Back to Journals » OncoTargets and Therapy » Volume 12
TNIP1 Inhibits Proliferation And Promotes Apoptosis In Clear Cell Renal Carcinoma Through Targeting C/Ebpβ
Authors Yang Y, Fan J, Han S, Li E
Received 17 May 2019
Accepted for publication 31 October 2019
Published 18 November 2019 Volume 2019:12 Pages 9861—9871
DOI https://doi.org/10.2147/OTT.S216138
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Yong Yang,1 Jinhai Fan,2 Shenglu Han,1 Enyuan Li1
1Department of Urology, The Ninth Hospital of Xi’an, Xi’an, Shaanxi, People’s Republic of China; 2Department of Urology, The First Affiliated Hospital of Xi’an JiaoTong University, Xi’an, Shaanxi, People’s Republic of China
Correspondence: Yong Yang
Department of Urology, The Ninth Hospital of Xi’an, 151 East Section of South Second Ring Road, Xi ‘an, Shaanxi 710054, People’s Republic of China
Tel +86 135 7197 6611
Email [email protected]
Background/Purpose: TNF-α-induced protein 3-interacting protein 1 (TNIP1) is active in various cancers, but its expression and function in renal cell carcinoma (RCC) have not been described. This study investigated the role of TNIP1 in clear cell renal cell carcinomas (ccRCC), which accounts for 75–80% of RCC and has a poor prognosis.
Methods: The expression of TNIP1 in human ccRCC tissues and cells was detected by real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR), Western blot (WB), and immunohistochemical (IHC) staining. Cell proliferation was assayed by a cell counting kit (CCK)-8 assay; cell cycle analysis and apoptosis assay were done by flow cytometry.
Results: TNIP1 is downregulated in both ccRCC human tissues and cells. Besides, TNIP1 downregulation promoted cell proliferation with more cell cycle entry, and inhibited apoptosis. TNIP1 downregulation was associated with increased of expression of the Bcl-2 anti-apoptosis gene and decreased expression of the Bax apoptosis-promoting gene and cleaved-caspase-3 by negatively regulating C/EBPβ expression.
Conclusion: TNIP1 acted as a tumor-inhibitor in ccRCC by targeting C/EBPβ. The results warrant study of TNIP1 as a potential diagnostic marker and therapeutic target of ccRCC.
Keywords: ccRCC, TNIP1, C/EBPβ, proliferation, apoptosis
Introduction
Renal cell carcinoma (RCC) is the most common urinary system cancer. It accounts for 90–95% of kidney cancers and 2–3% of all cancers. The morbidity and mortality of RCC are high, producing a heavy socio-economic burden and public health challenges worldwide.1,2 RCC includes four subtypes, clear cell, granular cell, mixed, and undifferentiated and clear cell renal cell carcinomas (ccRCC) accounts for 75–80% of the diagnoses.2,3 Early ccRCC is usually asymptomatic or produces only mild or vague systemic symptoms such as fever or fatigue until the tumor volume increases; papillary RCC type 1 and chromophobe RCC may be more indolent than ccRCC. Medical advances have led to diagnosis of RCC at earlier stages, but ccRCC can be difficult it identify histologically because it is often mixed with granular-cell and fusiform-cell carcinoma.4 Radical nephrectomy is generally the preferred treatment and can improve survival but metastasis occurs after surgery in about 30% of the patients.5,6 Effective adjuvant treatments for RCC would improve outcomes, but the availability is limited.7 Identification of novel biological targets for diagnosis and treatment of RCC is needed. As the most prevalent RCC subtype, ccRCC serves as a representative experimental model.
Tumor necrosis factor α-induced protein 3-interacting protein 1 (TNIP1) is a protein-coding gene also known as VAN, NAF1, ABIN-1, and nip40-1, which is located on chromosome 5q33 and encodes the A20-binding inhibitor that inhibits the activation of nuclear factor kappa-B (NF-κB) protein and is involved in autoimmunity, chronic inflammation, and cancer by controlling (NF-κB) activation and suppressing tumor.8–11 TNIP1 is present in both the cell cytoplasm and nucleus. Its expression is increased in esophageal cancer and decreased in prostate cancer. It influences cell apoptosis, proliferation, and differentiation.8 TNIP1 expression is downregulated in human keratinocytes and is associated with increased cell proliferation in severe psoriasis-like conditions,11 and the pathogenesis of myasthenia gravis and thymoma.12 However, its expression and function in ccRCC have not been described.
