Back to Journals » Nature and Science of Sleep » Volume 14
Timing of Sleep in the Break Between Two Consecutive Night-Shifts: The Effect of Different Strategies on Daytime Sleep and Night-Time Neurobehavioural Function
Authors Sargent C , Kosmadopoulos A , Zhou X, Roach GD
Received 30 August 2021
Accepted for publication 17 November 2021
Published 17 February 2022 Volume 2022:14 Pages 231—242
DOI https://doi.org/10.2147/NSS.S336795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sarah L Appleton
Charli Sargent,1 Anastasi Kosmadopoulos,2 Xuan Zhou,3 Gregory D Roach1
1Appleton Institute for Behavioural Science, Central Queensland University, Wayville, SA, Australia; 2Centre for Study and Treatment of Circadian Rhythms, Douglas Mental Health University Institute, Department of Psychiatry, McGill University, Montreal, QC, Canada; 3Centre for Quantitative Genetics and Genomics, Aarhus University, Aarhus, Denmark
Correspondence: Charli Sargent, Appleton Institute for Behavioural Science, Central Queensland University, PO Box 42, Goodwood, Wayville, SA, 5034, Australia, Tel +61 8 8378 4523, Email [email protected]
Objective: The aim of this study was to examine whether the timing of sleep in the break between consecutive night-shifts affects the quantity and quality of sleep obtained during the daytime and/or neurobehavioural function and self-perceived capacity during the night-time.
Methods: Participants (n = 12, all male, aged 22.9± 5.2 y) completed three randomised, counterbalanced conditions in a sleep laboratory, consisting of two consecutive 12-hour night-shifts (18:00– 06:00) with 7 hours in bed in the break between shifts. The three conditions differed only in the timing of the sleep opportunities – immediate (07:00– 14:00), delayed (10:00– 17:00), split (07:00– 10:30 and 13:30– 17:00). Neurobehavioural function (attention, memory, throughput) and self-perceived capacity (sleepiness, alertness, fatigue, mood) were assessed at 2-hour intervals during the night-shifts.
Results: Condition did not affect total sleep time (p = 0.465), but it did affect sleep onset latency (p < 0.001; W = 0.780; large effect), wake after sleep onset (p = 0.018; W = 0.333; moderate effect) and the amount of Stage N3 sleep (p < 0.001; η2=0.510; small effect). Compared to the immediate and delayed sleep conditions, the split sleep condition had less wake after sleep onset and more Stage N3 sleep; and compared to the delayed condition, the split sleep condition had longer latency to sleep onset. There was no effect of condition on measures of neurobehavioural function or self-perceived capacity during the second night-shift.
Conclusion: None of the three sleep strategies examined here – immediate, delayed or split – are clearly superior or inferior to the others in terms of the capacity to sleep during the daytime or to work at night. Therefore, those who work consecutive night-shifts should employ the strategy that best suits their personal preferences and/or circumstances.
Keywords: shift work, sleep duration, slow wave sleep, performance, sleepiness, split sleep
Introduction
Individuals who perform night work face considerable challenges in terms of obtaining adequate sleep during the day1–3 and maintaining acceptable levels of performance during the night.4,5 This is because it is more difficult to function effectively at night-time compared to daytime,6,7 and it is more difficult to obtain sleep during the day compared to night.6–9 Night work poses a significant risk because it almost doubles the likelihood of making errors, having an accident, and being injured.10–12
A number of countermeasures have been examined to improve alertness and performance during the night-shift. For example, caffeine administration during a shift reduces the physiological tendency for sleep,13 exposure to bright light in the work place improves alertness and cognitive performance,14 and a short 30-min nap during a night-shift (ie, ~02:00) reduces self-reported sleepiness and improves psychomotor performance.15 These countermeasures are effective, but in some workplaces, they are not practical or feasible. In such cases, an alternative approach could be to strategically organise daytime sleep around the work schedule.
