Back to Journals » International Journal of General Medicine » Volume 16
Thyroglobulin Antibody (TgAb) Positive is an Independent Risk Factor for Lymph Node Metastasis in Patients with Differentiated Thyroid Carcinoma
Authors Lai Y , Gu Y, Yu M, Deng J
Received 12 September 2023
Accepted for publication 5 December 2023
Published 19 December 2023 Volume 2023:16 Pages 5979—5988
DOI https://doi.org/10.2147/IJGM.S439919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yeqian Lai, Yihua Gu, Ming Yu, Jiaqin Deng
Department of Thyroid Surgery, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, Meizhou, People’s Republic of China
Correspondence: Yeqian Lai, Department of Thyroid Surgery, Meizhou People’s Hospital, Meizhou Academy of Medical Sciences, No. 63 Huangtang Road, Meijiang District, Meizhou, People’s Republic of China, Email [email protected]
Objective: To investigate the relationship between lymph node metastasis and the clinicopathologic features of differentiated thyroid carcinoma (DTC) patients with thyroglobulin antibody (TgAb) positive and negative.
Methods: A total of 443 patients with DTC were included in this study. Clinicopathological data of the patients were collected, including tumor size, clinical stage, calcification, Hashimoto’s thyroiditis, extra-membrane infiltration, BRAF V600E mutation status, and thyroid-related hormone and antibody levels. The relationship between of lymph node metastasis and clinicopathologic features was analyzed.
Results: There were 227(51.2%) TgAb negative and 216(48.8%) TgAb positive DTC patients. Compared with patients without lymph node metastasis, DTC patients with lymph node metastasis had a higher proportion of patients with < 55 years of age, maximum tumor diameter > 1cm, calcification, BRAF V600E mutation, and TgAb positive. Multivariate regression logistic analysis showed that < 55 years old (odds ratio (OR): 2.744, 95% CI: 1.665– 4.522, P< 0.001), maximum tumor diameter > 1cm (OR: 2.163, 95% CI: 1.431– 3.271, P< 0.001), BRAF V600E mutation (OR: 2.489, 95% CI: 1.397– 4.434, P=0.002), and TgAb positive (OR: 1.540, 95% CI: 1.020– 2.326, P=0.040) were risk factors for lymph node metastasis. Maximum tumor diameter > 1cm and BRAF V600E increased the risk by more than one fold for lymph node metastasis in TgAb-negative and TgAb-positive DTC patients.
Conclusion: Younger age (< 55 years old), maximum tumor diameter > 1cm, BRAF V600E mutation, and TgAb positive were independent risk factors for lymph node metastasis in DTC. And maximum tumor diameter > 1cm and BRAF V600E mutation were risk factors for lymph node metastasis both in TgAb positive and negative DTC patients.
Keywords: differentiated thyroid carcinoma, lymph node metastasis, thyroglobulin antibody, BRAF
Introduction
Thyroid carcinoma is the most common malignancy of the endocrine system, and accounts for about 3% of all malignancies in the human.1 Thyroid carcinoma is the most common malignant tumor of head and neck, and its incidence has been on the rise in recent decades.2 Thyroid carcinoma can be divided into differentiated, undifferentiated, and medullary thyroid carcinoma according to histological classification.3 The majority of thyroid cancer patients are differentiated thyroid carcinoma (DTC) patients derived from follicular epithelial cells, accounting for more than 90% of new cases, including papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC).4
For most DTC patients, the 10-year overall survival rate after surgical resection, radioiodine therapy, and endocrine suppression therapy was 94.8%.5 Although DTC is highly differentiated and progresses slowly, the incidence of cervical lymph node metastasis in patients with DTC is 30%-60%,6 and it is closely related to the increased risk of local recurrence.7 Regional lymph node metastasis is present in 40–90% of thyroid cancer patients, and 15% of lymph node metastasis cases have regional invasion, distant metastasis, and treatment tolerance.8 Prophylactic lymph node dissection is controversial for surgically treated thyroid cancer patients. Prophylactic lymph node dissection may lead to hypoparathyroidism in patients without lymph node metastasis, while no lymph node dissection may leave metastatic lymph nodes in high-risk patients.9 So, it is important to identify predictors of lymph node metastasis in patients with DTC. Asimakopoulos et al summarized some risk factors for lymph node metastasis, including age, tumor size, BRAF gene mutation.10 Other factors affecting lymph node metastasis of thyroid cancer need to be continuously explored and studied.
