Back to Journals » Journal of Inflammation Research » Volume 16
Time-Related Vascular Inflammatory Response to COVID-19 Assessed by 18F-FDG PET/CT in Follow-Up Tumor Patients
Authors Lin R , Yu J, Tian A, Wang X, Yuan X, Xu W, Xie W
Received 25 April 2023
Accepted for publication 12 July 2023
Published 24 July 2023 Volume 2023:16 Pages 3109—3117
DOI https://doi.org/10.2147/JIR.S415288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Video abstract presented by Wenli Xie.
Views: 52
Runlong Lin,1,2 Jing Yu,2 Aijuan Tian,2 Xiaomei Wang,2 Xin Yuan,2 Wengui Xu,1 Wenli Xie3
1Department of Molecular Imaging and Nuclear Medicine, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, Tianjin, People’s Republic of China; 2Department of Nuclear Medicine, The Second Hospital of Dalian Medical University, Dalian, People’s Republic of China; 3Department of Cardiovascular Medicine, The Second Hospital of Dalian Medical University, Dalian, People’s Republic of China
Correspondence: Wengui Xu, Department of Molecular Imaging and Nuclear Medicine, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Tianjin Key Laboratory of Cancer Prevention and Therapy, Tianjin’s Clinical Research Center for Cancer, No. 1 Huanhu West Road, Tianjin, 300060, People’s Republic of China, Tel +86-22-23340123, Email [email protected] Wenli Xie, Department of Cardiovascular Medicine, The Second Hospital of Dalian Medical University, No. 467 Zhongshan Road, Dalian, Liaoning, 116023, People’s Republic of China, Tel +86-411-84671291, Email [email protected]
Purpose: This study aimed to assess COVID-19’s effects on vascular inflammatory response, by evaluating 18-Fluorodeoxyglucose (18F-FDG) uptake via positron emission tomography/computed tomography (PET/CT) in the artery of diffuse large B cell lymphoma (DLBCL) patients before and after infection with COVID-19.
Patients and Methods: Thirty-five DLBCL patients administered the chemotherapy regimen R-CHOP and examined by oncological 18F-FDG PET/CT imaging twice from August 2022 to February 2023 for pre-treatment evaluation or assessment of treatment efficacy were enrolled. Seventeen patients were confirmed with COVID-19 within the study period. Arterial wall FDG uptake was semi-quantitatively analyzed as TBR (target-to-blood pool ratio) in 14 different vascular regions using oncological 18F-FDG PET/CT. Based on COVID-19 course and the two PET/CT scans, we further analyzed time-related FDG uptake for vascular walls in DLBCL patients with COVID-19.
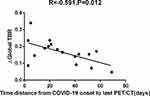
Results: Arterial TBRs were higher in the last PET/CT examination than previous ones in all patients with or without COVID-19. Besides the ascending aorta, ΔTBR (last PET/CT scanning’s TBR minus previous PET/CT scanning’s TBR) were not significantly different between the COVID-19 and Control groups. However, cases scanned ≤ 30 days from infection had remarkably higher ΔTBRs in comparison with those assessed > 30 days post-infection in the COVID-19 group (p< 0.05). A moderate inverse correlation was observed between ∆Global TBR (last PET/CT scanning’s average TBR value minus previous PET/CT scanning’s average TBR value) and time distance from COVID-19 onset to 18F-FDG PET/CT scan (Spearman’s rho=− 0.591, P=0.012). Interestingly, there were no differences of changes of TBR between different purpose of PET/CT examination group.
Conclusion: This work firstly suggested vascular inflammation is elevated in the early post-COVID-19 phase in DLBCL cases compared with prolonged post-COVID-19 phase or controls. Increasing attention should be paid to these patients and the protection of their vascular function and complications in early COVID-19.