Night-shift workers generally adopt one of three sleep ‘strategies’ in the breaks between consecutive night-shifts: (i) ~45% obtain a single sleep episode in the morning soon after night work (ie, immediate strategy); (ii) ~15% attempt to sleep in the afternoon, preferring to delay sleep after work in a manner similar to day workers (ie, delayed strategy); and (iii) ~40% choose to divide their sleep across their breaks, obtaining some sleep in the morning and some sleep later in the afternoon/evening (ie, split strategy).16–19 Each strategy has advantages and disadvantages, but their relative efficacy to sustain alertness and performance during night-shifts has not been systematically compared.20
The impact of a delayed sleep strategy on subsequent performance and sleepiness during consecutive night-shifts has been examined in both younger21 and older healthy adults.22,23 Individuals on a delayed sleep strategy—where sleep is scheduled to end 1–2 hours before the start of the subsequent night-shift—obtain more sleep23 and perform better21–23 and report less sleepiness over consecutive night-shifts21–23 compared to individuals on an immediate sleep strategy—where sleep is scheduled to begin as soon as the night-shift ends. One of the benefits of a delayed sleep strategy compared to an immediate strategy is that individuals spend less time awake prior to the start of the night-shift because the sleep period ends close to the start of the work period (ie, wake duration prior to the start of the shift is ~1-2 h with a delayed strategy compared to ~6-7 h with an immediate strategy). The reduction in the duration of wake prior to beginning night work is the most likely explanation for the improvements associated with a delayed strategy. However, in two of these studies, the delayed sleep strategy also involved exposure to bright light during the night-shifts. The light exposure was timed to promote an advance of the circadian system, which most likely facilitated sleep in the early evening.21,22
In the absence of exposure to bright light, a delayed sleep strategy still appears advantageous in terms of night-time performance and alertness.23 However, this has only been established in older adults who may be suited to a delayed strategy because of advanced rhythms of core body temperature and melatonin.24 Furthermore, in all three of the aforementioned studies, only consolidated strategies were examined. A split sleep strategy could be advantageous in that it provides some of the benefits of both immediate and delayed strategies—ie, immediate relief from being awake overnight and reduced prior wakefulness prior to the start of the subsequent night-shift. To date, however, the amount of sleep obtained during a daytime split sleep strategy, and its subsequent effect on night-time performance, has not been ascertained. The aim of this study was to determine the best sleep strategy (immediate, delayed or split) to adopt between consecutive night-shifts in healthy adults by comparing the effectiveness of each at sustaining performance at night and maximising sleep quantity and quality during the day.
Materials and Methods
Participants
Twelve male volunteers (22.9±5.2 y; mean±SD) gave written, informed consent to participate in the study. Participants were in good mental and physical health; were non-smokers, medication free and had not undertaken shift work or flight across more than two time zones in the three months prior to the study. The study was approved by CQUniversity’s Human Research Ethics Committee (H12/11-206) and was conducted in accordance with the Declaration of Helsinki.
Setting
The study was conducted in a sound-attenuated, windowless sleep laboratory. Participants were isolated from environmental time cues during the protocol but they had access to clocks. Ambient light levels were ~300 lux during wake periods. Lights were extinguished during all sleep periods.
Experimental Design
A randomised, counter-balanced, repeated-measures design was employed. Participants attended the laboratory on three separate occasions approximately one week apart. On each occasion, participants spent three consecutive days in the laboratory and completed one of three experimental conditions. Each visit consisted of a 9.5-hour night-time sleep opportunity, a 1-hour afternoon nap, two 12-hour simulated night-shifts separated by a 7-hour sleep opportunity, and one daytime recovery sleep (Figure 1). The only difference between conditions was the timing of the sleep opportunity that was provided in the 12-hour break between the two night-shifts (ie, immediate, delayed, split). The participants were not permitted to leave the laboratory during the protocol, nor were they permitted to consume alcohol or caffeine.
Protocol
In the week prior to each visit, participants maintained a consistent sleep/wake schedule. This was verified using wrist activity monitors (Actical Z-series; Philips Respironics; Oregon, USA) in conjunction with self-report sleep diaries. There was no difference in the amount of sleep obtained at home in the week prior to the commencement of the immediate (407.0± 6.4 min), delayed (410.6±20.3 min) and split (443.0±30.1 min) conditions (F2,22 = 1.747; p = 0.198).
On the evening of the first visit, participants were trained on the performance tasks. Participants were given 9.5 hours in bed on Night 1 (22:30–08:00) and were given a 1-hour nap opportunity on Day 1 (16:00–17:00) to prepare for the first night-shift on Day 1 (18:00–06:00). At the start of Day 2, participants were given a 7-hour sleep opportunity. The timing of the sleep opportunity differed for each condition: immediate (07:00–14:00), delayed (10:00–17:00), and split (07:00–10:30 and 13:30–17:00). In the 30 min prior to sleep opportunities, polysomnography (PSG) electrodes were attached to participants. At the end of Day 2, participants performed their second night-shift (18:00–06:00). During the night-shift, participants completed a 30-min test battery every two hours, with the first test battery beginning at 18:30. At the start of Day 3, participants were given a 9-hour recovery sleep before exiting the laboratory.
