Back to Journals » Infection and Drug Resistance » Volume 16
Time to Sputum Culture Conversion and Its Predictors Among Multidrug Resistant Tuberculosis Patients in Tigray, Northern Ethiopia: Retrospective Cohort Study
Authors Weldemhret L , Atsbaha AH, Bekuretsion H , Desta A , Legesse L, Kahsay AG , Hagos D
Received 22 March 2023
Accepted for publication 25 May 2023
Published 9 June 2023 Volume 2023:16 Pages 3671—3681
DOI https://doi.org/10.2147/IDR.S413495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Letebrhan Weldemhret,1 Ataklti Hailu Atsbaha,1 Hadish Bekuretsion,1 Abraham Desta,1 Lemlem Legesse,1 Atsebaha Gebrekidan Kahsay,2 Dawit Hagos2
1Tigray Health Research Institute, Mekelle, Tigray, Ethiopia; 2Department of Medical Microbiology and Immunology, College of Health Sciences, Mekelle, University, Mekelle, Tigray, Ethiopia
Correspondence: Letebrhan Weldemhret, Email [email protected]
Background: Sputum culture conversion status is a cardinal index of treatment response and patient outcome for MDR TB patients on longer anti-TB drugs. But, there is limited information on time to sputum culture conversion of MDR TB patients on a longer anti-TB treatment regimen. Therefore, this study aimed to evaluate time to sputum culture conversion and its predictors among MDR TB patients in Tigray, Northern Ethiopia.
Methods: A retrospective cohort study was conducted from January 2017 through September 2020 among MDR TB patients in Tigray, Northern Ethiopia. Demographic and clinical characteristics including bacteriological data were extracted from the TB registration book and electronic database in Tigray Health Research Institute. Statistical analysis was performed using SPSS version 25. The time to initial sputum culture conversion was analyzed using the Kaplan–Meier method. Bivariate and multivariate Cox proportional hazards regression analyses were used to identify predictors for culture conversions. P < 0.05 was considered statistically significant.
Results: A total of 294 eligible study participants with a median age of 30 years (IQR: 22.75– 40) were included. The participants were followed for a total of 1066.7 person months. Sputum culture conversion was achieved in 269 (91%) of the study participants. The median time of sputum culture conversion was 64 days (IQR: 49– 86). In our multivariate model, HIV-positive (aHR=1.529, 95% CI: 1.096– 2.132, P=0.012), patients new to anti-TB treatment (aHR=2.093, 95% CI: 1.100– 3.982, P=0.024) and baseline AFB smear grading of +1 (aHR=1.982, 95% CI: 1.428– 2.750, P=0.001) significantly affected time to initial sputum culture conversion.
Conclusion: The median time of culture conversion was 64 days. Moreover, the majority of the study participants achieved culture conversion within the first six months of treatment commencement, which supports predefined standard treatment durations.
Keywords: sputum, smear, culture, conversion, tuberculosis, multi-drug resistance, Ethiopia
Background
Tuberculosis (TB) is one of the leading causes of death from a single infectious agent, higher even than HIV/AIDS.1,2 Approximately 4000 people die from TB and nearly 30,000 people fall ill daily.1 Globally, 10 million cases of TB were reported in 2019.1,3 According to the World Health Organization (WHO), the largest numbers of new TB cases were reported in the Southeast Asian region (44%), followed by theAfrican region (25%) and the Western Pacific region (18%).1 This data emphasized the need to tackle achieving the global target set by 2030 for a 90% reduction in the number of TB deaths and an 80% reduction in the TB incidence rate.1
Without treatment, the mortality rate from TB is high.1,3 An estimated 70% of individuals with sputum smear-positive pulmonary TB die within 10 years of being diagnosed, if left untreated.1 Different stakeholders, including respective high burden countries, are working very hard on TB elimination and decreasing emerging drug resistant-TB; however, multidrug-resistant (MDR) TB remains a public health concern and a health security threat globally.3–5 About 3.3% of new cases and 18% of previously treated cases were reported with MDR/RR TB in 2020.2,3 Of the patients on MDR TB treatment, about 57% were successfully treated worldwide.2,3
Multidrug resistant TB patients require two phases of treatment: the intensive phase, which suggests 8 months of treatment follow up, and the continuous phase, which can last up to 20 months until treatment completion.1,2,6,7 Moreover, shifting MDR TB patients on an anti-TB treatment regimen from intensive phase to the continuation phase is based on bacteriological status of patients’ sputum culture.7,8 Multidrug-resistant TB treatment management includes long-term drug regimens, expensive and toxic regimens or creating potential adverse drug reactions; patients are also prone to drug-resistant infections.2,6,9,10 Appropriate monitoring of responses to anti-TB treatment is vital to ensure all patients are responding to the prescribed treatment and achieving favorable treatment outcomes.6
