Back to Journals » Journal of Inflammation Research » Volume 16
TLR5 Signaling in the Regulation of Intestinal Mucosal Immunity
Authors Feng S, Zhang C, Chen S, He R, Chao G, Zhang S
Received 7 February 2023
Accepted for publication 23 May 2023
Published 14 June 2023 Volume 2023:16 Pages 2491—2501
DOI https://doi.org/10.2147/JIR.S407521
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Shuyan Feng,1,* Chi Zhang,1,* Shanshan Chen,2,* Ruonan He,1,* Guanqun Chao,3 Shuo Zhang4
1Zhejiang Chinese Medical University, Hangzhou, 310053, People’s Republic of China; 2The First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, 310006, People’s Republic of China; 3Sir Run Run Shaw Hospital of Zhejiang University, Hangzhou, 310018, People’s Republic of China; 4The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, 310005, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guanqun Chao; Shuo Zhang, Email [email protected]; [email protected]
Abstract: Toll-like receptor 5 (TLR5) is a pattern recognition receptor that specifically recognizes flagellin and consequently plays a crucial role in the control of intestinal homeostasis by activating innate and adaptive immune responses. TLR5 overexpression, on the other hand, might disrupt the intestinal mucosal barrier, which serves as the first line of defense against harmful microbes. The intestine symbiotic bacteria, mucous layer, intestinal epithelial cells (IECs), adherens junctions (such as tight junctions and peripheral membrane proteins), the intestinal mucosal immune system, and cytokines make up the intestinal mucosal barrier. Impaired barrier function has been linked to intestinal illnesses such as inflammatory bowel disease (IBD). IBD is a persistent non-specific inflammatory illness of the digestive system with an unknown cause. It is now thought to be linked to infection, environment, genes, immune system, and the gut microbiota. The significance of immunological dysfunction in IBD has received more attention in recent years. The purpose of this paper is to explore TLR5’s position in the intestinal mucosal barrier and its relevance to IBD.
Keywords: toll-like receptor 5, intestinal mucosal barrier, microbes, intestinal immune, inflammatory bowel disease
Introduction
The primary function of the immune system, comprised of interconnected innate and adaptive arms, is to launch a robust immune response towards pathogens while maintaining tolerance to self-antigens. The intestinal mucosal immune system is very complex, consisting of the mucus layer, intestinal epithelial cells (IECs), gut-associated lymphoid tissue (GALT), which includes lamina propria dendritic cells (LPDCs), and the enteric nervous system (ENS). Toll-like receptors (TLRs) are a class of transmembrane protein receptors and belong to pattern recognition receptors (PRRs). TLRs can recognize the microbe-associated molecular patterns (MAMPs) and play a central role in the immune response. TLR5, one of the most significant extracellular receptors interacting with the human gastrointestinal microbiome1 as well as a flagellin-specific receptor, plays a vital part in intestinal immunity.
The intestinal mucosal barrier is regarded as one of the most significant protective barriers in the human body, which can keep the gut microbiota in balance, and protect against bacterial translocation, endotoxemia, and its secondary damage. Intestinal disorders, such as IBD, are closely linked to impaired barrier function. IBD is a chronic nonspecific inflammatory illness of the digestive system that mainly encompasses Crohn’s disease (CD) and Ulcerative Colitis (UC). It is characterized by the accumulation of immune cells and pro-inflammatory cytokines in the intestinal mucosa. In recent years, the incidence of IBD has been on the rise in Asia2,3. However, the pathophysiology of IBD, which may be associated with barrier disorder, intestinal microecological disorder, and genetic susceptibility to the host autoimmunity, has not been comprehensively understood.
In this review, we provided an overview of TLR5 about its role and potential mechanisms under the circumstances of intestinal homeostasis or inflammation to further explain the occurrence and development of IBD.
Crosstalk Between IECs and LPDCs
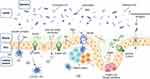
The intestinal mucosa is exposed to a large number of microorganisms and foreign food source antigens. Symbiotic bacteria not only defend against infection by activating mucosal innate immune defenses but also induce regulatory cells to inhibit detrimental inflammatory responses. At the same time, immune cells in the gut identify and resist bacteria and foreign antigens between bacteria and immune cells.4 Therefore, a tightly regulated barrier is crucial to maintain intestinal homeostasis. IECs, including absorptive cells, goblet cells (GCs), M cells, Paneth cells, and intestine endocrine cells, are differentiated from intestinal epithelial stem cells.5