Sleep
Sleep was assessed using PSG (Compumedics Grael; Melbourne, Victoria, Australia). The electroencephalogram (EEG) was recorded using a single central derivation of electrodes (C4–M1, C3–M2) in conjunction with two electrooculograms and two electromyograms. The use of a single central derivation (as opposed to a frontal derivation)26 is not likely to affect the measurement of sleep. In a recent study, the impact of using a montage including three EEG derivations (frontal, central, and occipital) versus a montage including one central EEG derivation on PSG sleep scoring summary statistics was assessed.25 The use of three EEG derivations instead of a single central EEG derivation resulted in a small increase in the duration of Stage N3 sleep (3 EEG derivation = 63.5 min; 1 EEG derivation = 52.8 min) and had no effect on inter-scorer or intra-scorer reliability.25 The authors concluded that there was no significant advantage in using multiple over single EEG derivations for scoring sleep. Furthermore, any potential effects of using one, instead of two or three, EEG derivations in the present study should be similar for the three conditions, as a repeated-measures design was employed.
Sleep records were blinded and analysed in 30-s epochs by a single technician using established criteria.26 The following dependent variables were calculated for each sleep record: (i) total sleep time; (ii) time spent in Stages N1, N2, N3 and rapid eye movement (Stage R) sleep; (iii) sleep onset latency; (iv) wake after sleep onset; (v) sleep efficiency; and (vi) number of arousals. For the split sleep strategy, values for each sleep episode (except for sleep onset latency and sleep efficiency) were summed and the single value was used in all analyses. Sleep onset latency was averaged across the two sleep episodes. Sleep efficiency was calculated as follows: (total sleep time episode 1 + total sleep time episode 2) divided by (time in bed episode 1 + time in bed episode 2) multiplied by 100.
Neurobehavioural Performance
Sustained attention was assessed using the psychomotor vigilance task (PVT-192; Ambulatory Monitoring Inc., New York, USA). The PVT is a hand-held device with an upper surface that contains an LED display and two push-button response keys. Participants attended to the LED display for 10 min and pressed the appropriate response key with their dominant thumb as quickly as possible after the appearance of a visual stimulus. The dependent measures derived from the PVT were reciprocal response time (RRT, 1/ms × 1000) and the number of lapses (ie, response latency exceeding 500 ms). For all analyses, anticipated responses (ie, response times less than 100 ms) were excluded.
Cognitive throughput was assessed using the digit symbol substitution task (DSST)27 and a serial addition/subtractions task (SAS). For the DSST, participants were provided with a pen and a sheet of paper containing a key associating the digits 0 to 9 with different symbols. The participants were given 90 seconds to make as many substitutions as possible. The dependent measures derived from the DSST included the total number of substitutions attempted and the percentage of correct substitutions. The SAS task is operated on a computer and requires participants to perform addition and subtraction using integers between 1 and 9. Operations were presented at a variable interval of 1 to 5 seconds. The participants were given five min to complete as many operations as possible. The dependent measures derived from the SAS task were RRT and the percentage of correct responses.
Self-Perceived Capacity
Sleepiness was assessed using the Karolinska Sleepiness Scale (KSS);28 alertness was assessed using a visual analogue scale (VAS alertness);29 fatigue was assessed using the Samn–Perelli fatigue scale;30 and mood was assessed using the Profile of Mood States (ie, total mood disturbance).31
Statistical Analyses
Variables were examined for normality using the Shapiro–Wilks test. Differences in normally distributed sleep variables between conditions were examined using separate repeated measures ANOVAs with “condition” (immediate, delayed, split) as a within-subjects factor. Differences in non-normally distributed sleep variables between conditions were examined using Friedman test. In addition, the probability distribution of sleep stages during the 7-hour sleep episodes in each condition was examined using a sleep histogram. Sleep stage probability was calculated as the percentage of epochs (in 5-min bins) scored as either Stage N1, Stage N2, Stage N3, Stage R or Wake. For normally distributed variables related to neurobehavioural performance and self-perceived capacity, separate repeated measures ANOVAs were conducted with “condition” (immediate, delayed, split) and “time of test session” (18:30, 20:30, 22:30, 00:30, 02:30, 04:30) as within-subjects factors. For non-normally distributed variables related to neurobehavioural performance and self-perceived capacity, differences between conditions and between test sessions were examined using Friedman test. Only neurobehavioural performance and self-perceived capacity data from the second night-shift (ie, the shift following each strategy) were included in the analyses. Sphericity was examined in all ANOVAs using Mauchly’s test of sphericity. If the assumption of sphericity was violated, p values were based on Greenhouse-Geisser’s corrected degrees of freedom, but the original degrees of freedom are reported. Where appropriate, pairwise comparisons were performed with a Bonferroni correction for multiple comparisons. Effect sizes for ANOVAs were calculated using partial eta squared (η2) and interpreted as small (0.01), medium (0.06), and large (0.14). Effect sizes for Friedman tests were calculated using Kendall’s W and interpreted as small (0.2), medium (0.5), and large (0.8). For all analyses, the critical level of alpha was set at p < 0.05. Statistical analyses were performed using SPSS (Version 27, IBM, Armonk, NY) and data are presented as mean ± SEM.