Sputum culture is regularly checked during entire treatment period to measure prognostic treatment outcome.6,9,11–13 Based on World Health Organization guidelines, culture conversion testing is preferable within the first six months of treatment for MDR TB patients.4,12 The duration may also extended up to 9 months at the intensive phase for individuals with no culture conversion despite receiving treatment.14–17 Delayed or lack of culture conversion indicates poor treatment response, and indicates the persistent possibility of MTB bacilli transmission from the patient to other people.15 One study indicated the optimum time for sputum culture conversion to be between four and six months to predict prognostic treatment outcome.18 However, other studies demonstrated that patients with identified risk factors remained culture positive even during prolonged anti-TB treatment.14,16,18–20 So far there are studies on time to culture conversion, however the conversion rate and contributing factors significantly vary among findings.10,18,19,21,22 The information available on these studies is also controversial.6,17,23,24 To our knowledge, there is no previous finding regarding this study in the region. Therefore, this study was intended to determine the time to sputum culture conversion, and related factors among MDR TB patients in Tigray, Northern Ethiopia.
Materials and Methods
Study Design, Setting and Participants
A retrospective cohort study was conducted from January 2017 to September 2020 in Tigray region, which is located in Northern Ethiopia. The region has seven administrative zones, one special zone, 52 districts and 799 kebeles. Tigray has an estimated total population of 5.1 million people, over an area of 50,078.64 square kilometers.25 The health system of the region includes 2 specialized referral hospitals, 15 general hospitals, 22 primary hospitals, 218 health centers, 710 health posts, one research institute and 500 private health facilities, which are serving the population of Tigray and neighboring regions of Afar and Amhara. The region has seven treatment initiation centers (TIC) for MDR TB patients. Moreover, Tigray Health Research Institute (THRI) is serving as a reference laboratory for MDR TB TICs in the region, including neighboring areas for further bacteriological MDR TB confirmation and treatment follow-up monitoring. As part of routine care, regular sputum specimens are collected from MDR/RR TB or presumptive MDR TB patients in TIC, and transported to THRI for culture and further drug susceptibility tests (DSTs) to monitor treatment outcome during their entire treatment follow up. Routine sputum culture is performed monthly for the first six months of treatment, and every other month up to treatment completion in Tigray Health Research Institute. MDR/RR TB patients who were bacteriologically confirmed at the commencement of treatment and enrolled on a longer anti-TB drug regimen, and had taken treatment for at least two months from January 2017 through September 2020 were included in the study.
Sampling Technique
All bacteriological confirmed MDR TB patients who had a baseline culture positive result and enrolled on a longer anti-TB treatment regimen and have received treatment for at least two months following an initial sputum culture positive result during the study period were included in the study. Patients who had incomplete data, and culture positive MDR TB patients followed for less than two months and those with a culture negative result at baseline were excluded. Moreover, patients diagnosed with MDR TB but who did not start treatment were excluded.
Data Collection Procedure
Demographic characteristics like sex and age, and clinical characteristics of HIV status, history of previous treatment category and history of anti-TB regimen receipt were collected. Bacteriological data for a baseline sputum smear and culture status, baseline smear grading, and follow-up culture results were also extracted from TB registration book and electronic database in Tigray Health Research Institute. Individuals who did not experience culture conversion were censored earlier than their last culture date or treatment outcome date. Time to sputum culture conversion was defined as the time from commencement of treatment to at least two consecutive negative culture results 30 days apart after treatment initiation.12 New to anti-TB treatment was indicated for patients who had no prior anti-TB treatment uptake or who had history of anti-TB treatment receipt of less than one month, whereas previously treated cases were defined as patients who have ever taken anti-TB treatment for one month or more. Moreover, sputum smear grading was collected based on the number of AFB (acid fast bacilli) observed and reported; negative indicated no AFB/100 high power field (HPF), actual (1-9/100 HPF), 1+ (10-99AFB/100HPF), 2+ (1-9AFB/HPF), and 3+ (> 9AFB/HPF).