Keywords: 18F-FDG PET/CT, vascular inflammation, COVID-19, endothelial dysfunction, non-Hodgkin lymphoma
Introduction
Coronavirus disease 2019 (COVID-19), a disease resulting from infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has a wide range of clinical patterns, from minimal to no symptoms, to mild symptoms, to severe infection, and to critical condition.1,2 Previous findings indicated that inflammatory responses can exacerbate disease progression and magnify clinical manifestations in COVID-19.3 The SARS-CoV-2 virus mostly affects the lung, but also dysregulates the immune system causing systemic inflammatory response, endothelial function impairment and coagulopathy, which predisposes to systemic complications such as hematologic and vascular events.4 COVID-19 can lead to immune disruption, renin-angiotensin-aldosterone (RAA) function and thrombotic balance, which all converge to vascular function impairment as a shared pathway.5,6 Cancer cases show high susceptibility to COVID-19 and associated mortality because of immunodeficiency. 18-Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), is originally an important method for diagnosing and assessing oncological indications and is currently recommended in various types of cardiovascular inflammatory response and infections, representing a possible factor for predicting outcomes in various settings.7,8 Glucose transporters are overexpressed in induced inflammatory cells, whose affinity for deoxyglucose is enhanced by cytokine and growth factor release at the injury site.9 In patients with lymphoma, repeated oncological PET/CT scans are often required to assess the disease condition.10 Could we evaluate tumors and observe the inflammatory response to COVID-19 in blood vessels simultaneously? This study aimed to investigate the effects of inflammatory response induced by COVID-19 on the vascular level, by examining the change of FDG uptake between two PET/CT examinations in different vascular regions of the same patient.
Methods
Patients
We conducted a retrospective analysis of diffuse large B cell lymphoma (DLBCL) cases administered the chemotherapy regimen R-CHOP and examined by oncological 18F-FDG PET/CT twice between August 2022 and February 2023 for pre-treatment evaluation or assessment of treatment efficacy. Patients administered radiotherapy were excluded. CHOP comprised cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2) and vincristine (1.4 mg/m2), all administered intravenously on day 1, plus prednisolone (100 mg/m2) injected intravenously on days 1 to 5. Rituximab was administered at 375 mg/m2. A chemotherapy cycle lasted 3 weeks. All patients were assessed for COVID-19 within the study period and the time from SARS-CoV-2 infection to last PET/CT examination and next cycle chemotherapy were recorded. Similarly, all patients were given symptomatic treatment to protect gastric mucosa and liver function, stop vomiting, and nourish myocardium. Throat swabs with positive COVID-19 test were used for real-time reverse transcription-polymerase chain reaction (RT-PCR).
18F-FDG PET/CT Imaging
18F-FDG PET/CT was performed after ≥12 h of fasting, with controlled glycemia; no patient showed blood glucose level above 11.1 mmol/L prior to injection. FDG was administered by intravenous injection (4.44 MBq/kg), followed by scanning 1 h after injection using Philips Ingenuity TF PET/CT. Low-dose CT scans in supine patients were utilized for attenuation correction and image fusion. The patients were injected no CT contrast agent. PET data reconstruction utilized the iterative proprietary BLOB-OS-TF algorithm from the PET/CT scanner’s manufacturer.
Image Analysis
Regions of interest (ROIs) around the arterial wall were delineated manually along the whole artery in consecutive axial sections at 4-mm intervals, minimizing the odds of including bordering FDG uptake in an ROI. Metabolic activities in the arterial ROIs were assessed by maximum standardized uptake value (SUVmax), for the carotid artery, subclavian artery, axillary artery, thoracic and abdominal aortas, and iliac and femoral arteries; bilateral arteries were assessed twice and all 3 parts of the thoracic aorta were considered. An ROI was delineated in the center of the blood pool of the inferior cava vein to generate a venous SUVmean value. Each arterial target-blood pool ratio (TBR) was obtained as arterial SUVmax divided by the inferior vena cava’s SUVmean.11 Overall, 14 TBRs were calculated. Global TBR was the sum of TBRs for all above TBRs divided by 14. ΔTBR was last PET/CT TBR minus the first PET/CT TBR.
Statistical Analysis
Continuous data are mean±standard deviation (SD) or median (interquartile range), and were compared by the unpaired two-sample Wilcoxon rank-sum (Mann–Whitney) test, Student’s t-test or Paired t-test, as appropriate. Categorical variables were presented as number and percentage and compared by the Fisher’s exact test. Pearson’s correlation coefficients (r) were derived to investigate possible associations among continuous data. Two-sided P<0.05 indicated statistical significance. SPSS 26.0 was utilized for data analysis.
