Innes S, Jackson J, Van Der Geest KSM, Dasgupta B. Role of the halo sign in the assessment of giant cell arteritis: a systematic review and meta-analysis. Rheumatol Adv Pract. 2021;5(3). doi:10.1093/rap/rkab059
18. Conticini E, Falsetti P, Baldi C, Bardelli M, Cantarini L, Frediani B. Superb microvascular imaging in giant cell arteritis. Clin Exp Rheumatol. 2022;40(4):860–861. doi:10.55563/clinexprheumatol/ygcvaz
19. Ponte C, Monti S, Scirè CA, et al. Ultrasound halo sign as a potential monitoring tool for patients with giant cell arteritis: a prospective analysis. Ann Rheum Dis. 2021;80(11):1475–1482. doi:10.1136/annrheumdis-2021-220306
20. Castan P, Dumont A, Deshayes S, et al. Impact of Glucocorticoid Cumulative Doses in a Real-Life Cohort of Patients Affected by Giant Cell Arteritis. J Clin Med. 2022;11(4):1034. doi:10.3390/jcm11041034
21. Albrecht K, Huscher D, Buttgereit F, et al. Long-term glucocorticoid treatment in patients with polymyalgia rheumatica, giant cell arteritis, or both diseases: results from a national rheumatology database. Rheumatol Int. 2018;38(4):569–577. doi:10.1007/s00296-017-3874-3
22. Nannini C, Niccoli L, Sestini S, Laghai I, Coppola A, Cantini F. Remission maintenance after tocilizumab dose-tapering and interruption in patients with giant cell arteritis: an open-label, 18-month, prospective, pilot study. Ann Rheum Dis. 2019;78(10):1444–1446. doi:10.1136/annrheumdis-2019-215585
23. Saito S, Okuyama A, Okada Y, et al. Tocilizumab monotherapy for large vessel vasculitis: results of 104-week treatment of a prospective, single-centre, open study. Rheumatology. 2020;59(7):1617–1621. doi:10.1093/rheumatology/kez511
24. Calderón-Goercke M, Loricera J, Aldasoro V, et al. Tocilizumab in giant cell arteritis. Observational, open-label multicenter study of 134 patients in clinical practice. Semin Arthritis Rheum. 2019;49(1):126–135. doi:10.1016/j.semarthrit.2019.01.003
25. Reichenbach S, Adler S, Bonel H, et al. Magnetic resonance angiography in giant cell arteritis: results of a randomized controlled trial of tocilizumab in giant cell arteritis. Rheumatology. 2018;57(6):982–986. doi:10.1093/rheumatology/key015
26. Quinn KA, Grayson PC. The Role of Vascular Imaging to Advance Clinical Care and Research in Large-Vessel Vasculitis. Curr Treatm Opt Rheumatol. 2019;5(1):20–35. doi:10.1007/s40674-019-00114-0
27. Calderón-Goercke M, Castañeda S, Aldasoro V, et al. Tocilizumab in refractory giant cell arteritis. Monotherapy versus combined therapy with conventional immunosuppressive drugs. Observational multicenter study of 134 patients. Semin Arthritis Rheum. 2021;51(2):387–394. doi:10.1016/j.semarthrit.2021.01.006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