GCs, as secretory intestinal epithelial cells, can synthesize and secrete mucin. Mucin is a high molecular weight glycoprotein that serves as the first line of defense against physical and chemical damage and prevents the intestinal epithelium from the invasion by pathogens. The predominant component of the intestine mucin is Muc2.6,7 As the factory of mucins, GCs play an important role in maintaining intestinal immunological homeostasis by transmitting luminal antigens to LPDCs. Previous in vitro investigations have revealed that LPDCs can extend beyond the intestinal epithelium to the intestinal lumen via trans-epithelial dendrites (Ted) to detect microorganisms.8 However, this phenomenon was not found in vivo experiments in mice, because LPDCs could hardly pass through the intestinal mucous layer via Ted. Studies have shown that goblet cell-associated antigen passages (GAPs) is one of the ways for CD103 + LPDCs to obtain intraluminal antigens.9–11 Recently, some GCs have been identified as sentinel goblet cells (senGCs) with nonspecific endocytosis at the entrance of the colonic crypt. They can activate the downstream NOD-like receptor protein (NLRP) 6 inflammasome of the TLR/MyD88-dependent Nox/Duox reactive oxygen species synthesis, nonspecifically endocytose, and react to the TLR2/1, TLR4, and TLR5 ligands to increase Muc2 secretion, hence expelling bacteria.12
Paneth cells are granular secretory cells only found at the base of the small intestinal crypt. They can produce various kinds of antimicrobial peptides (AMPs), such asα-defensins, lysozyme, lipopolysaccharide (LPS)-binding protein, RegIII, and several pro-inflammatory mediators including interleukin-17A (IL-17A), tumor necrosis factor α (TNF-α), and IL-1b,13 which can stimulate intestinal innate immunity and regulate intestinal microorganism. Hence, Paneth cells are regarded as the fundamental regulator of intestinal homeostasis and intestinal microorganism.14 Many CD-related genes are expressed by Paneth cells, including ATG16-like 1 (Atg16L1), transcription factor 4 (Tcf4), NOD2, and immune-associated GTPase family M protein (IRGM).14 Mutations in Atg16L1 and another CD risky gene X-box-binding protein-1 (Xbp1) can lead to abnormal particle morphology and reduce particle number in Paneth cells of genetically deficient mice and CD patients.15 Xbp1 deletion in intestinal epithelial cells (IECs) results in spontaneous enteritis that will induce endoplasmic reticulum stress and then contribute to spontaneous colitis.16 Moreover, Paneth cell dysfunction and decreased antimicrobial peptide release have been shown to enhance the formation and development of IBD.17–19
GALT is the main site to initiate the gut-adaptive immune response. Over the surface of the GALT is a layer of follicle-associated epithelium (FAE) containing M cells. M cells absorb and transport granular antigens from the lumen to dendritic cells (DCs) and promote T cell activation and secretory immunoglobulin A (SIgA) synthesis.20,21 SIgA can effectively restrain pathogenic bacteria from adhering to the intestinal mucosa by stimulating immune responses specific to pathogens, increasing intestinal mucus secretion, and enhancing mucus layer flow. Mice that lack intestinal M-cell gene have a delayed IgA secretion response,21 lower IL-17A production, and are more susceptible to colitis.22
In addition to intestinal epithelial cells, tight junction (TJ) plays a critical role in maintaining the epithelial barrier of the intestinal mucosa. Being at the top of IECs, TJs consist of transmembrane protein Occludin, Claudins, junctional adhesion molecule (JAM), cytoplasmic protein ZO-1, ZO-2, ZO-3, and cingulin.23 TJs are the most significant defense in the paracellular metastatic route and serve a critical function in preserving the paracellular permeability of IECs. Generally, TJs may selectively allow the flow of ions and small molecules and prevent harmful bacteria and antigen macromolecules in the intestinal cavity from passing through the intestinal mucosal barrier. However, changes in TJ protein expression and structure are associated with the occurrence of IBD.24 Furthermore, some pro-inflammatory cytokines, such as TNF-α,25 IL-8,26 and interferon -γ27 have been shown to increase the permeability of TJs and induce apoptosis in IECs. As a result, this impairs the barrier function and induces epithelial mucosal inflammation. Among patients with CD and UC, a strong relationship has also been established between intestinal permeability and mucosal inflammation while the permeability is directly proportional to the severity of diarrhea.28
LPDCs are a class of antigen-presenting cells (APCs) that play an important role in both innate and adaptive immunity. However, the way that intraluminal antigen transport to LPDCs is controversial. It is generally accepted that soluble antigens may be transported to LPDCs through intercellular or paracellular pathways,29,30 while the insoluble granular antigens are taken up by M cells on follicle-associated epithelium.31 As mentioned above, LPDCs can acquire soluble and granular antigens through GAPs formed by GCs. LPDCs can also obtain antigens from apoptotic epithelial cells.32 Studies have shown that LPDCs can upregulate the chemokine receptor CCR7 and migrate to enteric-draining mesenteric lymph nodes (MLNs) after being activated, where they provide antigens to naive T cells33,34 to guide their differentiation into T-helper type 1 (Th1), type 2 (Th2), type 17 (Th17)35,36 or regulatory T cells (Treg)37 (Figure 1).