Results
Thirty-five individuals meeting the eligibility criteria were included. Mean patient age was 57.06±12.23 years, and there were seventeen males (48.6%). Eight patients had hypertension (22.9%), and three had diabetes (8.57%). Twenty-five patients had first PET/CT scan for pre-treatment evaluation and second PET/CT scan for efficacy evaluation after 4 cycles of chemotherapy. Ten patients had first PET/CT scan for efficacy evaluation following 4 cycles of chemotherapy and second PET/CT scan for efficacy evaluation after 8 cycles. In total, 17 patients had COVID-19.
BMI (body mass index), age, fasting blood-glucose, history of hypertension and diabetes, and purpose of PET/CT were comparable between COVID-19 cases and Controls (P>0.05), as shown in Table 1. Also, there was no difference in clinical staging of lymphoma based on Ann Arbor classification between COVID-19 cases and Controls. Arterial TBR was higher after 4 cycles of chemotherapy in all patients with or without COVID-19 (Table 2). Besides the ascending aorta, other arterial ΔTBR values were similar in the COVID-19 and Control groups (Table 3).
 |
Table 1 Clinical Features of DLBCL Cases |
 |
Table 2 18F-FDG PET/CT Data of DLBCL Cases After 4 Cycles of Chemotherapy |
 |
Table 3 TBR Changes Between COVID-19 Cases and Controls |
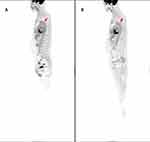
In the COVID-19 group, times from COVID-19 onset to last PET/CT examination were recorded. Median time between COVID-19 onset and last PET/CT was 23.5 days, meanwhile, median time from COVID-19 onset to next cycle chemotherapy was 5 days. Ten of the patients had an interval ≤30 days between COVID-19 infection and last PET/CT scan, with a median time of 15.1 days. The remaining seven patients had an interval of more than 30 days between COVID-19 infection and last PET/CT scan, with a median time of 50.57 days. Between ≤30 days and >30 days group, there was no difference of the median time from COVID-19 onset to next cycle chemotherapy. BMI, age, fasting blood glucose, history of hypertension and diabetes, purpose of PET/CT, clinical staging of lymphoma and severity of COVID-19 based on NIH COVID-19 Treatment Guidelines were comparable between different groups based on times from COVID-19 onset to last PET/CT (P>0.05), as shown in Table 4. TBRs were also higher for the last PET/CT versus the first PET/CT in patients of both groups of COVID-19 cases (Table 5). In the left carotid, right subclavian, left subclavian and left iliac arteries, and ascending and descending aortas, ΔTBRs were significantly higher in patients with ≤30 days between COVID-19 infection and last PET/CT scan compared with the >30 days group. Particularly, ΔGlobal TBR was significantly higher in the ≤30 days group (P<0.05)(Table 6). As shown in Figure 1, ΔGlobal TBR was moderately correlated with time from COVID-19 onset to 18F-FDG PET/CT scan (Spearman’s rho −0.591, P=0.012). Interestingly, there were no differences in ΔTBR between the cumulative chemotherapy dose groups (P>0.05)(Table 7). Figure 2 shows a representative DLBCL case with COVID-19 imaged by 18F-FDG PET/CT examination prior to and after infection.
 |
Table 4 Clinical Characteristics of DLBCL Cases with COVID-19 |
 |
Table 5 18F-FDG PET/CT Findings Between Different Groups Based on Time Interval from COVID-19 Onset to Last PET/CT |
 |
Table 6 Target-to-Blood Pool Ratio Changes Between Different Groups Based on Time Interval from COVID-19 Onset to Last PET/CT |
 |
Table 7 Target-to-Blood Pool Ratio Changes Between Different Chemotherapy Stages at 18F-FDG PET/CT Examination |
Discussion
This retrospective study firstly investigated vascular inflammation in DLBCL patients with COVID-19 assessed twice by fourteen different vascular FDG uptake evaluations. Although changes in Global TBR were similar in the COVID-19 and Control groups, patients with time from COVID-19 onset to last PET/CT scan ≤30 days had markedly higher values than the >30 days group. In addition, a negative correlation was determined between ΔGlobal TBR and time from COVID-19 onset to 18F-FDG PET/CT scan. Interestingly, TBRs for all vascular regions and Global TBRs were higher at the last PET/CT scan compared with the first one, but different groups based on chemotherapy cycles had no significant differences. This excludes the effect of chemotherapy on FDG uptake by the vascular wall. These data suggest that DLBCL patients scanned by 18F-FDG PET/CT less than 30 days post-COVID-19 infection have enhanced vascular inflammation compared with the >30 days group.