Thyroglobulin antibody (TgAb) is a common autoantibody in serum of patients with autoimmune thyroid disease, such as chronic lymphocytic thyroiditis (CLT).11 TgAb principally comprising immunoglobulin G (IgG), is mainly derived from B lymphocytes in the thyroid gland, and the rest is produced by immune cells in the neck lymph node and bone marrow.12 Study has found that TgAb-positive Hashimoto thyroiditis patients have a higher risk of papillary thyroid cancer.13 High TgAb level was significantly associated with central lymph node metastasis in PTC patients with Hashimoto’s thyroiditis.14 On the contrary, another study suggested that the incidence of central lymph node metastasis was reduced in thyroid peroxidase antibody (TPOAb) positive and TgAb positive PTC patients.15 In addition, there was no difference in serum TgAb levels in patients with metastatic PTC compared with the non-metastatic patients.16 TgAb positive was not identified as risk factor of lymph node metastasis of thyroid cancer.17 Clinically, the relationship between the condition of TgAb and lymph node metastasis in DTC patients has not been recognized.18 And the relationship between lymph node metastasis and other clinicopathological features (such as extra-membrane infiltration and BRAF mutation, etc) in TgAb-positive and TgAb-negative patients with DTC is rarely reported. To assess the association between TgAb and lymph node metastasis in DTC patients, we studied a consecutive case series of DTC from Meizhou People’s Hospital, China.
Materials and Methods
Subjects
The study included 443 patients with DTC who were admitted to Meizhou People’s Hospital from January 2018 to December 2021. Inclusion criteria: (1) complete demographic and clinical data of patients; (2) the histological diagnosis was consistent with the diagnostic criteria for differentiated thyroid carcinoma; (3) no diseases such as dysfunction of important organs were complicated. Exclusion criteria: (1) patients without DTC; (2) patients with dysfunction of vital organs; (3) complicated with other tumor diseases. This study was supported by the Ethics Committee of the Meizhou People’s Hospital (Clearance No.: 2021-C-123).
BRAF V600E Mutation Analysis
DNA was extracted from the tissue specimens by Tissue DNA separation Kit (Amoy Diagnostics, Xiamen, China). BRAF V600E mutation was detected by real-time amplification refractory mutation system (ARMS)-PCR with the BRAF V600E Mutation Fluorescence PCR Diagnostic Kit (Amoy Diagnostics, Xiamen, China), as previously described.19
Thyroid-Related Hormone and Antibody Levels Analysis
About 5 mL of the patient’s venous blood was drawn using a vacuum collection vessel with separation glue. Thyroid-related hormones and antibodies were determined by electrochemiluminescence immunoassay (Abbott Immulite 2000). Serum thyroid stimulating hormone (TSH) less than or equal to 4.94uIU/mL defined TSH as negative, and serum thyrotropin higher than 4.94uIU/mL defined TSH as positive, according to the reference range detected by the kit. Free triiodothyronine (FT3) and free thyroxine (FT4) levels higher than 3.91pg/mL and 1.48ng/dl were defined as FT3 positive and FT4 positive, respectively. Similarly, TgAb and anti-thyroperoxidase antibody (TPOAb) levels higher than 4.11IU/mL and 5.61IU/mL were defined as TgAb positive and TPOAb positive, respectively.
Statistical Analysis
The clinical information of these subjects (such as gender, age, maximum tumor diameter, disease stage, Hashimoto’s thyroiditis, calcification, and extra-membrane infiltration) was collected. SPSS statistical software version 21.0 (IBM Inc., USA) was used for data analysis. Association between lymph node metastasis and the clinical features of DTC patients was evaluated by Fisher’s exact test. Logistic regression analysis was applied to assess the covariates in risk assessment of lymph node metastasis of all DTC patients, TgAb positive DTC patients, and TgAb negative DTC patients, respectively. P <0.05 was set as statistically significant.