CCAAT/enhancer-binding protein β (C/EBP β), also known as transcriptional activator protein (LAP), is overexpressed in diverse tumor cell lines and in tumor-associated myeloid-derived suppressor cells (MDSCs). MDSCs comprise a subset of innate immune cells that are active in adaptive immunity and generate immunodepression.13 Overexpression of C/EBPβ promotes cell invasiveness and migration, NF-κB translocation, and epithelial-mesenchymal transition, reduced E-cadherin, and increased vimentin expression in 786-O human renal carcinoma cells. Under expression has the opposite effects.14 Additionally, C/EBPβ and TNIP1 expression are negatively correlated, consistent with C/EBPβ as a target of and directly regulated by TNIP1.11 This study investigated TNIP1 as a tumor-suppressor in ccRCC via targeting of C/EBPβ.
Materials And Methods
Patients And Tumor Tissues
Human primary ccRCC tumor tissues and adjacent normal control tissues were collected from 40 patients at the Department of Urology of the Ninth Hospital of Xi’an, Xi’an, Shaanxi, China. Thirty-three were men and seven were women. The mean age was 55.4 years (range, 44–70 years). The diagnosis was pathologically confirmed, all eligible patients had been treated by nephrectomy and none had received any treatment before surgery. The tissues were immediately snap-frozen in liquid nitrogen after resection and stored at −80°C until RNA and protein extraction and immunohistochemical (IHC) staining of TNIP1 and C/EBPβ. Patient sex, age, extent of tumor differentiation, Fuhrman stage, tumor stage, lymphatic metastasis, and postoperative recurrence, were retrieved from their medical records. The study was approved by the institutional Clinical Ethical Committee of the Ninth Hospital of Xi’an, Xi’an and was conducted following the ethical guidelines of the Declaration of Helsinki. All enrolled patients provided written informed consent before the start of the study.
Cell Culture
OS-RC-2, 786-O, 769-P, Caki-1, and Caki-2 human ccRCC cell lines and the HK-2 human proximal tubule cells that were used as a control were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cells were grown in Dulbecco’s modified Eagle’s medium, Sigma, St Louis, USA) or Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Shanghai, China), with 10% fetal bovine serum (FBS; Gibco) and 1% (v/v) penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and maintained in a humidified 5% CO2 atmosphere at 37°C.
RNA Interference And Transfection Assay
TNIP1 overexpression was induced in 786-O by transfection of a pcDNA3.1 plasmid vector. Transfection was performed by seeding 1x106 cells in each well of a 6-well plate, adding 1.5 µg TNIP1 cDNA and transfected the cells with an E.Z.N.A Endo-free plasmid DNA Maxi Kit (Biology co., Ltd. Zhejiang, China) and Lipofectamine 2000 (Invitrogen, USA) for 24 h at 37°C. An empty vector was the control. Short hairpin (sh)RNAs against human TNIP1 (NM_006058.4, Shanghai Sunbio, Shanghai, China), was used to knockdown TNIP1. The sequences were sense: 5′-ccgg GAT GAG AAG GCA AGA GAA CTC GAG TTC TCT TGC CTT CTC CTC ATC TTT TTTg-3′, antisense: 5′-aat tca aaa aaG ATG AGG AGA AGG CAA GAG AAC TCG AGT TCT CTT GCC TTC TCC TCA TC-3′. The sequences of the negative control shRNA were sense: 5′- Ccgg TTC TCC GAA CGT GTC ACG TTT CAA GAG AAC GTG ACA CGT TCG GAG AAT TTT Tg-3′, antisense: 5′- aat tca aaa aTT CTC CGA ACG TGT CAC GTT CTC TTG AAA CGT GAC ACG TTC GGA GAA-3′. pMagic 4.1 lentiviral vectors accompanied by TNIP1 shRNAs or negative control shRNAs were inserted into recombinant TNIP1 (rTNIP1). The recombinant lentiviral vectors, pHelper vector 1.0 and pHelper vector 2.0, were cotransfected into 293T cells using a transfection reagent (Shanghai Sunbio). The lentiviral particles were adapted to infect 786-O cells following the kit manufacturer’s instructions. The construction of 786-O cells that under expressed TNIP1 was previously described by Chen et al.11 To silence C/EBPβ in 786-O cells, C/EBPβ siRNAs (oligonucleotide sequence 5′-CGT GGT GTT ATT TAA AGA A-3′) and a nonsilencing siRNA negative control (5′-TTC TCC GAA CGT GTC ACG T-3′), were constructed from the full length human C/EBPβ cDNA sequence (NM_005194, Shanghai Genechem Co. Ltd., Shanghai, China). Transfection was with Lipofectamine 2000 (Invitrogen, USA). The construction of 786-O cells that underexpressed C/EBPβ was previously described by Chen et al.11