Growing evidence reveals systemic inflammatory response and vascular damage caused by COVID-19, especially in lung and brain blood vessels.12 Endothelial cells are directly infected by the virus or damaged by the immune response involving inflammation, which triggers vascular leakage and activates coagulation and results in thrombosis and vascular occlusion.13 Excessive secretion of cytokines and exacerbated neutrophilic host immune response to COVID-19 causes neutrophil infiltration into endothelial cells.14 Impaired vascular function might result in serious complications, including arterial thrombosis, venous thrombosis, and coronary and aortic dissection.15 Vascular involvement is commonly reported in COVID-19 cases, and even COVID vaccines may cause transient endothelial dysfunction.16 Moreover, cancer patients are more likely to show corresponding vascular complications after COVID-19 infection due to immune deficiency resulting from the tumor and/or chemotherapy. 18F-FDG PET/CT is a noninvasive tool for the diagnosis and monitoring of malignancies and infections.17 In acute infection, neutrophils rely on anaerobic glycolysis for the maintenance of cellular activities, and inflammation induces the production of cytokines and causes inflammatory cells to express high levels of glucose transporters.18 Therefore, 18F-FDG PET/CT that detects inflammation in the whole body might help assess vascular anomalies in individuals affected by COVID-19.
However, limited reports examining whole body 18F-FDG PET/CT in individuals with post-acute COVID-19 revealed anomalies mostly in the lungs, bone marrow, vascular wall, and joints versus healthy controls.19 Sollini et al utilized 18F-FDG PET/CT to assess persistent inflammation in adults who recovered from COVID-19 but had unexplained symptoms for >30 days.20 In the COVID-19 group, FDG uptake in various aortic regions and the right iliac and femoral arteries (reflected by TBRs) showed significant elevations compared with the control group, indicating COVID-19 may be responsible for such persistent inflammation. A further COVID-19 trial assessing aortic inflammation and the associated time-dependent trend revealed index aortic segment TBR (IAS-TBR) was moderately associated with high-sensitivity CRP and time between admission and FDG PET/CT. IAS-TBR was markedly elevated in individuals imaged ≤60 days from admission compared with control cases, and returned to levels similar to control values at >60 days following admission for COVID-19. As COVID-19 may cause important damage to the whole body, whole body FDG PET/CT scan might help evaluate the disease extent and quantify disease severity in diverse organs,21 particularly in immunodeficient individuals, eg, patients with cancer or autoimmune diseases.
This study had potential limitations. First, the present study had a retrospective design and examined COVID-19’s effects on the vascular wall in a group of patients administered the same chemotherapy regimen for DLBCL during two 18F-FDG PET/CT examinations. Although vascular inflammation due to COVID-19 was firstly observed by oncological 18F-FDG PET/CT, large prospective multicenter trials are warranted to validate the current results. Indeed, a small sample size was used, although we took into account confounding factors such as chemotherapy and examination time, to finally conclude that COVID-19 can enhance vascular inflammation during chemotherapy in DLBCL patients.
Conclusion
In conclusion, increasing attention should be paid to immunodeficient patients and the protection of their vascular function and complications during COVID-19, testing for stroke, pulmonary embolism, acute myocardial infarction and so on. Although the observed mild vascular inflammation may not produce clinical symptoms, it also requires further investigation.
Ethics Statement
This study had approval from the Ethics Committee of the second hospital of Dalian Medical University, and signed informed consent was provided by each patient. The study was conducted according to the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The current work was funded by the Basic Scientific Research Program of Education Department of Liaoning Province (No. LJKMZ20221288) and Dalian Medical Science Research Program (No.2012027 and No.2112011).