TLR5 Signal
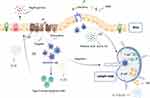
TLR5 is one of the main extracellular receptors interacting with the human gastrointestinal microbiota, and it is involved in the innate and adaptive immune responses to flagellin.3 TLR5 gene is located on chromosome 1 and can be expressed in monocytes, immature DCs, and epithelial cells. In IECs, TLR5 is mainly expressed in the basal part of cells.38,39 CD103+ CD11b+ LPDCs are the main cells expressing TLR5 in the small intestine.40,41 TLR5 consists of a Leucine-rich repeat (LRR) domain that mediates pathogen-associated molecular patterns (PAMPs) recognition, a transmembrane domain, and a cytoplasmic Toll/IL-1 receptor (TIR) domain that initiates downstream signaling.42 TLR5, as a specific receptor for flagellin of intestinal symbiotes and pathogenic bacteria, can recognize the conserved domain of different types of G+ and G- flagellin.43 The flagellin arginine residues in the D1 domain (BS flagellin R89) and the adjacent residues (BS flagellin E114 and L93) form complementary structural and chemical interactions with LRR which activates TLR5 and maximize the flagellin-mediated cellular activity with the assistance of the D0 domain.44
Flagellin binds directly to the lateral surfaces of TLR5 in a symmetrical arrangement, leading to the formation of a 2/2 complex.45 After activated by flagellin, a conformational change in the TIR domain of TLR5 on IECs recruits MyD88, which subsequently forms a complex with IL-1 receptor-associated kinase (IRAK) 4 and IRAK1/2 kinase called Myddosome.46 Myddosome induces the binding and activation of E3 ubiquitin (Ub) ligase TNF receptor-associated factor 6 (TRAF6).46 Then, the polyubiquitin chain produced by TRAF6 interacts with the complex formed by TAK1-binding protein 2 (TAB2) and 3 (TAB3) to activate the TGF-β activated kinase 1 (TAK1).47 After being sensitized, TAK1 activates the MAPK pathway and the IKK complex is composed of catalytic subunits IKKα, IKKβ, and regulatory subunit NEMO (also known as IKKγ). IKK complex phosphorylates nuclear factor-kappa B (NF-κB) inhibitory protein IκBα and leads to proteasome degradation, so that NF-κB can translocate into the nucleus to induce pro-inflammatory gene expression.48 IECs can also sense the endogenous luminal flagellin through TLR5-mediated signaling pathways to induce the expression of zinc-finger protein A20, which functions as the first-line defense against excessive innate immune responses in the early stage of inflammation.49 The A20 can inhibit the NOD2 signaling pathway stimulated by MDP, which ultimately activates the NF-κB signaling pathway.50
TLR5 signaling can lead to extensive transcriptional activation of at least 500 genes,51 including Janus kinase (JAK)/signal sensor and transcriptional activator (STAT), mitogen-activated protein kinase (MAPK), extracellular signal-associated kinase (ERK), as well as C-Jun N-terminal kinase (JNK),52–54 and also activate anti-apoptotic or proliferation pathways.55 This activation of anti-apoptotic gene expression enables cells to survive when facing deadly damage. Erk1/2, JNK, p38MAPK, and ERK5 are the four confirmed cascades of MAPKs. The ERK cascade preferentially regulates proliferation, differentiation and metastasis. The P38 cascade and the JNK cascade have similar functions in regulating stress response, inflammation, apoptosis, and proliferation. The ERK5 cascade is mainly involved in stress response and proliferation.56 MEKl and MEK2 are activators of ERKl/2, so as MEK5 to ERK5, MKK4 and MKK7 to JNK, and MKK3 and MKK6 to P38MAPKs.57 Ap-1, a co-product of P38, JNK, and ERK, activates downstream signaling molecules through kinase, leading to the release of various inflammatory cytokines and immune modulators (Figure 2).
TLR5 Signaling in IECs: The First Responder to Flagellin
IECs are a crucial line of defense against gut bacteria. However, PRRs on IECs do not always lead to inflammatory activation, because it depends on whether the stimulation received by PRRs comes from the apical side or the basolateral side.58 That is, PRRS can selectively inhibit or initiate inflammation. TLR5 is specifically expressed in the basal side of IECs and forms physical isolation from the lumen contents, which enables it to respond to the flagellin of translocative bacteria, such as Salmonella, but not to the non-translocative symbiotic Escherichia coli.59 Hence, inflammation occurs only when microorganisms penetrate the sterile room. This separation of inflammatory signal transduction helps to prevent uncontrolled inflammation caused by symbiotic microorganisms. Studies have found that the tolerance of polarized IECs to flagellin seems to be achieved through the internalization of TLR5, which blocks the activation of NF-κB, MAPK, and phosphoinositol 3-kinase (PI3K) signaling pathway, thereby preventing the secretion of pro-inflammatory cytokine IL-8. After incubation with flagellin for 24 hours, polarized monolayers showed a 10–50% reduction in TLR5 expression on the cell surface, avoiding the threshold of inflammatory activation and inhibiting pro-inflammatory responses.60 Interleukin-1 receptor-associated kinase (IRAK) is a key factor in the downstream TLR and IL-1R signaling pathways and plays an important role in systemic inflammatory responses.61 In the flagellin-tolerant state, TLR5 inhibits inflammation through the inhibition of IRAK4 phosphorylation.60 Thus, flagellin tolerance appears to help protect the host epithelium from uncontrolled immune responses and contribute to the healing process after initial inflammatory damage.
The expression of TLR5 in IECs can regulate the composition and localization of the intestinal microbiome, thus preventing diseases related to intestinal inflammation. The high expression of TLR5 on cells makes TLR5 signaling an important regulatory factor in the host immune response to flagellate microorganisms and maintenance of intestinal homeostasis.62 The symbiotic microbiome may evade TLR5-mediated intestinal immune surveillance by down-regulating flagellin expression.63 On the other side, a lack of TLR5 will lead to intestinal microbiome disorder, low-level inflammation, metabolic syndrome, and susceptibility to colitis.64–66 TLR5 deficient mice were animal models susceptible to the development of spontaneous colitis,67 suggesting that TLR5 is critical for the regulation of potentially harmful bacterium. As was shown in an experiment by Kinnebrew MA et al, systemic application of flagellin to antibiotic-treated mice significantly reduced vancomycin-resistant Enterococcus colonization.68
Flagellin of symbiotic microbiome also played an antibacterial role through the TLR5-regIIIγ pathway. RegIIIγ is an antibacterial peptide of the C-type lectin family expressed in the intestine,69 which is synthesized by IECs. RegIIIγ can exert direct antibacterial activity by specifically binding to bacterial peptidoglycan.69 Studies have shown that the RegIIIγ expression requires the activation of the TLR-MyD88-mediated signaling pathway in IECs when in intestinal homeostasis, which is directly activated by microbial products released by the intestinal microbiome. RegIIIγ expression may affect mucosal homeostasis and sensitivity to intestinal G+ pathogens through the composition of a symbiotic microbiome.70
Studies have shown that when the steady state is broken, the specific activation of TLR5 can significantly decrease the epithelial barrier resistance and alter the expression of TJ proteins, leading to increased intestinal permeability,71 translocation of bacteria, and antigens.72 Flagellin-stimulated endothelial cells rapidly induce proinflammatory mediators, such as the chemokine CXCL1, CXCL2, CXCL5, CXCL8, CCL2, CCL20, or CXCL10, which in turn attract immune cells belonging to the myeloid and lymphoid lineages,73,74 and pass on the baton (Figure 3).