Results
Sleep Prior to the First Night-Shift
There was no difference in the amount of sleep obtained on the night prior to the first night shift between conditions (χ22=3.500; p = 0.174); or in the amount of time spent in Stage N1 (χ22=3.910; p = 0.822), Stage N2 (χ22=0.667; p = 0.717), Stage N3 (F2,22=0.766; p = 0.472) or Stage R (F2,22=1.917; p = 0.175). On the night prior to the first night shift, average sleep duration was 493.5±25.2 min for the immediate condition; 526.3±8.7 min for the delayed condition; and 514.1±8.7 min for the split condition.
There was no difference in the amount of sleep obtained during the afternoon nap prior to the first night-shift in each condition (χ22=0.298; p = 0.862); or in the amount of time spent in Stage N1 (χ22=3.511; p = 0.173), Stage N2 (F2,22=1.715; p = 0.206), Stage N3 (F2,22=0.822; p = 0.447) or Stage R (χ22=0.300; p = 0.861). Average sleep duration during the 1-hour nap was 53.6±0.9 min in the immediate condition; 46.5±5.3 min in the delayed condition; and 52.6±2.1 min in the split condition.
Sleep During the Immediate, Delayed and Split Conditions
There was no effect of condition on any of the sleep measures, except for sleep onset latency (W = 0.882; large effect), the amount of Stage N3 (η2=0.510; small effect), and WASO (W = 0.333; moderate effect) (Table 1). Sleep onset latency was longer in the split condition than for both the immediate (p = 0.032) and delayed (p < 0.001) conditions; the amount of Stage N3 was greater in the split condition than for both the immediate (p < 0.001) and delayed (p = 0.042) conditions; the amount of Stage N3 was greater in the delayed condition compared to the immediate condition (p = 0.018); and WASO was lower in the split condition compared with both the immediate (p = 0.043) and delayed (p = 0.043) conditions.
 |
Table 1 Sleep Parameters Derived from Polysomnography for the Immediate, Delayed and Split Conditions |
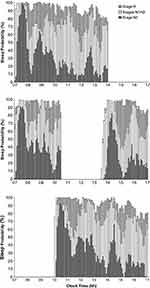
The probability distribution of sleep stages for each condition is illustrated in Figure 2. In all three conditions, Stage N3 was predominant in the first 3.5 hours of the sleep episode. In the immediate and delayed conditions, Stage N3 gradually declined over the 7-hour sleep episode. However, in the split condition there was a burst of Stage N3 at the start of the second sleep episode. Towards the end of the sleep episode in the immediate and delayed conditions, and to a lesser extent in the second sleep in the split condition, there was a gradual increase in wake.
Neurobehavioural Performance During the Immediate, Delayed and Split Conditions
There was no effect of condition on PVT RRT (χ22=0.044; p = 0.978), PVT lapses (χ22=0.605; p = 0.739), SAS RRT (χ22=0.320; p = 0.852), SAS correct number of responses (χ22=1.319; p = 0.517), DSST total attempted (χ22=1.830; p = 0.401), or DSST percentage of correct responses (χ22=1.647; p = 0.439) (Figure 3). There was a significant effect of time of test session on PVT RRT (χ25=28.608; p < 0.001; W = 0.477; moderate effect), PVT lapses (χ25=16.129; p = 0.006; W = 0.269; small effect), SAS RRT (χ25=12.714; p = 0.026; W = 0.212; small effect), and DSST total number attempted (χ25=12.602; p = 0.027; W = 0.210; small effect), but not on SAS percentage of correct responses (χ25=5.073; p = 0.407) or DSST percentage of correct responses (χ25=6.099; p = 0.297). Response time during the PVT and the SAS progressively slowed between the first and last test sessions; the number of attempts during the DSST decreased between the first and last test sessions; and the number of lapses during the PVT increased between the first and last test sessions (Figure 3).