Disclosure
The authors have no conflicts of interest in this work.
References
1. Pascarella G, Strumia A, Piliego C, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi:10.1111/joim.13091
2. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–840. doi:10.1001/jamacardio.2020.1286
3. Manson JJ, Crooks C, Naja M, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2(10):e594–e602. doi:10.1016/S2665-9913(20)30275-7
4. Szekely Y, Gilead R, Réa A, Lawler PR. An evolving understanding of the basis and management of vascular complications of COVID-19: where do we go from here. Can J Cardiol. 2023. doi:10.1016/j.cjca.2023.03.019
5. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi:10.1056/NEJMoa2015432
6. Yao S, Luo N, Liu J, et al. Elevated serum levels of progranulin and soluble vascular cell adhesion molecule-1 in patients with COVID-19. J Inflamm Res. 2021;14:4785–4794. doi:10.2147/JIR.S330356
7. Walter MA, Melzer RA, Schindler C, Müller-Brand J, Tyndall A, Nitzsche EU. The value of [18F]FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32(6):674–681. doi:10.1007/s00259-004-1757-9
8. Jamar F, Gormsen LC, Yildiz H, Slart RH, van der Geest KS, Gheysens O. The role of PET/CT in large vessel vasculitis and related disorders: diagnosis, extent evaluation and assessment of therapy response. Q J Nucl Med Mol Imaging. 2022;66(3):182–193. doi:10.23736/S1824-4785.22.03465-3
9. Treglia G, Albano D, Dondi F, Bertagna F, Gheysens O. A role of FDG PET/CT for response assessment in large vessel disease. Semin Nucl Med. 2023;53(1):78–85. doi:10.1053/j.semnuclmed.2022.08.002
10. Kurch L, Hüttmann A, Georgi TW, et al. Interim PET in Diffuse Large B-Cell Lymphoma. J Nucl Med. 2021;62(8):1068–1074. doi:10.2967/jnumed.120.255034
11. Slart R. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45(7):1250–1269. doi:10.1007/s00259-018-3973-8
12. Dudouet P, Cammilleri S, Guedj E, Jacquier A, Raoult D, Eldin C. Aortic (18)F-FDG PET/CT hypermetabolism in patients with long COVID: a retrospective study. Clin Microbiol Infect. 2021;27(12):1873–1875. doi:10.1016/j.cmi.2021.09.020
13. Melillo F, Napolano A, Loffi M, et al. Myocardial injury in patients with SARS-CoV-2 pneumonia: pivotal role of inflammation in COVID-19. Eur J Clin Invest. 2022;52(1):e13703. doi:10.1111/eci.13703
14. Evans PC, Rainger GE, Mason JC, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116(14):2177–2184. doi:10.1093/cvr/cvaa230
15. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi:10.1016/S0140-6736(20)30937-5
16. Terentes-Printzios D, Gardikioti V, Solomou E, et al. The effect of an mRNA vaccine against COVID-19 on endothelial function and arterial stiffness. Hypertens Res. 2022;45(5):846–855. doi:10.1038/s41440-022-00876-6
17. Kusmirek JE, Magnusson JD, Perlman SB. Current applications for nuclear medicine imaging in pulmonary disease. Curr Pulmonol Rep. 2020;9(3):82–95. doi:10.1007/s13665-020-00251-1
18. Jamar F, Buscombe J, Chiti A, et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54(4):647–658. doi:10.2967/jnumed.112.112524
19. Sollini M, Morbelli S, Ciccarelli M, et al. Long COVID hallmarks on [18F]FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021;48(10):3187–3197. doi:10.1007/s00259-021-05294-3
20. Sollini M, Ciccarelli M, Cecconi M, et al. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [(18)F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging. 2021;48(5):1460–1466. doi:10.1007/s00259-020-05084-3
21. Alavi A, Werner TJ, Gholamrezanezhad A. The critical role of FDG-PET/CT imaging in assessing systemic manifestations of COVID-19 infection. Eur J Nucl Med Mol Imaging. 2021;48(4):956–962. doi:10.1007/s00259-020-05148-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.