TLR5 in DCs: The Key to Response to Flagellin
DC activation is a key parameter to determine whether intestinal tolerance or protective immunity is induced. After maturation under the action of danger signals (including MAMPs) or cytokines, cDC matures and up-regulates C chemokine receptor 7 (CCR7), which interacts with ligand CCL21 on lymphatic endothelial cells.75 This interaction enables cDC to enter the lymphatic vessels in both stable and inflammatory states and migrate directionally to draining lymph nodes to draining lymphoid tissue for antigen presentation,76,77 providing appropriate signals for the development of CD4 + T helper cells (Th), regulatory T cells (Treg), CD8 + T cells, and B cell responses. As mentioned above, CD103+ CD11b+ LPDCs (cDC2) are the main cells expressing TLR5 in the small intestine, while colonic DCs are concentrated mainly in isolated lymphoid follicles, few of which are present in the lamina propria. Therefore, we will focus on the effects of TLR5 + LPDC on CD4 + T cells. Interestingly, TLR5 exerts a novel MyD88-independent, endocytic receptor function by enhancing the presentation of the flagellar peptide to antigen-specific CD4 + T cells.78 The enhancement of flagellin processing and/or presentation by TLR5 seems likely to be caused solely as a consequence of increased antigen uptake by specific surface receptors.79
Under physiological conditions, the migration of cDC from intestinal LP mainly results in “harmless” antigen presentation to immature T cells in MLN. Intestinal LPDC presents flagellin in a tolerant manner, which catalyzes the conversion of vitamin A metabolite retinaldehyde to retinoic acid and secretion of IL-33 by expressing high levels of aldehyde dehydrogenase, thereby inducing immature CD4+T cells to differentiate into Foxp3+Treg.80,81
However, when there are inflammatory stimuli such as flagellin, cDCs will mature and release inflammatory cytokines and initiate protective acquired immunity. After being stimulated, LPDCs migrate into lymphatic vessels and induce the differentiation of naive B cells into plasma cells that produce IgA and interleukins (IL-17 and IL-22), facilitating early defense against pathogen invasion40,82 and positively regulating the differentiation of Th17-producing cells.36,40 Meanwhile, the involvement of TLR5 limits the production of Treg cells.62 Therefore, the high expression level of TLR5 on LPDCs ensured that TLR5+ LPDCs play an important role in inducing effector T cells to respond to the invasion of flagellated pathogens. CD103+ CD11b+ LPDCs can rapidly activate antibacterial inflammation after flagellin enters lamina propria by producing IL-23 through the flagellin-TLR5 signal pathway, thereby stimulating group 3 innate lymphoid cells (ILC3) to produce IL-22, promoting IEC recovery and inducing RegIIIγ expression.83 Contrary to the activation of lymphocytes caused by migratory DC in lymphoid tissue, ILC3 activation is immediate and heavily dependent on tissue-resident DC75 (Figure 3).
Expression of TLR5 in IBD
The onset of IBD is not caused by a single factor but by many factors. Among them, the intestinal barrier damage and immune response deficiency caused by innate genes include T cell differentiation (IL-10, IL-21), Th17 cell maintenance (IL-23R, JAK2, PTPN2), and NF-κB activation (TNF signal gene).84–86 The imbalance of key host–microbe interactions is an important cause of the pathogenesis of IBD. T-cell infiltration is a key feature of chronic intestinal inflammation,87 and T-cell-derived cytokines play an important role in the occurrence and development of IBD.88
Flagellin has been proven to be the major antigen of the adaptive immune response associated with IBD.89 Increasing evidence supports the critical role of flagellin/TLR5 in the pathogenesis of IBD, especially CD.72 Increased intestinal permeability is a common problem in IBD.90 When the barrier integrity gets impaired, the basolateral receptors of cells will be exposed to potential pathogenic ligands, including flagellin.3
Michael and his team91 used the molecular technique of serum expression cloning (SEC) to identify specific bacterial antigens which lead to IBD. The dominant antigens they identified were related to novel flagellin families, and they could activate the innate immunity through TLR5. Dubinsky and Targan et al came to a similar conclusion to Lodes.92,93 Gewirtz AT et al also found that the polymorphism of TLR5 termination codon reduced the adaptive immune response of anti-flagellin (CBir1) and played a positive role in protecting CD, which meant that TLR5 regulated the immune response to flagellin.94
Some studies indicate that TLR5 expression level is not positively correlated with the incidence of IBD. Abreu et al showed that the expression levels of TLR2 and TLR5 are not high in the colonic IECL of patients with IBD.89 Stanislawowski M et al have found that the expression of the TLR5 gene and protein was decreased in mucous of patients with moderate and severe active UC, suggesting that the down-regulation of TLR5 may be caused by an increase of ligand molecules near epithelial cells in inflammatory tissues.95,96
Therefore, we believe that it may be necessary to dynamically track the expression of intestinal TLR5 in patients with IBD. More research is needed to determine the role of TLR5 in the adaptive immune system in order to promote the development of IBD treatment drugs or diagnostic tools. The most important thing in the next stage is how to carry out clinical transformation targeting TLR5 on an existing basis, that is, how to use diet or drugs to accurately regulate intestinal flora or its metabolites to regulate the expression of TLR5, to achieve the goal of treating diseases through the interaction of innate immunity and adaptive immunity.