Statistical Analysis
Descriptive statistics and time to initial sputum culture conversion were calculated using SPSS version 25. Time to initial sputum culture conversion was analyzed using Kaplan–Meier method. Patients whose sputum cultures did not convert before the last follow up were censored. Moreover, MDR TB patients who stopped treatment while culture positive, and study time completion before culture conversion were censored. Survival times across the strata were evaluated with log rank test. Bivariate and multivariate Cox proportional hazards regression analyses were used to identify predictors of culture conversion. Time of entry into the cohort was the date of diagnosis for bacteriological confirmed MDR TB within the study period, with at least two sputum culture follow-up results, and exit time was the date of collecting the first negative culture result.12 The adjusted hazard ratio (AHR), with its respective 95% confidence interval (CI), was reported to show the strength of association. Confounding factors and interactions were assessed in multivariate models. P <0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
This study included a total of 294 MDR TB patients. Most of the participants were male 174 (59%). The median age of the study participants was 30 years (IQR: 22.75–40). Of the study participants, 280 had known HIV status. Of these, 54 (18%) were positive for HIV infection. Regarding treatment history with anti-TB drugs, 95 (32%) of the participants were new to anti-TB drugs and 199 (68%) had prior history of anti-TB drugs receipt (Table 1). Majority of the study participants 150 (65%) had prior first-line anti-TB drug treatment uptake. Of the study participants, 243 (83%) were positive for baseline AFB smear status (Table 1).
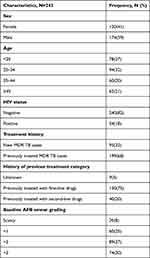 |
Table 1 Demographic and Clinical Characteristics of the Study Participants |
Time to Sputum Culture Conversion
Sputum culture conversion was achieved in 269 (91%) of the study participants. Twenty-five (9%) of the participants did not convert to culture negative: 4 treatment failures, 10 defaulters (lost to follow up), and 11 who did not convert to culture negative by the end of the study period. The participants were followed for a total of 1066.7 person months (88.9 person years). Amongst the participants with sputum culture conversion, 276 (94%) had shown a culture conversion within the first six months of treatment initiation. The median time to initial sputum culture conversion was 64 days (interquartile range (IQR): 49–86). The median times to initial sputum culture conversion in HIV-positive and -negative MDR TB patients were 55 days (IQR: 44–82) and 65 days (IQR: 51–90), respectively. Individuals new to anti-TB treatment had shown a median time of 55 days (IQR: 40–72) time to initial sputum culture conversion. MDR TB patients who had previously received anti-TB treatment receipt showed a median time of 69 days (IQR: 52–96) time to initial sputum culture conversion. The median time to sputum culture conversion of patients who had a baseline AFB smear grading of +1 was 55 days (IQR: 40–70).
The Kaplan–Meier survival curve showed faster and steeper time to initial sputum culture conversion within the first 4 months of treatment commencement, and there was little change thereafter (Figure 1). The cumulative probability of survival on time to initial sputum culture conversion was 0.85 at the end of one month, 0.59 at two months, 0.24 at 3 months, 0.19 at 4 months, 0.08 at six months and 0.02 at the end of 8 months (Figure 1).
 |
Figure 1 Kaplan–Meier survival plot of time to initial sputum culture conversion. |
Predictors of Time to Sputum Culture Conversion
Being in the younger age groups, HIV-positive, new to anti-TB treatment, and having a baseline AFB smear grading of actual, +1, and +2 were predictors of time to initial sputum culture conversion in bivariate analysis (Table 2). In the multivariate model, being HIV-positive (aHR=1.529, 95% CI: 1.096–2.132, P=0.012), new to anti-TB treatment (aHR=2.093, 95% CI: 1.100–3.982, P=0.024) and having a baseline AFB smear grading of +1 (aHR=1.982, 95% CI: 1.428–2.750, P=0.001) were significantly associated with time to initial sputum culture conversion (Table 2). Patients new to anti-TB treatment were twice as likely to experience a shorter time to initial sputum culture conversion compared to patients who had history of anti-TB drugs uptake. MDR TB patients with a baseline AFB smear grading of +1 were twice as likely to experience decreased time to initial sputum culture conversion compared with those individuals with a baseline AFB smear grade of +3. The time to initial sputum culture conversion was also shorter among individuals whose baseline AFB smear grading of scanty and +2, although no significant association was observed. HIV-positive MDR TB patients were 1.5 times more likely to experience decreased time to initial sputum culture conversion compared with those who were HIV-negative (Table 2).