TNIP1 And C/EBPβ Immunohistochemical (IHC) Staining
Immunohistochemical staining for TNIP1 and C/EBPβ were performed and described according to previously reported studies.15–17 Briefly, formaldehyde-fixed paraffin-embedded ccRCC tumor tissues and the paired control tissues were sectioned at 3 µm. The sections were deparaffinized in xylene for 25 min and rehydrated in 100, 100, 95, 85, and 75% ethanol for 5 min, respectively. Antigen retrieval treatment was done in 0.01M citrate buffer in a microwave oven for 3 min at high power and 10 min at low power, and endogenous peroxidase was blocked by incubation in 3% H2O2 for 10 mins at room temperature. Then, the sections were washed in PBS solution subsequent and blocked with 10% goat serum (ZhongShanBiotechnology, China) for 30 min, followed by incubation with primary mice anti-human TNIP1 (1:200; eBioscience) and anti-C/EBPβ antibody (1:250; sc-150, Santa Cruz Biotechnology) overnight at 4 ◦C. The sections were washed in PBS solution three times and incubated with a HRP-conjugated secondary antibody for 30 min at room temperature. All slides were counterstained diaminobenzidine (DAB) solution and 20% hematoxylin, and dehydrated. Immunohistochemical staining tissue sections were reviewed and scored by two independent pathologists. The score was calculated according to the proportion of stained tumor cells and intensity of cellular staining according to previous study.17 The final score was ranged from 0 to 12 scores.
Real-Time Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
Expression of TNIP1 and C/EBPβ mRNA was assayed by qRT-PCR. Total RNA was extracted from ccRCC tumor tissues and cell lines using Trizol reagent (Invitrogen, USA) following the manufacturer’s instructions and then reverse transcribed into cDNA using a cDNA chain sythesis kit (Thermofizrre). The qPCR experiment was performed using a Light Roche 480 System. cDNA was amplified using the following primer sequences: TNIP1: forward, 5ʹ- CAG AAT GAG TTG CTG AAA CA −3ʹ; reverse, 5ʹ- TCT CCT CAT CTT TGA ATG CT −3ʹ; C/EBPβ: forward, 5ʹ-CTC GCA GGT CAA GAG CAA G-3ʹ; reverse, 5ʹ-CTC GCA GGT CAA GAG CAA G-3ʹ. GAPDH was used as internal control gene with the primer sequences: forward, 5ʹ-CGGAGTCAACGGATTTGGTCGTAT-3ʹ; reverse, 5ʹ-AGCCTTCTCCATGGTGGTGAAGAC-3ʹ. The relative quantification was estimated using the ΔΔ CT method.
Western Blotting (WB)
ccRCC tumor tissues or cultured cell were homogenized in lysis buffer containing protease inhibitors, centrifuged at 12,000 g for 15 min at 4°C, and the protein concentration in the supernatant was determined with a bicinchoninic acid assay kit (Beyotime Institute). The precipitated proteins (80 μg) were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) for 2 h at 75V and transferred to polyvinylidene difluoride (PVDF) membranes on wet transfer equipment at 350 mA for 2 h. The membranes were blocked with 5% skim milk in TBS for 1 h at room temperature and then incubated overnight with rabbit polyclonal anti-TNIP1 (LSBio, USA, 1:1000), rabbit polyclonal anti-C/EBPβ (GeneTex, 1:1000), rabbit polyclonal anti-B-cell lymphoma 2 (Bcl-2, Proteintech, 1:500), rabbit monoclonal anti-Bcl-2-associated X (Bax, Proteintech, Chicago, USA, 1:1000), or rabbit monoclonal anti-cleaved-caspase 3 (Cell Signaling Technology, Inc., Danvers, MA, USA, 1:1000) primary antibodies at 4°C. Proteins were visualized by incubation with horseradish peroxidase (HRP)-conjugated secondary goat anti-rabbit IgG antibody (ZSGB-BIO, China, 1:5000) for 1h. Color was developed by electrochemiluminescence (ECL) and the images were scanned with an Alpha Innotech gel imaging system (Bio-Rad) and captured. GAPDH (mouse, 1:1000, Santa Cruz Biotechnology) was used as an internal control.