Results
Clinicopathological Features of DTC Patients
Among the 443 subjects included in the study, 371 (83.7%) were female and 354 (79.9%) were younger than 55 years old, indicating that the majority of DTC patients were young women. There were 242 (54.6%) and 201 (45.4%) patients with maximum tumor diameter ≤1cm and >1cm, respectively. The main disease stage was I-II (n= 405, 91.4%). The rates of calcification, Hashimoto’s thyroiditis, extra-membrane infiltration and BRAF V600E mutation were 34.3%, 11.1%, 9.3% and 85.3%, respectively. There were 227(51.2%) DTC patients with TgAb negative and 216(48.8%) DTC patients with TgAb positive (Table 1).
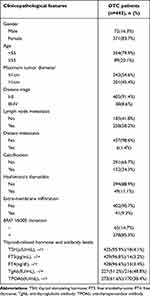 |
Table 1 The Clinicopathological Features of DTC Patients |
Association Between Lymph Node Metastasis and Clinicopathological Features of DTC Patients
In this study, there were 185(41.8%) DTC patients without and 258(58.2%) DTC patients with lymph node metastasis. Compared with patients without lymph node metastasis, DTC patients with lymph node metastasis had a higher proportion of patients with <55 years of age (86.0% vs 71.4%) (P<0.001) and a higher proportion of patients with maximum tumor diameter >1cm (53.9% vs 33.5%) (P<0.001). The proportion of tumor tissue calcification (38.4% vs 28.6%) (P=0.034), BRAF V600E mutation (88.4% vs 81.1%) (P=0.041), and TgAb positive (53.1% vs 42.7%) (P=0.034) in DTC patients with lymph node metastasis was higher than that in DTC patients without lymph node metastasis, respectively. There was no statistically significant association between lymph node metastasis and other clinicopathological features of DTC, such as sex distribution, proportion of patients with Hashimoto’s thyroiditis, and extra-membrane infiltration (Table 2).
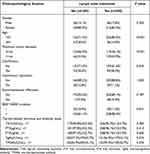 |
Table 2 Association Between Lymph Node Metastasis and Clinicopathological Features of DTC Patients |
Logistic Regression Analysis of Risk Factors of Lymph Node Metastasis of DTC
To investigate the effect of age, tumor size, calcification, BRAF V600E mutation, and TgAb status on lymph node metastasis in DTC, we performed a univariate analysis and multivariate regression logistic analysis to measure the association between these parameters and the presence of lymph node metastasis. The results of univariate analysis showed that younger age (<55 years old) (odds ratio (OR): 2.476, 95% CI: 1.540–3.981, P<0.001), maximum tumor diameter >1cm (OR: 2.317, 95% CI: 1.567–3.427, P<0.001), calcification (OR: 1.551, 95% CI: 1.034–2.327, P=0.034), BRAF V600E mutation (OR: 1.773, 95% CI: 1.044–3.011, P=0.034), and TgAb positive (OR: 1.519, 95% CI: 1.039–2.222, P=0.031) were significantly associated with lymph node metastasis in DTC. And the results of multivariate regression logistic analysis showed that age (<55 years old) (OR: 2.744, 95% CI: 1.665–4.522, P<0.001), maximum tumor diameter >1cm (OR: 2.163, 95% CI: 1.431–3.271, P<0.001), BRAF V600E mutation (OR: 2.489, 95% CI: 1.397–4.434, P=0.002), and TgAb positive (OR: 1.540, 95% CI: 1.020–2.326, P=0.040) were still significantly associated with lymph node metastasis in DTC (Table 3).