 |
Table 2 Bivariate and Multivariate Cox Proportional Hazard Regression Analysis of Determinants of Time to Sputum Culture Conversion Among MDR TB Patients |
The log rank test showed statistically significant survival distribution between HIV-positive and HIV-negative MDR TB patients: X2(2)=5.989, P=0.014 (Figure 2). The observed sputum culture conversion time was also rapid among HIV-positive individuals compared with those who were HIV-negative (Figure 2).
 |
Figure 2 Time to initial sputum culture conversion comparisons by HIV status. |
Discussion
Time to sputum culture conversion following treatment commencement is a key index of treatment success in patients who are on long-term anti-TB drug regimen follow up. This study emphasized the optimum time to initial sputum culture conversion of MDR TB patients on a prolonged anti-TB treatment regimen whose baseline sputum culture results were positive. The median age of the study participants was 30 years. This is consistent with other, similar studies.22,26 Of the study participants, 18% were co-infected with HIV. Most of the study participants (68%) had been previously treated with anti-TB drugs. This finding is comparable with other studies.18,22,26
The study participants were followed for a total of 1066.7 person months. Sputum culture conversion was achieved in 91% of the study participants who were under follow up during the study period. The median time to initial culture conversion was 64 days. This is in line with other findings.10,27–29 However, the median time to sputum culture conversion was shorter compared with other studies.19,20,26,30 The variation time to culture conversion is related to the way the outcome variable is defined, as the time to culture conversion is considered by two consecutive negative culture results, 30 days apart.6,12 But other studies may consider a period beyond this cut off value, and others may have taken the first initial sputum culture conversion.19,20,23 The median time to initial sputum culture conversion was decreased among HIV co-infected individuals compared to those who were HIV-negative. This is similar to other previous studies.26,31 Rapid time to sputum culture conversion is essential for treatment success and thus to decrease the transmission of the disease from the patient to other people.
Moreover, the majority (94%) of the study participants attained initial sputum culture conversion within the first six months of effective treatment. This finding is consistent with other studies.10,18,22,28 The Kaplan–Meier curve indicated rapid and steeper time to initial sputum culture conversion within the first 4 months of treatment commencement, and little change thereafter. Likewise, the cumulative probability of survival on time to initial sputum culture conversion was 0.85 at one month, 0.59 at two months, 0.19 at 4 months, 0.08 at six months and 0.02 at the end of 8 months. This is comparable with other findings.22,28 This shows that there is little change in survival time to initial sputum culture conversion after six months, which supports the treatment duration recommended by WHO.11,12 Though this needs a further well-designed prospective study, the possible reason for the lower conversion rate later is that the patient may be immune compromised and less likely to experience culture conversion on any drug regimen. Other studies found that patients identified with indicators of severe diseases experienced delayed time to culture conversion even on longer anti-TB drug regimens.19,20 Lack of sputum culture conversion at the end of the stated months by the programmer following anti-TB treatment implies continuous transmission of the infection to the community, particularly in those following poor infection prevention control practices.
Rapid time to culture conversion is the main indicator of treatment efficacy in patients following longer anti-TB treatment regimens. In our study, being new to anti-TB drugs and HIV-positive and having a baseline AFB smear grading were identified as possible factors affecting time to initial sputum culture conversion. Patients new to anti-TB treatment showed shorter time to initial sputum culture conversion compared to patients who had history of anti-TB drug receipt. This is consistent with other studies.19,28 Moreover, research findings show that patients retreated with anti-TB drugs have high risk of poor treatment outcome compared to individuals with less exposure to prior anti-TB drugs.32–34 Likewise, a systematic review suggested patients with no prior anti-TB treatment receipt showed successful treatment outcomes compared to patients with previous anti-TB drug receipt.35 A WHO report revealed that previously treated TB patients are more likely to be identified with poor treatment outcomes and a high risk of drug resistant acquisition when retreated with ant-TB drugs compared to new TB cases. This emphasized that greater culture conversion is attained among new TB cases than in previously treated TB cases, as culture conversion is the key indicator of successful treatment outcome.2,3,6,12
MDR TB patients whose baseline AFB smear grading of +1 showed decreased time to initial sputum culture conversion compared with those individuals with a baseline AFB smear grading of +3 or high bacillary density. This implied that individuals with lower bacillary density on their smear experienced rapid smear conversion and earlier clearance of the infection, with a short intensive period after treatment initiation. Likewise, sputum culture conversion time is decreased as the bacillary load on their sputum smear decreases. This is comparable with other findings.19,36,37 On the other hand, other studies indicated that patients diagnosed with a high bacillary load before treatment initiation had a reduced possibility of culture conversion and experienced a prolonged time to culture conversion, with a longer isolation period after treatment commencement.22,36,38–40 These findings emphasize the importance of prompt detection and receipt of appropriate treatment for MDR TB patients.