Conclusion
Innate and adaptive immunity in humans is significantly influenced by the intestinal mucosal barrier. IECs and LPDCs collaborate to develop the gut immune system. PPRs play an important role in the crosstalk between the two.
TLR5, a particular recognition receptor of flagellin, is an essential component of intestinal immunity. It mediates immune tolerance to symbiotic flagellin under intestinal homeostasis by recognizing bacterial flagellin and mediating dynamic interactions between the microbiota and the host’s innate immunity. This is a two-way communication and balance. On the one hand, symbiotic bacteria down-regulate the expression of flagellin by detecting the TLR5 signal on IECs, and on the other hand, Intestinal homeostasis is maintained by the asymmetric distribution of TLR5, the internalization of a fraction of the basolateral TLR5 and the crosstalk between IECs and LPDCs——TLR5 signaling in the FAE promotes antigen transport from the M cells to the immature DC,97 and then stimulate IgA secretion to inhibit bacterial motility. After the intestinal immune homeostasis is broken, IECs, which receive flagellin stimulation, secrete chemokine through the activation of the TLR5 signal to recruit myeloid and lymphatic immune cells. Then, cDC matured and up-regulated CCR7, migrated into the draining lymphoid tissue for antigen presentation, and promoted the activation of CD4+T cells and B cells. However, it is still not clear why flagellin can mediate T cell differentiation in different directions, and whether it is related to how to prevent further expansion of the immune cascade at the beginning of inflammation is still worth pondering. Similarly, whether different types of dendritic cells play a synergistic role in the immune process induced by flagellin is also a question worth exploring.
IBD is a disease related to intestinal mucosal barrier damage, intestinal flora disorder, and T cell differentiation, which fits well with the intestinal immune disorder mediated by flagellin-TLR5. Flagellin is indeed the main antigen of adaptive immune response associated with inflammatory bowel disease. It has been proved by a large number of studies that there are differences in the expression of TLR5 in inflammatory bowel disease, but the role of TLR5 in the disease is still unknown. It may be the fuse of the immune cascade, the defender of intestinal homeostasis, or both but have different identities with different stages of the disease.
To summarize, addressing the gut microbiota-TLR5 axis offers enormous potential for clinical prognosis and treatment of IBD, but major efforts are required to expand our understanding of molecular links and integrate them into clinical practice.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81973598, 82074214).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hug H, Mohajeri MH, La Fata G. Toll-like receptors: regulators of the immune response in the human gut. Nutrients. 2018;10(2):203. doi:10.3390/nu10020203
2. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology. 2013;145(1):158–165. doi:10.1053/j.gastro.2013.04.007
3. Zhao JN. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of “western” disease. Inflamm Bowel Dis. 2013;19(9):1839–1845. doi:10.1097/MIB.0b013e31828a6551
4. Sun M, He C, Cong Y, Liu Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015;8(5):969–978. doi:10.1038/mi.2015.49
5. Noah TK, Donahue B, Shroyer NF. Intestinal development and differentiation. Exp Cell Res. 2011;317(19):2702–2710. doi:10.1016/j.yexcr.2011.09.006
6. Arike L, Holmén-Larsson J, Hansson GC. Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology. 2017;27(4):318–328. doi:10.1093/glycob/cww134
7. Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. P Natl Acad Sci USA. 2011;2011:4659–4665.
8. Soloff AC, Barratt-Boyes SM. Enemy at the gates: dendritic cells and immunity to mucosal pathogens. Cell Res. 2010;20(8):872–885. doi:10.1038/cr.2010.94
9. Kulkarni DH, Gustafsson JK, Knoop K, et al. Goblet cell associated antigen passages support the induction and maintenance of oral tolerance. Mucosal Immunol. 2020;13(2):271–282. doi:10.1038/s41385-019-0240-7
10. McDole JR, Wheeler LW, McDonald KG, et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483(7389):345–349. doi:10.1038/nature10863
11. Kulkarni DH, McDonald KG, Knoop K, et al. Goblet cell associated antigen passages are inhibited during Salmonella typhimurium infection to prevent pathogen dissemination and limit responses to dietary antigens. Mucosal Immunol. 2018;11(4):1103–1113. doi:10.1038/s41385-018-0007-6
12. Birchenough George MH, Nyström EEL, Johansson MEV, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352(6293):1535–1542. doi:10.1126/science.aaf7419
13. Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroen. 2010;26(6):547–553. doi:10.1097/MOG.0b013e32833dccde
14. Clevers C, Bevins B. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol. 2013;75:289–311. doi:10.1146/annurev-physiol-030212-183744