 |
Table 3 Logistic Regression Analysis of Risk Factors of Lymph Node Metastasis of DTC |
Association Between Lymph Node Metastasis and Clinicopathological Features of DTC Patients with TgAb Positive and TgAb Negative, Respectively
In DTC patients with TgAb negative, the proportion of maximum tumor diameter >1cm (46.3% vs 28.3%) (P=0.006), tumor tissue calcification (36.4% vs 23.6%) (P=0.043), and BRAF V600E mutation (95.0% vs 86.8%) (P=0.035) in DTC patients with lymph node metastasis was higher than that in DTC patients without lymph node metastasis, respectively. In DTC patients with TgAb positive, the proportion of patients with <55 years of age (87.6% vs 65.8%) (P<0.001), and maximum tumor diameter >1cm (60.6% vs 40.5%) (P=0.005) in DTC patients with lymph node metastasis was higher than that in DTC patients without lymph node metastasis, respectively (Table 4).
 |
Table 4 Association Between Lymph Node Metastasis and Clinicopathological Features of DTC Patients with TgAb (+) and TgAb (-), Respectively |
The results of logistic regression analysis showed that maximum tumor diameter >1cm (OR: 2.080, 95% CI: 1.166–3.711, P=0.013), and BRAF V600E mutation (OR: 3.364, 95% CI: 1.190–9.505, P=0.022) were risk factors for lymph node metastasis in TgAb negative DTC patients. And maximum tumor diameter >1cm (OR: 2.276, 95% CI: 1.251–4.142, P=0.007), and BRAF V600E mutation (OR: 2.239, 95% CI: 1.097–4.572, P=0.027) were risk factors for lymph node metastasis in TgAb positive DTC patients (Table 5). That is to say, tumor size >1cm and BRAF V600E mutation were risk factors for lymph node metastasis both in TgAb positive and negative DTC patients.
 |
Table 5 Logistic Regression Analysis of Risk Factors of Lymph Node Metastasis in DTC Patients with TgAb (+) and TgAb (-) |
Discussion
Thyroid cancer cells can spread to lymph nodes through the thyroid endolymphatic vessels, most commonly in cervical lymph nodes, and lymph node metastasis occurs in 20–90% of DTC patients.20 At follow-up after treatment, 5–20% of DTC patients will develop local or regional recurrence, which is associated with incomplete initial treatment or a more aggressive tumor, and 10–15% of DTC patients will develop distant metastases, usually in the lung and bone, and less frequently in the brain, liver, and skin. Compared with PTC, local lymph nodes of follicular thyroid carcinoma are less involved but have a higher probability of distant metastasis.21 Prophylactic lymph node dissection is controversial for patients with DTC who are suitable for surgical treatment. Prophylactic lymph node dissection may lead to hypoparathyroidism in patients without lymph node metastasis, while no lymph node dissection may leave metastatic lymph nodes in high-risk patients.9
In recent years, the relationship between Hashimoto’s thyroiditis and thyroid cancer has been mentioned. It is believed that thyroid cancer with Hashimoto’s thyroiditis is less aggressive and has a lower overall recurrence rate.22 However, whether high levels of TgAb affect lymph node metastasis in patients with thyroid cancer remains controversial. Currently, several studies have reported that the presence of TgAb is associated with lymph node metastasis in DTC patients, and the lymph node metastasis rate in TgAb positive DTC patients is higher than that in TgAb negative patients.23 The mechanism of action of TgAb in lymph node metastasis of DTC remains unclear. TgAb level is higher in DTC patients with lymph node metastasis, which may be potentially caused by stronger immune response generated by tumors in lymph nodes and improved immunogenicity of TgAb production.24 In addition, some studies suggested that the increase of TgAb in Hashimoto’s thyroiditis complicated with thyroid cancer would accelerate lymph node metastasis.25 However, Giagourta et al pointed out that Hashimoto’s thyroiditis was negatively correlated with lymph node or distant metastasis of PTC, and those with Hashimoto’s thyroiditis had lower rates of lymph node and distant metastasis than those without Hashimoto’s thyroiditis.26 These studies speculated that the possible mechanism might be the atrophy and fibrosis of thyroid caused by inflammation caused by Hashimoto’s thyroiditis,27 which involves related damage of peripheral lymphatic vessels, thus hindering the lymphatic diffusion of thyroid cancer and ultimately reducing the possibility of lymph node metastasis in patients with thyroid cancer.28 In this study, TgAb positive was a risk factor for lymph node metastasis in DTC, but this result did not appear to be associated with Hashimoto’s thyroiditis. And further research may be needed to confirm the exact mechanism.