Cell Proliferation Assay
A cell counting Kit-8 kit (CCK-8; Beyotime, Shanghai, China) was used to assay cell proliferation following the manufacturer’s instructions. Briefly, 786-O cells were seeded in 96-well plates at 5x103/well and incubated for 3 h at 37°C with 10 µL CCK-8 solution in 100 μL fresh media. The optical density was read at an absorbance wavelength of 450 nm with a full wavelength microplate analyzer (Molecular Devices). Each result was the mean of triplicate assays.
Flow Cytometry
Cell cycle and apoptosis analysis were performed by flow cytometry as previously described.18 Briefly, after transfection for 72 h, single-cell suspensions of 786-O cells adjusted 1 × 106 cells/mL were centrifuged for 10 min at 1500 r/min. After discarding the supernatant, 2 mL phosphate buffered saline (PBS) was added for every 1 mL of the initial suspension. After centrifuging 1 mL of the suspension, the supernatant as removed and the cells were fixed overnight at 4°C in prechilled 70% ethanol. For cell cycle analysis conducted the next day, 50 µg propidium iodide (PI), a fluorescent nucleic acid dye, containing RNAase was added to every 100 µL of cell suspension. The cells were washed twice with PBS, allowed to react for 30 min in the dark, and then filtered through a 100-mesh nylon membrane. A FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) scanned red fluorescence at an excitation wavelength of 488 nm; BD Cell-Fit software analyzed the cell cycle.
For the cell apoptosis assay, cells were cultured in a CO2 incubator for 48 h at 37°C, washed twice with PBS solution, centrifuged and resuspended in 200 µL buffer solution. The cells were stained with 10 µL Annexin V-fluorescein isothiocyanate (FITC, ab14085; Abcam) and 5 µL PI (Beyotime, Beijing, China) and for 15 min in the dark and at room temperature. The percentage of apoptotic cells in 300 µL buffer was determined with a FACSCalibur flow cytometer and CellQuest software (BD Biosciences) at an excitation wavelength of 488 nm.
Statistical Analysis
All the data were performed statistical analysis using SPSS 16.0 software and described as mean ± standard deviation (SD). In present study, the Student t-test, nonparametric Mann–Whitney U-test or Fisher exact test were adopted for comparation of the statistical differences between two groups. P < 0.05 was deemed statistically significant.
Results
TNIP1 Expression Was Reduced In Tumor Tissues Of ccRCC Patients And Its Clinical Significance
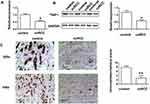
qRT-PCR, Western blots, and IHC staining all showed that TNIP1 expression was decreased in ccRCC tumor tissue compared with adjacent normal control tissue (P < 0.05, Figure 1). TNIP1 was present in both the nuclei and cytoplasm of epithelial cells of renal tubules in the kidney parenchyma (Figure 1C). Decreased TNIP1 expression in the nucleus and cytoplasm of renal tubule epithelial cells was associated with the occurrence and development of ccRCC.
The association of TNIP1 expression with the clinicopathological characteristics of ccRCC patients is summarized in Table 1. Increased malignancy was significantly associated with the decreased TNIP1 expression (P < 0.05 or P < 0.01). Malignancy decreased with differentiation, low >medium > high; Fuhrman stage, III–IV > I–II; tumor stage, T3+T4 > T1+T2; lymphatic metastasis, yes > no; and postoperative recurrence: yes > no. TNIP1 expression in human ccRCC was correlated with differentiation, Fuhrman stage, tumor stage, lymphatic metastasis and postoperative recurrence (P < 0.05) but not sex or age (P > 0.05, Table 1).
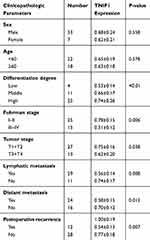 |
Table 1 Associations Of TNIP1 Expression With The Clinicopathological Features In 40 ccRCC Patients. (Mean ± SD) |
C/EBPβ Expression Was Increased In Tumor Tissues Of ccRCC Patients
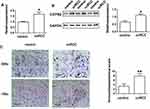
Compared with normal control tissue, qRT-PCR, Western blot assays, and IHC staining all found that C/EBPβ expression was enhanced in ccRCC tumor tissue (P < 0.05, Figure 2). IHC confirmed that the abnormal expression occurred in both the nucleus and cytoplasm of epithelial cells in the renal tubules of the kidney parenchyma (Figure 2C). C/EBPβ expression was increased in RCC clear cells in the nucleus and cytoplasm of renal tubular epithelial cell, possibly participating in the progression of ccRCC.