MDR TB patients with HIV infection showed rapid time to initial sputum culture conversion compared with HIV-negative individuals. This finding may be attributed to the pauci-bacillary state of TB and HIV coinfection, with a lower amount of bacilli per milliliter of sputum sample in HIV-infected patients.41 This is consistent with previous findings.26,28 The survival distribution between patients with and without HIV was also statistically significant (log rank, P=0.014). A possible explanation for early culture conversion among HIV-infected patients may be the fully integrated MDR-TB/HIV care they receive, notably in high burden settings that prioritize ART initiation, continuous follow up and patient management. In contrast to our finding, one study showed that HIV co-infected individuals experience significantly longer time to culture conversion compared with HIV-negative individuals.19 On the other hand, other studies have not found any significant difference in time to culture conversion between those with HIV infection and those without.27,30,31,42 To clear up these contradictory findings, a controlled prospective interventional study is needed. This study is not without limitations, mainly that it was unable to determine the rate of and time to sputum culture conversion because the study design is retrospective and failed to identify such a pattern. Moreover, the culture conversion time was not directly related to treatment outcome status due to data scarcity.
Conclusions
The median time to initial culture conversion of MDR TB is slightly lower than the WHO three-month guideline for drug-resistant TB. The study participants in large part achieved culture conversion within the first six months of treatment commencement. However, rapid and steeper times to culture conversion were observed within the first four months of treatment initiation. HIV co-infected individuals, patients with less exposure to prior anti-TB drugs and individuals with fewer baseline bacilli on their smear experienced shorter times to initial sputum culture conversion.
Data Sharing Statement
All data supporting the findings of this study are available from Tigray Health Research Institute. Moreover, the data are available from Tigray Health Research Institute’s Institutional Review Board (IRB) via [email protected] upon reasonable request.
Ethical Approval and Informed Consent
Ethical clearance and approval was obtained from Tigray Health Research Institute’s Institutional Review Board (THRI-IRB). Likewise, a permission letter was obtained from Tigray Health Research Institute. This study has no direct contact with patients or patient samples, as all data used are available on Tigray Health Research Institute’s database from routine sputum smears and culture follow-up activities. The study was also conducted in compliance with Helsinki Declaration (www.wma.net/en/30publications/10policies/b3/).
Patient Data Confidentiality and Compliance
This study was conducted in accordance with institutional ethical standards and with the 1964 Helsinki Declaration. Each study participant was identified with a unique code before the data extraction process started. Privacy and confidentiality of the participants were kept secure.
Acknowledgments
The authors are grateful to the staff working in tuberculosis bacteriology section for their cooperation in accessing the required data. We are also thankful to the Tigray Health Research Institute for giving us permission to conduct the study.
Author Contributions
All authors made a significant contribution to the conception, study design, execution, and acquisition of data, analysis and interpretation. All authors were involved in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there is no competing interest.
References
1. Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/336069.
2. World Health Organization. World TB day; 2020. Available from: www.who.int›campaigns›world-tb-day.
3. Chakayaa J, Khan M, Ntoumie F, et al. Global tuberculosis report 2020 – reflections on the global TB burden, treatment and prevention efforts. Int J Infect Dis. 2020. doi:10.1016/j.ijid.2021.02.107
4. World Health Organizations. Global Tuberculosis Report. Geneva: World Health Organization; 2018. Available from: https://apps.who.int/iris/handle/10665/274453.
5. Federal Democratic Republic of Ethiopia, Ministry of Health. Health Sector Transformation Plan –I.annual Performance Report 2016/17. Addis Ababa, Ethiopia: FMOH; 2017:53–54.
6. Federal Democratic Republic of Ethiopia Ministry of Health. Guideline for Management of TB, DR-TB and Leprosy in Ethiopia.