15. Cadwell K, Liu JY, Miyoshi H, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–263. doi:10.1038/nature07416
16. Li M, Zhang S, Qiu Y, et al. Upregulation of miR-665 promotes apoptosis and colitis in inflammatory bowel disease by repressing the endoplasmic reticulum stress components XBP1 and ORMDL3. Cell Death Dis. 2017;8(3):e2699. doi:10.1038/cddis.2017.76
17. Yu S, Balasubramanian I, Laubitz D, et al. Paneth cell-derived lysozyme defines the composition of mucolytic microbiota and the inflammatory tone of the intestine. Immunity. 2020;53(2):398–416. doi:10.1016/j.immuni.2020.07.010
18. Liu T-C, Gurram B, Baldridge MT, et al. Paneth cell defects in Crohn’s disease patients promote dysbiosis. JCI Insight. 2016;1(8):e86907. doi:10.1172/jci.insight.86907
19. Adolph TE, Mayr L, Grabherr F, Tilg H. Paneth Cells and their Antimicrobials in Intestinal Immunity. Curr Pharm Design. 2018;24(10):1121–1129. doi:10.2174/1381612824666180327161947
20. Kanaya T, Williams IR, Ohno H. Intestinal M cells: tireless samplers of enteric microbiota. Traffic. 2020;21(1):34–44. doi:10.1111/tra.12707
21. Rios D, Wood MB, Li J, et al. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016;9(4):907–916. doi:10.1038/mi.2015.121
22. Ramakrishnan SK, Zhang H, Ma X, et al. Intestinal non-canonical NFκB signaling shapes the local and systemic immune response. Nat Commun. 2019;10(1):660. doi:10.1038/s41467-019-08581-8
23. Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Bio. 2016;17(9):564–580. doi:10.1038/nrm.2016.80
24. Malik A, Guy CS, Karki R, Vogel P, Kanneganti T-D. Pyrin inflammasome regulates tight junction integrity to restrict colitis and tumorigenesis. Gastroenterology. 2018;154(4):948–968. doi:10.1053/j.gastro.2017.11.276
25. Zhang C, Yan J, Xiao Y, et al. Inhibition of autophagic degradation process contributes to claudin-2 expression increase and epithelial tight junction dysfunction in TNF-α treated cell monolayers. Int J Mol Sci. 2017;18(1).
26. Yu H, Huang X, Ma Y, et al. Interleukin-8 regulates endothelial permeability by down-regulation of tight junction but not dependent on integrins induced focal adhesions. Int J Biol Sci. 2013;9(9):966–979. doi:10.7150/ijbs.6996
27. Sayoc-Becerra A, Krishnan M, Fan S, et al. The JAK-inhibitor tofacitinib rescues human intestinal epithelial cells and colonoids from cytokine-induced barrier dysfunction. Inflamm Bowel Dis. 2020;26(3):407–422. doi:10.1093/ibd/izz266
28. Gleeson JP, Estrada HQ, Yamashita M, et al. Development of physiologically responsive human iPSC-derived intestinal epithelium to study barrier dysfunction in IBD. Int J Mol Sci. 2020;21(4):25.
29. Knoop K, Miller M, Newberry RD. Transepithelial antigen delivery in the small intestine: different paths, different outcomes. Curr Opin Gastroen. 2013;29(2):112–118. doi:10.1097/MOG.0b013e32835cf1cd
30. Knoop K, Kulkarni DH, McDonald KG, et al. In vivo labeling of epithelial cell-associated antigen passages in the murine intestine. Lab Anim. 2020;49(3):79–88. doi:10.1038/s41684-019-0438-z
31. Nakamura Y, Kimura S, Hase K. M cell-dependent antigen uptake on follicle-associated epithelium for mucosal immune surveillance. Inflamm Regen. 2018;38(1). doi:10.1186/s41232-018-0072-y
32. du Pre MF, Samsom JN. Adaptive T-cell responses regulating oral tolerance to protein antigen. Allergy. 2011;66(4):478–490. doi:10.1111/j.1398-9995.2010.02519.x
33. Tordesillas L, Berin MC. Mechanisms of oral tolerance. Clin Rev Allerg Immu. 2018;55(2):107–117. doi:10.1007/s12016-018-8680-5
34. Brandum EP, Jørgensen AS, Rosenkilde MM, Hjortø GM. Dendritic cells and CCR7 expression: an important factor for autoimmune diseases, chronic inflammation, and cancer. Int J Mol Sci. 2021;22(15):8340. doi:10.3390/ijms22158340
35. Hilligan KL, Ronchese F. Antigen presentation by dendritic cells and their instruction of CD4+ T helper cell responses. Cell Mol Immunol. 2020;17(6):587–599. doi:10.1038/s41423-020-0465-0
36. Liu H, Chen F, Wu W, et al. TLR5 mediates CD172α(+) intestinal lamina propria dendritic cell induction of Th17 cells. Sci Rep-UK. 2016;6:22040. doi:10.1038/srep22040
37. Waisman A, Lukas D, Clausen BE, Yogev N. Dendritic cells as gatekeepers of tolerance. Semin Immunopathol. 2017;39(2):153–163. doi:10.1007/s00281-016-0583-z
38. Yu S, Gao N. Compartmentalizing intestinal epithelial cell toll-like receptors for immune surveillance. Cell Mol Life Sci. 2015;72(17):3343–3353. doi:10.1007/s00018-015-1931-1
39. Hamonic G, Pasternak JA, Wilson HL. Recognizing conserved non-canonical localization patterns of toll-like receptors in tissues and across species. Cell Tissue Res. 2018;372(1):1–11. doi:10.1007/s00441-017-2767-9
40. Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9(7):769–776. doi:10.1038/ni.1622
41. Fujimoto K, Karuppuchamy T, Takemura N, et al. A new subset of CD103+CD8alpha+ dendritic cells in the small intestine expresses TLR3, TLR7, and TLR9 and induces Th1 response and CTL activity. J Immunol. 2011;186(11):6287–6295. doi:10.4049/jimmunol.1004036
42. Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020;180(6):1044–1066. doi:10.1016/j.cell.2020.02.041
43. Smith KD, Andersen-Nissen E, Hayashi F, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4(12):1247–1253. doi:10.1038/ni1011
44. Song S, Jeon J, Namgung B, Hong M, Yoon S-I. A conserved TLR5 binding and activation hot spot on flagellin. Sci Rep-UK. 2017;7:40878. doi:10.1038/srep40878
45. Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol. 2014;14(8):546–558. doi:10.1038/nri3713
46. Balka KR, De Nardo D. Understanding early TLR signaling through the Myddosome. J Leukocyte Biol. 2019;105(2):339–351. doi:10.1002/JLB.MR0318-096R
47. Ajibade A, Wang HY, Wang R-F. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol. 2013;34(7):307–316. doi:10.1016/j.it.2013.03.007
48. West AP, Koblansky AA, Ghosh S. Recognition and signaling by toll-like receptors. Annu Rev Cell Dev Bi. 2006;22(1):409–437. doi:10.1146/annurev.cellbio.21.122303.115827
49. Oshima N, Ishihara S, Rumi MA, et al. A20 is an early responding negative regulator of Toll-like receptor 5 signalling in intestinal epithelial cells during inflammation. Clin Exp Immunol. 2010;159(2):185–198. doi:10.1111/j.1365-2249.2009.04048.x
50. Hitotsumatsu O, Ahmad RC, Tavares R, et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity. 2008;28(3):381–390. doi:10.1016/j.immuni.2008.02.002
51. Zeng H, Carlson AQ, Guo Y, et al. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171(7):3668–3674. doi:10.4049/jimmunol.171.7.3668
52. Yan J, Shen S, He Y, Li Z. TLR5 silencing reduced hyperammonaemia-induced liver injury by inhibiting oxidative stress and inflammation responses via inactivating NF-κB and MAPK signals. Chem Biol Interact. 2019;299:102–110. doi:10.1016/j.cbi.2018.11.026
53. Lin Y, Papp G, Miskey C, et al. The Flagellin: Allergen Fusion Protein rFlaA: Betv1 Induces a MyD88− and MAPK-Dependent Activation of Glucose Metabolism in Macrophages. Cells-Basel. 2021;10(10):2614. doi:10.3390/cells10102614
54. Zhang W, Wang L, Sun X, et al. Toll-like receptor 5-mediated signaling enhances liver regeneration in mice. Mil Med Res. 2021;8(1). doi:10.1186/s40779-021-00309-4
55. Zeng H, Wu H, Sloane V, et al. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290(1):G96–G108. doi:10.1152/ajpgi.00273.2005
56. Plotnikov A, Zehorai E, Procaccia S, Seger R. The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophys Acta. 2011;1813(9):1619–1633. doi:10.1016/j.bbamcr.2010.12.012
57. Strniskova M, Barancik M, Ravingerova T. Mitogen-activated protein kinases and their role in regulation of cellular processes. Gen Physiol Biophys. 2002;21(3):231–255.
58. Stanifer ML, Mukenhirn M, Muenchau S, et al. Asymmetric distribution of TLR3 leads to a polarized immune response in human intestinal epithelial cells. Nat Microbiol. 2020;5(1):181–191. doi:10.1038/s41564-019-0594-3
59. Gewirtz AT, Navas TA, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167(4):1882–1885. doi:10.4049/jimmunol.167.4.1882
60. Sun J, Fegan PE, Desai AS, Madara JL, Hobert ME. Flagellin-induced tolerance of the Toll-like receptor 5 signaling pathway in polarized intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G767–G778. doi:10.1152/ajpgi.00447.2006
61. Pereira M, Gazzinelli RT. Regulation of innate immune signaling by IRAK proteins. Front Immunol. 2023;14. doi:10.3389/fimmu.2023.1133354
62. Feng T, Cong Y, Alexander K, Elson CO. Regulation of Toll-like receptor 5 gene expression and function on mucosal dendritic cells. PLoS One. 2012;7(4):e35918. doi:10.1371/journal.pone.0035918
63. Niedergang F, Didierlaurent A, Kraehenbuhl JP, Sirard JC. Dendritic cells: the host Achille’s heel for mucosal pathogens? Trends Microbiol. 2004;12(2):79–88. doi:10.1016/j.tim.2003.12.011
64. Carvalho F, K O, Goodrich J, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12(2):139–152. doi:10.1016/j.chom.2012.07.004
65. Vijay-Kumar M, Aitken JD, Carvalho FA, et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328(5975):228–231. doi:10.1126/science.1179721
66. Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology. 2014;147(6):1363–1377. doi:10.1053/j.gastro.2014.08.033
67. Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63(7):1069–1080. doi:10.1136/gutjnl-2013-304909
68. Kinnebrew M, Ubeda C, Zenewicz L, Smith N, Flavell R, Pamer E. Bacterial flagellin stimulates toll-like receptor 5–dependent defense against vancomycin-resistant enterococcus infection. J Infect Dis. 2010;201(4):534–543. doi:10.1086/650203
69. Udomsopagit T, Miwa A, Seki M, et al. Intestinal microbiota transplantation reveals the role of microbiota in dietary regulation of RegIIIβ and RegIIIγ expression in mouse intestine. Biochem Bioph Res Co. 2020;529(1):64–69. doi:10.1016/j.bbrc.2020.05.150