Self-Perceived Capacity During the Immediate, Delayed and Split Conditions
There was no effect of condition on sleepiness (F2,22=0.121; p = 0.887), fatigue (F2,22=0.474; p = 0.529), alertness (F2,22=1.609; p = 0.223) or mood (χ22=0.500; p = 0.779) (Figure 3). There was an effect of time of test session on sleepiness (F2,22=47.7; p < 0.001), fatigue (F2,22=47.5; p < 0.001), alertness (F2,22=44.5; p < 0.001) and mood (χ25=39.844; p < 0.001; W = 0.664; large effect). Sleepiness, fatigue and mood disturbance increased between the first and last test sessions; and alertness decreased between the first and last test sessions (Figure 4). There were no interactions between condition and time of test session for any measures of self-perceived capacity.
Discussion
In the present study, sleep was scheduled in the break between two consecutive 12-hour night-shifts to mimic the immediate, delayed, and split sleep strategies commonly used by shiftworkers.16–19 The aim was to determine which of these strategies was most effective for maximising sleep during the break between the night shifts and sustaining performance during the second night shift. The main findings were that (i) the total amount of sleep obtained did not differ between the three strategies, but more slow wave sleep was obtained using the split strategy than the immediate and delayed strategies, (ii) it took longer to fall asleep, but there was less wake after sleep onset, using the split strategy than the immediate and delayed strategies, and (iii) neurobehavioural performance and self-perceived capacity during the night-shift did not differ between the three strategies. The results indicate that if sufficient sleep is obtained, individuals on 12-hour schedules have some flexibility when arranging their sleep times, because none of the strategies are clearly superior or inferior to the others.
A split sleep strategy is often used to obtain some of the benefits of both the immediate and delayed strategies.16 However, a potential limitation of this strategy is that it may affect the composition and restorative capacity of sleep. In the present study, the sleep opportunity in the split strategy was divided into equal halves, with one episode in the morning and one episode in the evening. Sleep duration was preserved in the split strategy and was not different from the immediate or delayed strategies. This finding is consistent with results of other laboratory studies in which split sleep strategies appear to result in similar durations of sleep to that obtained using a consolidated sleep strategy—regardless of whether the split sleep episodes are scheduled as one night-time sleep opportunity in combination with one daytime sleep opportunity32,33 or whether the split sleep episodes occur at any time of day or night.34,35 The results indicate that split sleep schedules do not appear to result in reduced quality or quantity of sleep.
The amount of slow-wave sleep obtained during 7 hours in bed differed between the immediate, delayed and split conditions. This may seem peculiar, given that the expectation – based on the two-process model of sleep/wake regulation36 – would be for a similar amount of slow-wave sleep to be obtained in sleep opportunities of similar duration after a night of sustained wakefulness. However, forced desynchrony studies have shown that (a) slow-wave sleep definitely has a strong homeostatic component such that the drive for slow-wave sleep is highest in the 1–2 hours after sleep is initiated6,37 and (b) slow-wave sleep may have a weak circadian component such that the drive for slow-wave sleep is highest near the daily maximum in core body temperature,36 which typically occurs in the mid-late afternoon. These homeostatic and circadian influences provide a good explanation for the current findings – the most amount of slow-wave sleep was obtained in the split condition, in which sleep was initiated twice and the second sleep occurred in the mid-late afternoon; the second-most amount of slow-wave sleep was obtained in the delayed sleep condition, in which sleep was initiated only once, but the second half of the sleep period occurred in the mid-late afternoon; and the least amount slow-wave sleep was obtained in the immediate condition, in which sleep was initiated only once and the sleep ended in the early afternoon. These findings are also consistent with those of a previous forced desynchrony study, which found that split sleep periods result in a greater amount of slow-wave sleep than consolidated sleep periods when total time in bed is similar.33
One of the challenges for individuals who undertake nightwork is obtaining good-quality sleep during the daytime when the wake-promoting signal from the circadian system is strong.38 In the present study, there was some evidence that the level of disruption in daytime sleep differed between the conditions. In particular, it took longer to fall asleep in the split condition than in the immediate and delayed conditions, whereas there was more wake after sleep onset in the immediate and delayed conditions than in the split condition. Longer sleep onset latency and greater wake after sleep onset are indicators of poorer sleep quality39 and the size of the between-groups effects were large and moderate, respectively. However, sleep onset latency and wake after sleep onset were in the normal ranges, and were well below the clinical/pathological thresholds, for all three conditions, so the differences between the conditions are unlikely to be meaningful in practice.39–41
Despite the differences in some of the sleep variables between sleep strategies, the participants in the present study generally slept very well during daytime sleep episodes (average sleep efficiency >90%). This value is much higher compared to sleep efficiencies reported by shiftworkers sleeping in their own homes.42 There are several explanations for this finding. First, the participants were young healthy males with no history of sleep disorders. Second, the sleeping environment was well controlled—the bedrooms were cool, dark and quiet—and may have protected participants from the usual disturbances that interfere with daytime sleep. In addition, the participants were not permitted to consume caffeine or alcohol during the protocol, both of which can negatively affect sleep.43,44 Finally, the participants were required to remain in bed for the full duration of each sleep episode. If the participants were free to choose when to get out of bed, sleep duration may have been different between conditions22,23 and this may have led to differences in performance and/or capacity during the night-shift following each strategy.