TNIP1 Was Down-Regulated In Human ccRCC Cell Lines
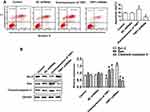
Western blot and qRT-PCR assays confirmed that TNIP1 expression was downregulated in OS-RC-2, 786-O, 769-P, Caki-1, and Caki-2 human ccRCC cells compared with HK-2 human proximal tubular cells (P < 0.05, Figure 3). The lowest relative TNIP1 expression was in 786-O cells (P < 0.01), which were used in subsequent experiments.
Overexpression Of TNIP1 Inhibits Cell Proliferation, Cell Cycle Entry And C/EBPβ Expression In 786-O Human ccRCC Cells In Vitro
Compared with cells transfected with the empty vector, TNIP1 overexpression led to a decrease of C/EBPβ expression (P < 0.05). Transfection of TNIP1-specific shRNA significantly reduced TNIP1 expression and significantly increased C/EBPβ expression compared with cells transfected with control shRNA (P < 0.01). The qRT-PCR and Western blot assay results were consistent (Figure 4A and B). In the CCK-8 assay, relative absorbance at 450 nm was lower in cells overexpressing TNIP1 than in the control cells (P < 0.05) and significantly higher cells transfected with TNIP1-specific shRNA than in cells transfected with control shRNA (P < 0.01, Figure 4C). Flow cytometry of PI-stained cells revealed that TNIP1 overexpression increased the number cells in G0/G1, and decreased the numbers in S and G2/M compared with the controls (P < 0.05). The opposite effects were seen in cells transfected with TNIP1-specific shRNA. The number of cells in G0/G1 was reduced and the numbers of cells in S and G2/M were increased compared with cells transfected with control shRNA (P < 0.01, Figure 4D). The results indicated that overexpression of TNIP1 inhibited cell proliferation and may have been associated with inhibition of cell cycle entry and the C/EBPβ expression induced in cells overexpressing TNIP1.
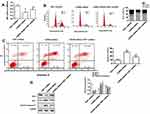
Overexpression Of TNIP1 Promotes Apoptosis Connected With Descendent Bcl-2 And Enhancive Bax And Cleaved-Caspase-3 Expressions In 786-O Human ccRCC Cells In Vitro
Flow cytometry with Annexin V-FITC/PI staining found that TNIP1 overexpression increased the apoptosis of 786-O cells compared with the control group (P < 0.05). TNIP1-specific shRNA significantly decreased the apoptosis rate compared with the control shRNA (P < 0.01, Figure 5A). Western blotting confirmed the decrease of Bcl-2 expression and the increase of both Bax and cleaved-caspase-3 expression in cells overexpressing TNIP1 compared with the control cells (P < 0.05). The opposite results were seen in cells with TNIP1-specific compared with control shRNA (P < 0.01, Figure 5B).