70. Brandl K, Plitas G, Schnabl B, DeMatteo RP, Pamer EG. MyD88-mediated signals induce the bactericidal lectin RegIII gamma and protect mice against intestinal Listeria monocytogenes infection. J Exp Med. 2007;204(8):1891–1900. doi:10.1084/jem.20070563
71. Lunardi C, Bason C, Dolcino M, et al. Antiflagellin antibodies recognize the autoantigens Toll-Like Receptor 5 and Pals 1-associated tight junction protein and induce monocytes activation and increased intestinal permeability in Crohn’s disease. J Intern Med. 2009;265(2):250–265. doi:10.1111/j.1365-2796.2008.02013.x
72. Lopetuso L, Jia R, Wang X-M, et al. Epithelial-specific toll-like receptor (TLR)5 activation mediates barrier dysfunction in experimental ileitis. Inflamm Bowel Dis. 2017;23(3):392–403. doi:10.1097/MIB.0000000000001035
73. Ramos HC, Rumbo M, Sirard J-C, et al. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 2004;12(11):509–517. doi:10.1016/j.tim.2004.09.002
74. Rumbo M, Nempont C, Kraehenbuhl J-P, et al. Mucosal interplay among commensal and pathogenic bacteria: lessons from flagellin and Toll-like receptor 5. FEBS Lett. 2006;580(12):2976–2984. doi:10.1016/j.febslet.2006.04.036
75. Vijayan A, Rumbo M, Carnoy C, et al. Compartmentalized antimicrobial defenses in response to flagellin. Trends Microbiol. 2018;26(5):423–435. doi:10.1016/j.tim.2017.10.008
76. Braun A, Worbs T, Moschovakis GL, et al. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol. 2011;12(9):879–887. doi:10.1038/ni.2085
77. De Winde CM, Munday C, Acton SE. Molecular mechanisms of dendritic cell migration in immunity and cancer. Med Microbiol Immun. 2020;209(4):515–529. doi:10.1007/s00430-020-00680-4
78. Coombes JL, Maloy KJ. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Semin Immunol. 2007;19(2):116–126. doi:10.1016/j.smim.2007.01.001
79. Letran SE, Lee S-J, Atif SM, Uematsu S, Akira S, McSorley SJ. TLR5 functions as an endocytic receptor to enhance flagellin-specific adaptive immunity. Eur J Immunol. 2011;41(1):29–38. doi:10.1002/eji.201040717
80. Hung LY, Tanaka T, Herbine K, et al. Cellular context of IL-33 expression dictates impact on anti-helminth immunity. Sci Immunol. 2020;5(53):19.
81. Hall JA, Cannons JL, Grainger JR, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34(3):435–447. doi:10.1016/j.immuni.2011.03.003
82. Frosali S, Pagliari D, Gambassi G, Landolfi R, Pandolfi F, Cianci R. How the intricate interaction among toll-like receptors, microbiota, and intestinal immunity can influence gastrointestinal pathology. J Immunol Res. 2015;2015:489821. doi:10.1155/2015/489821
83. Kinnebrew M, Buffie C, Diehl G, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276–287. doi:10.1016/j.immuni.2011.12.011
84. Momozawa Y, Mni M, Nakamura K, et al. Resequencing of positional candidates identifies low frequency IL23R coding variants protecting against inflammatory bowel disease. Nat Genet. 2011;43(1):43–47. doi:10.1038/ng.733
85. Glocker E-O, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361(21):2033–2045. doi:10.1056/NEJMoa0907206
86. Chen X, Liu G, Yuan Y, Wu G, Wang S, Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019;10(12). doi:10.1038/s41419-019-2157-1
87. Shale M, Schiering C, Powrie F. CD4 + T-cell subsets in intestinal inflammation. Immunol Rev. 2013;252(1):164–182. doi:10.1111/imr.12039
88. Miura N, Yamamoto M, Fukutake M, et al. Anti-CD3 induces bi-phasic apoptosis in murine intestinal epithelial cells: possible involvement of the Fas/Fas ligand system in different T cell compartments. Int Immunol. 2005;17(5):513–522. doi:10.1093/intimm/dxh231
89. Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant-negative TLR5 polymorphism reduces adaptive immune response to flagellin and negatively associates with Crohn’s disease. Am J Physiol-Gastr L. 2006;290(6):G1157–G1163.
90. Chang J, Leong RW, Wasinger VC, et al. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology. 2017;153(3):723–731. doi:10.1053/j.gastro.2017.05.056
91. Vijay-Kumar M, Gewirtz AT. Role of flagellin in Crohn’s disease: emblematic of the progress and enigmas in understanding inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(5):789–795. doi:10.1002/ibd.20734
92. Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113(9):1296–1306. doi:10.1172/JCI200420295
93. Dubinsky MC. What is the role of serological markers in the diagnosis of IBD? Inflamm Bowel Dis. 2008;14:S185–S186. doi:10.1002/ibd.20585
94. Targan SR, Landers CJ, Mayer L, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128(7):2020–2028. doi:10.1053/j.gastro.2005.03.046
95. Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10(2):131–144. doi:10.1038/nri2707
96. Stanislawowski M, Wierzbicki PM, Golab A, et al. Decreased Toll-like receptor-5 (TLR-5) expression in the mucosa of ulcerative colitis patients. J Physiol Pharmacol. 2009;2009:71–75.
97. Chabot S, Shawi M, Eaves-Pyles T, Neutra M. Effects of flagellin on the functions of follicle-associated epithelium. J Infect Dis. 2008;198(6):907–910. doi:10.1086/591056
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.