Performance and self-perceived capacity declined across the night-shift, consistent with the effects of increasing homeostatic sleep pressure and circadian misalignment.6,7,34 However, there was no difference in the rate of decline to suggest a protective effect of any of the sleep strategies. The absence of a difference in performance during a night-shift between the immediate sleep strategy and the delayed sleep strategy is in contrast with previous results.21–23 This discrepancy could be explained by the addition of bright light exposure during the 8-hour night-shifts in previous studies (which was timed to induce a phase advance and facilitate sleep in the early evening)21,22 and/or the inclusion of older adults23 who may be more suited to a delayed sleep strategy because of advanced rhythms of core body temperature and melatonin.24 It is also possible that the shorter break duration between consecutive night-shifts in the present study (12 hours) compared to the shift duration employed in previous studies (16 hours) accounts for the lack of differentiation between sleep strategies. For example, the start of the sleep episodes for a morning and delayed sleep strategy in an 8-hour night-shift (with a 16-hour break) vary by 6 hours. In the current study, the start and end times for the immediate and delayed sleeps differed by only 3 hours. The delayed sleep strategy may not be substantially better than any other strategy for shift schedules with long shifts (eg, >8 hours) because the difference in the onset times of sleep episodes between strategies is small. In future, it may be important to establish whether similar results are observed for shift lengths shorter than 12 hours.
When considering the results, it must be recognised that the study was conducted with young, healthy, male participants under controlled laboratory conditions. Outside the laboratory, sleeping environments may not always be conducive to good sleep and other factors (eg, social and family demands) may interfere with individuals’ adherence to an optimal sleep schedule. In addition, the simulated shiftwork protocol included only one day sleep and one night-shift. It is possible that a difference between the sleep strategies may not have emerged until after multiple consecutive shifts.21–23 Finally, the simulated night-shift was 12 hours in duration. In Australia, and other countries, night-shifts typically last for either 8 or 12 hours.45 It is possible that the sleep strategies could have had a different outcome on an 8-hour schedule and thus the findings here may not generalise to night-shifts of other durations.
In conclusion, this laboratory-based simulation indicates that the three major strategies used by shiftworkers to time sleep opportunities in the break between two night-shifts – immediate, delayed, split – produce similar outcomes in terms of the quantity/quality of sleep obtained during the daytime and the level of cognitive function achieved at night. Shiftworkers should be informed of the range of strategies available and encouraged to employ the strategy (or strategies) that best suits their personal preferences and/or circumstances. It is yet to be determined whether the current findings generalise to natural settings or to circumstances where shiftworkers work more than three consecutive night-shifts, spend less than 7 hours in bed between consecutive night-shifts and/or work night-shifts that are less than 12 hours in duration.
Abbreviations
PSG, polysomnography; EEG, electroencephalogram; R sleep, rapid eye movement sleep; PVT, psychomotor vigilance task; RRT, reciprocal response time; DSST, digit symbol substitution task; SAS, serial addition/subtraction task; KSS, Karolinska Sleepiness Scale; VAS, visual analogue scale.
Data Sharing Statement
The datasets generated from the current study are available from the corresponding author upon reasonable request.
Consent for Publication
Written informed consent was obtained from all participants who volunteered to take part in the study.