7. Federal Ministry of Health Ethiopia. Guidelines for Clinical and Programmatic Management of TB, Leprosy and TB/HIV in Ethiopia.
8. Parsons LM, Somosko¨vi K, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24(2):314–350. doi:10.1128/CMR.00059-10
9. American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J RespirCrit Care Med. 2000;161(4):1376–1395. doi:10.1164/ajrccm.161.4.16141
10. Assemiea MA, Alenea M, Petruckab P, Leshargied CT, Ketemaa DB. Time to sputum culture conversion and its associated factors among multidrug-resistant tuberculosis patients in Eastern Africa: a systematic review and meta-analysis. Int J Infect Dis. 2020;98:230–236. doi:10.1016/j.ijid.2020.06.029
11. World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision: updated December 2014 and January 2020. Geneva: World Health Organization; 2020. Available from: https://apps.who.int/iris/handle/10665/79199.
12. World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health organization; 2014. Available from: https://apps.who.int/iris/handle/10665/130918.
13. Kakchapati S, Gyawali BN, Jha RK, Choonpradu C. Treatment outcome of multidrug-resistant Mycobacterium tuberculosis in Nepal. Asia Pac J Public Health. 2014;24:631–640. doi:10.1177/1010539511408067
14. Federal Ministry of Health Ethiopia. Manual for Tuberculosis, Leprosy and TB/HIV Prevention and Control Program.
15. Ghimire S, Karki S, Maharjan B, et al. Treatment outcomes of patients with MDR-TB in Nepal on a current programmatic standardised regimen: retrospective single-centre study. BMJ Open Resp Res. 2020;7:e000606. doi:10.1136/bmjresp-2020-000606
16. Molie T, Teklemariam Z, Klinkenberg E, et al. Intensive phase treatment outcome and associated factors among patients treated for multi drug resistant tuberculosis in Ethiopia: a retrospective cohort study. BMC Infect Dis. 2019;19(1). doi:10.1186/s12879-019-4411-7
17. Su WJ, Feng JY, Chiu YC, Huang SF, Lee YC. Role of 2-month sputum smears in predicting culture conversion in pulmonary tuberculosis. Eur Respir J. 2011;37:376–383. doi:10.1183/09031936.00007410
18. Alene KA, Viney K, Yi H, et al. Comparison of the validity of smear and culture conversion as a prognostic marker of treatment outcome in patients with multidrug-resistant tuberculosis. PLoS One. 2018;13(5):e0197880. doi:10.1371/journal.pone.0197880
19. Kurbatova EV, Gammino VM, Bayona J, et al. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16(10):1335–1343. doi:10.5588/ijtld.11.0811
20. Liu Q, Lu P, Martinez L, et al. Factors affecting time to sputum culture conversion and treatment outcome of patients with multidrug-resistant tuberculosis in China. Liu et al. BMC Infect Dis. 2018;18:114. doi:10.1186/s12879-018-3021-0
21. CalderwoodI CJ, WilsonI JP, FieldingI KL, et al. Dynamics of sputum conversion during effective tuberculosis treatment: a systematic review and meta-analysis. A systematic review and meta-analysis. PLoS Med. 2021;8(4):e1003566. doi:10.1371/journal.pmed.1003566
22. Akalu TY, Muchie KF, Gelaye KA, Munderloh UG. Time to sputum culture conversion and its determinants among multi-drug resistant tuberculosis patients at public hospitals of the Amhara Regional State: a multicenter retrospective follow up study. A multicenter retrospective follow up study. PLoS One. 2018;13(6):e0199320. doi:10.1371/journal.pone.0199320
23. Horne DJ, Johnson CO, Oren E, Spitters C, Narita M. How soon can smear positive TB patients be released from inpatient isolation? Infect Control Hosp Epidemiol. 2010;31(1):78–84. doi:10.1086/649022
24. Li Q, MSa L, MSa M, et al. Time to sputum culture conversion and its predictors among patients with multidrug resistant tuberculosis in Hangzhou, China. A retrospective cohort study. Medicine. 2020;50:e23649. doi:10.1097/MD.0000000000023649
25. Population projection for Ethiopia, 2007-2037. Addis Abeba: Central Statistical Agency; 2013. Available from: searchworks.Standard. edu>view>10980513.