C/EBPβ siRNA Suppresses The Effects Of TNIP1 shRNAs On Proliferation, Cell Cycle Entry And Apoptosis In 786-O Human ccRCC Cells In Vitro
Silencing the expression of C/EBPβ by transfection of C/EBPβ-specific siRNA confirmed that the effects of TNIP1–shRNA on 786-O cell proliferation, cell cycle entry and apoptosis were by regulating C/EBPβ expression. The CCK-8 assay showed that the C/EBPβ–siRNAs inhibited the promotion of proliferation and cell cycle entry and decreased apoptosis in 786-O cells cotransfected with TNIP1-specific shRNA. The relative absorbance at 450 nm was lower in TNIP1–shRNA+C/EBPβ–siRNA-cotransfected than in TNIP1 shRNA-transfected cells (P < 0.05), and higher in TNIP1–shRNA+C/EBPβ–siRNA-transfected than in C/EBPβ–siRNA-transfected cells (P < 0.05). The 450 nm relative absorbance was decreased by C/EBPβ–siRNA compared with TNIP1–shRNA transfection (P < 0.05, Figure 6A). Flow cytometry and PI staining found that more 786-O cells were arrested in G0/G1 and in S and G2/M in TNIP1–shRNA+C/EBPβ–siRNA-cotransfected than with TNIP1–shRNA-transfected cells (P < 0.05). Fewer 786-O cells were in G0/G1 and more were in S and G2/M in TNIP1–shRNA+C/EBPβ–siRNA-transfected cells than in C/EBPβ–siRNA-transfected cells (P < 0.05). Transfection with C/EBPβ–siRNA resulted in more 786-O cells arrested at G0/G1 and fewer arrested at S and G2/M phase compared with TNIP1–shRNA transfection (P < 0.05, Figure 6B). Flow cytometry with Annexin V-FITC/PI staining and Western blotting found that apoptosis of 786-O cells was increased, Bcl-2 expression was decreased, and Bax and cleaved-caspase-3 expression were increased more with TNIP1–shRNA+C/EBPβ–siRNA transfection than with C/EBPβ–siRNAs transfection (P < 0.05). Apoptosis was decreased, Bcl-2 was increased, and Bax and cleaved-caspase-3 expression were lower with TNIP1–shRNA+C/EBPβ–siRNA than with TNIP1–shRNA transfection (P < 0.05). Apoptosis and Bax and cleaved-caspase-3 expression were increased and Bcl-2 expression was decreased, more with C/EBPβ–siRNA transfection than with TNIP1–shRNA transfection (Figure 6C and D).
Discussion
We believe this is the first study to demonstrate TNIP1 downregulation in human ccRCC tissues and in human RCC cell lines. TNIP1 is an NF-κB inhibitor protein also known as ABIN1.10 It is known to be involved in inflammatory diseases, hepatitis B virus-related hepatocellular carcinoma,19,20 autoimmune diseases including systemic lupus erythematosus,21 systemic sclerosis,22 rheumatoid arthritis,23 gastric carcinoma,9 and colorectal cancer.24 TNIP1 was distributed in both the nuclei and cytoplasm of epithelial cells in the renal tubules of the kidney parenchyma (Figure 1C). It has previously been found in the nucleus and cytoplasm of normal skin keratinocytes (12). Our study is also in line with methods described by Khanolkar et al who inhibited TNIP1 with siRNA.10
TNIP1 expression was lower in 786-O cells than in the other human ccRCC cell lines (OS-RC-2, 769-P, Caki-1 and Caki-2). This study is the first to identify TNIP1 as a tumor suppressor in human ccRCC. Overexpression of TNIP1 inhibited cell proliferation and cell cycle entry. It also promoted cell apoptosis and expression of the apoptosis-promoting Bax and gene and cleaved-caspase-3 expression. It inhibited expression of the Bcl-2 anti-apoptosis gene. Decreased TNIP1 expression was associated with increased ccRCC malignancy and worse prognosis (Table 1). Chen et al reported that downregulation of TNIP1 promoted cell proliferation and inhibited apoptosis.8,11,25 We found that downregulation of TNIP1 promoted entry into the cell cycle leading to increased cell proliferation.
C/EBPβ is overexpressed in human intestinal-type gastric cancers,26,27 human gastric cancer,28 ovarian cancer,29 colorectal cancer,30,31 etc. It promotes cell proliferation, invasion, migration and metastasis inhibits cell differentiation and apoptosis promotes inflammation, immune suppression, and pro-oncogenic functions.14,28,31–33 Overexpression of C/EBPβ promoted cell invasive and migratory activity; downregulation of C/EBPβ suppressed malignant activities of 786-O cells. Downregulation of TNIP1 increased C/EBPβ expression; overexpression of TNIP1 decreased C/EBPβ expression in HaCaT cell, which are spontaneously immortalized human keratinocytes.11 Whether C/EBPβ is also overexpressed in human ccRCC tissues, and the effects of C/EBPβ on cell proliferation, the cell cycle, and apoptosis in human ccRCC is unknown. This study is the first to find that C/EBPβ was overexpressed in human ccRCC tissues, and was negatively regulated by TNIP1, leading to increased cell proliferation, entry into the cell cycle, and inhibition of apoptosis. Bcl-2 expression was increased; Bax and cleaved-caspase-3 expression in human 786-O cells were decreased.
In Conclusion, our finds indicate that TNIP1 was downregulated and C/EBPβ was upregulated in human ccRCC tissue and human ccRCC cell lines, especially in 786-O cells. TNIP1 overexpression inhibited cell proliferation and cycle entry and promoted apoptosis by inhibiting C/EBPβ expression. We believe that this is the first study to identify TNIP1 as tumor-suppressor in ccRCC via negative regulation of C/EBPβ. The results identify TNIP1 as a potential biomarker of ccRCC diagnosis and a possible treatment target.