Acknowledgments
The authors are grateful for the time and contribution of the participants.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding
The study was financially supported by an internal merit grant from CQUniversity.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Åkerstedt T, Kecklund G, Knutsson A. Spectral analysis of sleep electroencephalography in rotating three-shift work. Scand J Work Environ Health. 1991;17(5):330–336. doi:10.5271/sjweh.1694
2. Knauth P, Landau K, Dröge C, et al. Duration of sleep depending on the type of shift work. Int Arch Occup Environ Health. 1980;46(2):167–177. doi:10.1007/BF00378195
3. Korsiak J, Tranmer J, Leung M, Borghese MM, Aronson KJ. Actigraph measures of sleep among female hospital employees working day or alternating day and night shifts. J Sleep Res. 2018;27(4):1–8. doi:10.1111/jsr.12579
4. Folkard S, Tucker P. Shift work, safety and productivity. Occup Med. 2003;53(2):95–101. doi:10.1093/occmed/kqg047
5. Niu SF, Chu H, Chen CH, et al. A comparison of the effects of fixed- and rotating-shift schedules on nursing staff attention levels: a randomised trial. Biol Res Nurse. 2012;15(4):443–450. doi:10.1177/1099800412445907
6. Wyatt JK, Cecco AR, Czeisler CA, et al. Circadian temperature and melatonin rhythms, sleep, and neurobehavioural function in humans living on a 20-h day. Am J Physiol. 1999;277(4 Pt 2):R1152–R1163. doi:10.1152/ajpregu.1999.277.4.r1152
7. Zhou X, Ferguson SA, Matthews RW, et al. Sleep, wake and phase dependent changes in neurobehavioural function under forced desynchrony. Sleep. 2011;34(7):931–941. doi:10.5665/SLEEP.1130
8. Sargent C, Darwent D, Ferguson SA, et al. Sleep restriction masks the influence of the circadian process on sleep propensity. Chronobiol Int. 2012;29(5):565–571. doi:10.3109/07420528.2012.675256
9. Paech GM, Ferguson SA, Sargent C, et al. The relative contributions of the homeostatic and circadian processes to sleep regulation under conditions of severe sleep restriction. Sleep. 2012;35(7):941–948. doi:10.5665/sleep.1956
10. Gold DR, Rogacz S, Bock N, et al. Accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82(7):1011–1014. doi:10.2105/AJPH.82.7.1011
11. Horwitz IB, McCall BP. The impact of shift work on the risk and severity of injuries for hospital employees: an analysis using Oregon workers’ compensation data. Occup Med. 2004;54(8):556–563. doi:10.1093/occmed/kqh093
12. Nielsen HB, Larsen AD, Dyreborg J, et al. Risk of injury after evening and night work – findings from the Danish working hour database. Scand J Work Environ Health. 2018;44(4):385–393. doi:10.5271/sjweh.3737
13. Muehlbach MJ, Walsh JK. The effects of caffeine on simulated night-shift work and subsequent daytime sleep. Sleep. 1995;18(1):22–29. doi:10.1093/sleep/18.1.22
14. Campbell SS, Dawson D. Enhancement of nighttime alertness and performance with bright ambient light. Physiol Behav. 1990;48(2):317–320. doi:10.1016/0031-9384(90)90320-4
15. Smith SS, Kilby S, Jorgensen G, et al. Napping and nightshift work: effects of a short nap on psychomotor vigilance and subjective sleepiness in health workers. Sleep Biol Rhythms. 2007;5(2):117–125. doi:10.1111/j.1479-8425.2007.00261.x
16. Åkerstedt T. Is there an optimal sleep-wake pattern in shift work? Scand J Work Environ Health. 1998;24:18–27.
17. Harrison EM, Easterling AP, Yablonsky AM, Glickman GL. Sleep-scheduling strategies in hospital shiftworkers. Nat Sci Sleep. 2021;13:1593–1609. doi:10.2147/NSS.S321960
18. Roach GD, Dawson D, Reid K, et al. The time-of-day that breaks occur between consecutive duty periods affects the sleep strategies used by shiftworkers. Chronobiol Int. 2016;33(6):653–656. doi:10.3109/07420528.2016.1167716
19. Tepas DI. Shiftworker sleep strategies. J Human Ergol. 1982;1:325–336.
20. Knauth P, Rutenfranz J. Duration of sleep related to the type of shift work. In: Reinberg A, Vieux N, Andlauer P, editors. Night and Shift Work: Biological and Social Aspects. Oxford, UK: Pergamon Press; 1981:161–168.