BRAF V600E mutation has been considered as an important molecular marker of thyroid carcinoma,29 but the mutation rate of BRAF V600E in thyroid cancer is obviously different in different countries.28 Therefore, different studies on the relationship between BRAF gene mutation status and lymph node metastasis of thyroid cancer may have inconsistent results. Tumors with the BRAF V600E mutation were likely to exhibit aggressive pathological features, including lymph node metastasis.30 BRAF V600E mutation was a predictor for central lymph node metastasis in PTC with tumor size 2.0–4.0 cm.31 Lymph node metastasis associated mortality risk was associated with the BRAF V600E status in PTC patients.32 BRAF V600E was a prognostic marker for tumor progression in DTC patients.33 A study showed that BRAF V600E mutation was associated with lymph node metastasis but not with other clinicopathological features.34 However, another study has found that BRAF V600E mutation was not related to the incidence of lymph node metastasis of thyroid carcinoma.35 BRAF V600E mutation was not associated with central lymph node metastasis in PTC patients.36
Younger age (<55 years old) was a risk factor for lymph node metastasis in DTC in this study. A study has showed that younger age was an independent predictor of lymph node metastasis in thyroid carcinoma,37 however another study has found that age was not associated with lymph node metastasis.38 On the contrary, another study showed lymph node metastasis was more common in older patients.39 In addition, maximum tumor diameter >1cm was significantly associated with lymph node metastasis in DTC in this study. Study has found that large tumor size is a risk factor for lymph node metastasis of thyroid cancer.40 There is not much controversy about the research on this relationship.
This study is one of the few that have examined the relationship between TgAb levels and lymph node metastasis of thyroid cancer. To sum up, the differences in the results of related studies may be related to the differences in sample size and tumor heterogeneity in these studies, and the confirmation of this relationship needs to be further verified through the data of multi-population and multi-research centers. There are some limitations in this study. First of all, this study is a single-center study, with a small number of included studies and possible population selection bias. Future studies will include more subjects. Secondly, this study only studied the serum TgAb level of patients, and did not analyze the TgAb level of tumor tissue. Whether the relationship between the TgAb level of tumor tissue and lymph node metastasis is more meaningful needs further research. Finally, this study did not analyze the relationship between the change trend and degree of TgAb level before and after treatment and the prognosis of DTC patients. Future studies need to make up for the above deficiencies and conduct comprehensive design for analysis.
Conclusions
In this study, age (<55 years old), maximum tumor diameter >1cm, BRAF V600E mutation, and TgAb positive were independent risk factors for lymph node metastasis in DTC. In other words, lymph node dissection and routine excision of lymph and adipose tissue are important for young DTC patients with preoperative BRAF V600E mutation and TgAb positive. On the other hand, the results of this study also suggest that maximum tumor diameter >1cm and BRAF V600E mutation were risk factors for lymph node metastasis both in TgAb positive and negative DTC patients. It suggests that the detection of TgAb level, and BRAF gene would be beneficial for the risk assessment of lymph node metastasis in patients with DTC.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the Ethics Committee of Medicine, Meizhou People’s Hospital. All participants signed informed consent in accordance with the Declaration of Helsinki.
Acknowledgments
The author would like to thank other colleagues whom were not listed in the authorship of Department of Thyroid Surgery, Meizhou People’s Hospital for their helpful comments on the manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Science and Technology Program of Meizhou (Grant No.: 2019B0202001).
Disclosure
The authors declare that they have no competing interests.
References
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Cheng F, Xiao J, Shao C, et al. Burden of thyroid cancer from 1990 to 2019 and projections of incidence and mortality until 2039 in China: findings from global burden of disease study. Front Endocrinol. 2021;12:738213. doi:10.3389/fendo.2021.738213
3. Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol. 2020;11:102. doi:10.3389/fendo.2020.00102
4. Lee K, Anastasopoulou C, Chandran C, Cassaro S. Thyroid Cancer. In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC; 2023. PMID: 29083690.