Ethics Approval And Consent To Participate
The study was approved by the Clinical Ethics Committee of the Ninth Hospital of Xi’an and was conducted following the ethical principles of the Declaration of Helsinki. All enrolled patients provided written informed consent before initiation of the study.
Availability Of Data And Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kuthi L, Jenei A, Hajdu A, et al. Prognostic factors for renal cell carcinoma subtypes diagnosed according to the 2016 WHO renal tumor classification: a study involving 928 patients. Pathol Oncol Res. 2017;23(3):689–698. doi:10.1007/s12253-016-0179-x
2. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi:10.1002/ijc.29210
3. Moch H. An overview of renal cell cancer: pathology and genetics. Semin Cancer Biol. 2013;23(1):3–9. doi:10.1016/j.semcancer.2012.06.006
4. Thomas JS, Kabbinavar F. Metastatic clear cell renal cell carcinoma: a review of current therapies and novel immunotherapies. Crit Rev Oncol Hematol. 2015;96(3):527–533. doi:10.1016/j.critrevonc.2015.07.009
5. Hutson TE, Figlin RA. Novel therapeutics for metastatic renal cell carcinoma. Cancer. 2009;115(S10):2361–2367. doi:10.1002/cncr.v115.10s
6. Randall JM, Millard F, Kurzrock R. Molecular aberrations, targeted therapy, and renal cell carcinoma: current state-of-the-art. Cancer Metastasis Rev. 2014;33(4):1109–1124.
7. Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(5):1744–1748. doi:10.1016/j.ijrobp.2011.02.040
8. Gurevich I, Zhang C, Francis N, Aneskievich BJ. TNIP1, a retinoic acid receptor corepressor and A20-binding inhibitor of NF-κB, distributes to both nuclear and cytoplasmic locations. J Histochem Cytochem. 2011;59(12):1101–1112. doi:10.1369/0022155411427728
9. Liu Z, Shi Y, Na Y, et al. Genetic polymorphisms in TNIP1 increase the risk of gastric carcinoma. Oncotarget. 2016;7(26):40500–40507. doi:10.18632/oncotarget.9637
10. Khanolkar RC, Kalogeropoulos M, Lawrie A, Roghanian A, Vickers MA, Young NT. Leukocyte Ig-like receptor B1 restrains dendritic cell function through increased expression of the NF-κB regulator ABIN1/TNIP1. J Leukoc Biol. 2016;100(4):737–746.
11. Chen Y, Yan H, Song Z, et al. Downregulation of TNIP1 expression leads to increased proliferation of human keratinocytes and severer psoriasis-like conditions in an imiquimod-induced mouse model of dermatitis. PLoS One. 2015;10(6):e0127957.
12. Geng Y, Song Y, Zhang Z, Zhang H, Huang Y, Wang Y. The role of TNIP1 in the pathogenesis of myasthenia gravis among patients with thymoma. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2016;33(5):615–618. doi:10.3760/cma.j.issn.1003-9406.2016.05.007
13. Lechner MG, Megiel C, Russell SM, et al. Functional characterization of human Cd33+ and Cd11b+ myeloid-derived suppressor cell subsets induced from peripheral blood mononuclear cells co-cultured with a diverse set of human tumor cell lines. J Transl Med. 2011;9:90. doi:10.1186/1479-5876-9-90
14. Xie F, Li X, Wei C, Liang G, Dang Y, Shan Z. C/EBPβ promotes NF-κB-mediated invasion and migration of human renal carcinoma 786-O cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2015;31(11):1483–1487.
15. Yang FQ, Liu M, Xu YF, et al. GATA-3 is down-regulated in patients with clear cell renal carcinoma. Actas Urol Esp. 2013;37(8):489–497.
16. Shim M, Powers KL, Ewing SJ, Zhu S, Smart RC. Diminished expression of C/EBPalpha in skin carcinomas is linked to oncogenic Ras and reexpression of C/EBPalpha in carcinoma cells inhibits proliferation. Cancer Res. 2005;65(3):861–867.