26. Shibabaw A, Gelaw B, Wang SH, Tessema B. Time to sputum smear and culture conversions in multidrug resistant tuberculosis at University of Gondar Hospital, Northwest Ethiopia. PLoS One. 2018;13(6):e0198080. doi:10.1371/journal.pone.0198080
27. Rieu R, Chang C, Collin SM, et al. Time to detection in liquid culture of sputum in pulmonary MDR-TB does not predict culture conversion for early discharge. J Antimicrob Chemother. 2015;71(3):803–806. doi:10.1093/jac/dkv407
28. Tierney DB, Franke MF, Becerra MC, et al. Time to culture conversion and regimen composition in multidrug-resistant tuberculosis treatment. PLoS One. 2014;9:e108035. PMID: 25238411. doi:10.1371/journal.pone.0108035
29. Kim J, Kwak N, Lee HY, et al. Effect of drug resistance on negative conversion of sputum culture in patients with pulmonary tuberculosis. Int J Infect Dis. 2016;42:64–68. PMID: 26692454. doi:10.1016/j.ijid.2015.11.018
30. Velayutham B, Nair D, Kannan T, et al. Factors associated with sputum culture conversion in multidrug-resistant pulmonary tuberculosis. Int J Tuberc Lung Dis. 2016;20(12):1671–1676. PMID: 27931345. doi:10.5588/ijtld.16.0096
31. Hafkin J, Modongo C, Newcomb C, et al. Impact of the human immunodeficiency virus on early multidrug-resistant tuberculosis treatment outcomes in Botswana. Int J Tuberc Lung Dis. 2013;17(3):348–353. doi:10.5588/ijtld.12.0100
32. Ma X, Mirutse G, Bayray A, Fang M, Ehtesham HS. Tuberculosis treatment outcome: the case of women in Ethiopia and China, ten-years retrospective cohort study. PLoS One. 2019;14(11):e0219230. doi:10.1371/journal.pone.0219230
33. Senedu B, Yimer GS. G tuberculosis case notification and treatment outcomes in West Gojjam Zone, Northwest Ethiopia: a five-year retrospective study. J Tuberc Res. 2016;4:23–33. doi:10.4236/jtr.2016.41004
34. Joseph P, Rao Desai VB, Mohan NS, et al. Outcome of standardized treatment for patients with MDR-TB from Tamil Nadu, India. Indian J Med Res. 2011;133:529–534. PMID: 21623039.
35. Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM, Pai M. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009;4(9):e6914. doi:10.1371/journal.pone.0006914
36. Caetano Mota P, Carvalho A, Valente I, Braga R, Duarte R. Predictors of delayed sputum smear and culture conversion among a Portuguese population with pulmonary tuberculosis. Rev Port Pneumol. 2012;18(2):72–79. doi:10.1016/j.rppneu.2011.12.005
37. Lee HY, Chae KO, Lee CH, et al. Culture conversion rate at 2 months of treatment according to diagnostic methods among patients with culture-positive pulmonary tuberculosis. PLoS One. 2014;9(8):e103768. PMID: 25105410. doi:10.1371/journal.pone.0103768
38. Kanda R, Nagao T, Tho NV, et al. Factors affecting time to sputum culture conversion in adults with pulmonary tuberculosis: a historical cohort study without censored cases. PLoS One. 2015;10(11):e0142607. doi:10.1371/journal.pone.0142607
39. Qazi F, Khan U, Khowaja S, et al. Predictors of delayed culture conversion in patients treated for multidrug-resistant tuberculosis in Pakistan. Int J Tuberc Lung Dis. 2011;15(11):1556. doi:10.5588/ijtld.10.0679
40. Rodriguez M, Monedero I, Caminero JA, et al. Successful management of multidrug-resistant tuberculosis under programme conditions in the Dominican Republic. Int J Tuberc Lung Dis. 2013;17:520–525. doi:10.5588/ijtld.12.0481
41. Johnson JL, Vjecha MJ, Okwera A, et al. Impact of human immunodeficiency virus type-1 infection on the initial bacteriologic and radiographic manifestations of pulmonary tuberculosis in Uganda. Makerere university-case western reserve university research collaboration. Int J Tuberc Lung Dis. 1998;2:397–404.
42. Brust JCM, Lygizos M, Chaiyachati K, et al. Culture conversion among HIV co-infected multidrug- resistant tuberculosis patients in Tugela Ferry, South Africa. PLoS One. 2011;6:e15841. PMID: 21253585. doi:10.1371/journal.pone.0015841
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