21. Santhi N, Aeschbach D, Horowitz TS, et al. The impact of sleep timing and bright light exposure on attentional impairment during night work. J Biol Rhythms. 2008;23(4):341–352. doi:10.1177/0748730408319863
22. Chinoy ED, Harris MP, Kim MJ, et al. Scheduled evening sleep and enhanced lighting improve adaptation to night shift work in older adults. Occup Environ Med. 2016;73(12):869–876. doi:10.1136/oemed-2016-103712
23. Isherwood CM, Chinoy ED, Murphy AS, et al. Scheduled afternoon-evening sleep leads to better night shift performance in older adults. Occup Environ Med. 2020;77(3):179–184. doi:10.1136/oemed-2019-105916
24. Dijk D-J, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17(3):285–311. doi:10.1081/CBI-100101049
25. Ruehland WR, O’Donoghue FJ, Pierce RJ, et al. The 2007 AASM recommendations for EEG electrode placement in polysomnography: impact on sleep and cortical arousal scoring. Sleep. 2011;34(1):73–81. doi:10.1093/sleep/34.1.73
26. Iber C, Ancoli-Israel S, Chesson AL, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications.
27. Wechsler D. Wechsler Adult Intelligence Scale–Revised. New York, NY: Psychological Corporation; 1981.
28. Åkerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Intern J Neurosci. 1990;52(1–2):29–37. doi:10.3109/00207459008994241
29. Dorrian J, Lamond N, Holmes AL, et al. The ability to self-monitor performance during a week of simulated night shifts. Sleep. 2003;26(7):871–877. doi:10.1093/sleep/26.7.871
30. Samn SW, Perelli P. Estimating aircrew fatigue: a technique with application to airlift operations. Brooks AFN, TX: USAF School of Aerospace Medicine 1982. Report No. 82-21, ADA 125319; 1982.
31. McNair D, Lorr M, Droppleman L. Profile of Mood States Manual. San Diego, CA: Educational and Industrial Testing; 1971.
32. Mollicone DJ, Van Dongen HPA, Dinges DF. Optimizing sleep/wake schedules in space: sleep during chronic nocturnal sleep restriction with and without diurnal naps. Acta Astronaut. 2007;60(4–7):354–361. doi:10.1016/j.actaastro.2006.09.022
33. Roach GD, Zhou X, Darwent D, et al. Are two halves better than one whole? a comparison of the amount and quality of sleep obtained by health adult males living on split and consolidated sleep-wake schedules. Accid Anal Prev. 2017;99:428–433. doi:10.1016/j.aap.2015.10.012
34. Kosmadopoulos A, Sargent C, Darwent D, et al. The effects of a split sleep-wake schedule on neurobehavioral performance and predictions of performance under conditions of forced desynchrony. Chronobiol Int. 2014;31(10):1209–1217. doi:10.3109/07420528.2014.957763
35. Jackson ML, Banks S, Belenky G. Investigation of the effectiveness of a split sleep schedule in sustaining sleep and maintaining performance. Chronobiol Int. 2014;31(10):1218. doi:10.3109/07420528.2014.957305
36. Borbély. AA. A two-process model of sleep regulation. Hum Neurobiol. 1982;1(3):195–204.
37. Dijk D-J, Duffy JF, Riel E, et al. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(2):611–627. doi:10.1111/j.1469-7793.1999.0611v.x
38. Czeisler CA, Weitzman E, Moore-Ede MC, et al. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210(4475):1264–1267. doi:10.1126/science.7434029
39. Ohayon M, Wickwire EM, Hirshkowitz M, et al. National sleep foundation’s sleep quality recommendations: first report. Sleep Health. 2017;3(1):6–19. doi:10.1016/j.sleh.2016.11.006
40. Carskadon MA, Dement WC. Monitoring and staging human sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine.
41. Ohayon MM, Carskadon MA, Guillenminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to healthy individuals: developing normative sleep values across the lifespan. Sleep. 2004;27(7):1255–1273. doi:10.1093/sleep/27.7.1255
42. Lajoie P, Aronson KJ, Day A, et al. A cross-sectional study of shift work, sleep quality and cardiometabolic risk in female hospital employees. BMJ Open. 2015;5(3):e007327. doi:10.1136/bmjopen-2014-007327
43. Carrier J, Fernando-Bolanos M, Robillard R, et al. Effects of caffeine are more marked on daytime recovery sleep than on nocturnal sleep. Neuropsychopharmacol. 2007;32(4):964–972. doi:10.1038/sj.npp.1301198
44. Feige B, Gann H, Brueck R, et al. Effect of alcohol on polysomnographically recorded sleep in healthy subjects. Alcohol Clin Exp Res. 2006;30(9):1527–1537. doi:10.1111/j.1530-0277.2006.00184.x
45. Ferguson SA, Dawson D. 12-h or 8-h shifts? it depends. Sleep Med Rev. 2012;16(6):519–528. doi:10.1016/j.smrv.2011.11.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.