17. Niu H, Li F, Wang Q, Ye Z, Chen Q, Lin Y. High expression level of MMP9 is associated with poor prognosis in patients with clear cell renal carcinoma. PeerJ. 2018;6:e5050. doi:10.7717/peerj.5050
18. Fan X, Liu M, Tang H, et al. MicroRNA-7 exerts antiangiogenic effect on colorectal cancer via ERK signaling. J Surg Res. 2019;240:48–59. doi:10.1016/j.jss.2019.02.035
19. Ramirez VP, Gurevich I, Aneskievich BJ. Emerging roles for TNIP1 in regulating post-receptor signaling. Cytokine Growth Factor Rev. 2012;23(3):109–118. doi:10.1016/j.cytogfr.2012.04.002
20. Cheng Y, Jiang X, Jin J, et al. TNIP1 polymorphisms with the risk of hepatocellular carcinoma based on chronic hepatitis B infection in Chinese Han Population. Biochem Genet. 2019;57(1):117–128. doi:10.1007/s10528-018-9882-5
21. Liu X, Qin H, Wu J, Xu J. Association of TNFAIP3 and TNIP1 polymorphisms with systemic lupus erythematosus risk: a meta-analysis. Gene. 2018;668:155–165. doi:10.1016/j.gene.2018.05.062
22. Bossini-Castillo L, Martin JE, Broen J, et al. Confirmation of TNIP1 but not RHOB and PSORS1C1 as systemic sclerosis risk factors in a large independent replication study. Ann Rheum Dis. 2013;72(4):602–607. doi:10.1136/annrheumdis-2012-201888
23. Oliveira RD, Fontana V, Junta CM, et al. Differential gene expression profiles may differentiate responder and nonresponder patients with rheumatoid arthritis for methotrexate (MTX) monotherapy and MTX plus tumor necrosis factor inhibitor combined therapy. J Rheumatol. 2012;39(8):1524–1532. doi:10.3899/jrheum.120092
24. Li C, Zhao Z, Zhou J, Liu Y, Wang H, Zhao X. Relationship between the TERT, TNIP1 and OBFC1 genetic polymorphisms and susceptibility to colorectal cancer in Chinese Han population. Oncotarget. 2017;8(34):56932–56941. doi:10.18632/oncotarget.18378
25. Verstrepen L, Carpentier I, Verhelst K, Beyaert R. ABINs: A20 binding inhibitors of NF-kappa B and apoptosis signaling. Biochem Pharmacol. 2009;78(2):105–114. doi:10.1016/j.bcp.2009.02.009
26. Regalo G, Canedo P, Suriano G, et al. C/EBPbeta is over-expressed in gastric carcinogenesis and is associated with COX-2 expression. J Pathol. 2006;210(4):398–404.
27. Sankpal NV, Moskaluk CA, Hampton GM, Powell SM. Overexpression of CEBPbeta correlates with decreased TFF1 in gastric cancer. Oncogene. 2006;25(4):643–649. doi:10.1038/sj.onc.1209081
28. Regalo G, Förster S, Resende C, et al. C/EBPβ regulates homeostatic and oncogenic gastric cell proliferation. J Mol Med (Berl). 2016;94(12):1385–1395.
29. Liu D, Zhang XX, Li MC, et al. C/EBPβ enhances platinum resistance of ovarian cancer cells by reprogramming H3K79 methylation. Nat Commun. 2018;9(1):1739. doi:10.1038/s41467-018-03590-5
30. Snezhkina AV, Krasnov GS, Lipatova AV, et al. The dysregulation of polyamine metabolism in colorectal cancer is associated with overexpression of c-Myc and C/EBPβ rather than enterotoxigenic bacteroides fragilis infection. Oxid Med Cell Longev. 2016;2016:2353560. doi:10.1155/2016/2353560
31. Ji Y, Li J, Li P, Wang L, Yang H, Jiang G. C/EBPβ promotion of MMP3-Dependent Tumor Cell Invasion and Association with Metastasis in Colorectal Cancer. Genet Test Mol Biomarkers. 2018;22(1):5–10. doi:10.1089/gtmb.2017.0113
32. Nerlov C. The C/ EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17(7):318–324. doi:10.1016/j.tcb.2007.07.004
33. Bonzheim I, Irmler M, Klier-Richter M, et al. Identification of C/EBPβ target genes in ALK+ anaplastic large cell lymphoma (ALCL) by gene expression profiling and chromatin immunoprecipitation. PLoS One. 2013;8(5):e64544. doi:10.1371/journal.pone.0064544
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.