5. Tam S, Boonsripitayanon M, Amit M, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and Eighth Editions. Thyroid. 2018;28(10):1301–1310. doi:10.1089/thy.2017.0572
6. Cracchiolo JR, Wong RJ. Management of the lateral neck in well differentiated thyroid cancer. Eur J Surg Oncol. 2018;44(3):332–337. doi:10.1016/j.ejso.2017.06.004
7. Zhi J, Wu Y, Hu L, et al. Assessment of the prognostic value and N1b changes of the eighth TNM/AJCC staging system for differentiated thyroid carcinoma. Int J Clin Oncol. 2020;25(1):59–66. doi:10.1007/s10147-019-01522-x
8. Zhang J, Yang Y, Zhao J, et al. Investigation of BRAF mutation in a series of papillary thyroid carcinoma and matched-lymph node metastasis with ARMS PCR. Pathol Res Pract. 2019;215(4):761–765. doi:10.1016/j.prp.2019.01.006
9. Alsubaie KM, Alsubaie HM, Alzahrani FR. Prophylactic central neck dissection for clinically node-negative papillary thyroid carcinoma. Laryngoscope. 2022;132(6):1320–1328. doi:10.1002/lary.29912
10. Asimakopoulos P, Nixon IJ, Shaha AR. Differentiated and medullary thyroid cancer: surgical management of cervical lymph nodes. Clin Oncol. 2017;29(5):283–289. doi:10.1016/j.clon.2017.01.001
11. Soh SB, Aw TC. Laboratory testing in thyroid conditions - pitfalls and clinical utility. Ann Lab Med. 2019;39(1):3–14. doi:10.3343/alm.2019.39.1.3
12. Li Y, Zhao C, Zhao K, et al. Glycosylation of Anti-Thyroglobulin IgG1 and IgG4 subclasses in thyroid diseases. Eur Thyroid J. 2021;10(2):114–124. doi:10.1159/000507699
13. Spencer CA. Clinical review: clinical utility of thyroglobulin antibody (TgAb) measurements for patients with differentiated thyroid cancers (DTC). J Clin Endocrinol Metab. 2011;96(12):3615–3627. doi:10.1210/jc.2011-1740
14. Min Y, Huang Y, Wei M, et al. Preoperatively predicting the central lymph node metastasis for papillary thyroid cancer patients with hashimoto’s thyroiditis. Front Endocrinol. 2021;12:713475. doi:10.3389/fendo.2021.713475
15. Li L, Shan T, Sun X, et al. Positive thyroid peroxidase antibody and thyroglobulin antibody are associated with better clinicopathologic features of papillary thyroid cancer. Endocr Pract. 2021;27(4):306–311. doi:10.1016/j.eprac.2020.10.017
16. Wu X, Liu Y, Li K, et al. Predictive Value of FNA-Tg and TgAb in cervical lymph node metastasis of papillary thyroid carcinoma. Technol Cancer Res Treat. 2022;21:15330338221127605. doi:10.1177/15330338221127605
17. Zhou Y, Sun Z, Zhou Y, et al. Thyroid antibody status exerts insignificant effect on lymph node metastasis of thyroid cancer. Transl Cancer Res. 2020;9(10):6423–6430. doi:10.21037/tcr-20-1941
18. Jo K, Lim DJ. Clinical implications of anti-thyroglobulin antibody measurement before surgery in thyroid cancer. Korean J Intern Med. 2018;33(6):1050–1057. doi:10.3904/kjim.2018.289
19. Lai Y, Gu Y, Yu M, Deng J. Younger Than 55 years old and BRAF V600E mutation are risk factors for lymph node metastasis in papillary thyroid carcinomas ≤1.0 cm but not in >1.0 cm. Int J Gen Med. 2023;16:1403–1414. doi:10.2147/IJGM.S408588
20. So YK, Kim MJ, Kim S, Son YI. Lateral lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg. 2018;50:94–103. doi:10.1016/j.ijsu.2017.12.029
21. Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338(5):297–306. doi:10.1056/NEJM199801293380506
22. Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012;269(3):1013–1017. doi:10.1007/s00405-011-1732-6
23. Lee ZJO, Eslick GD, Edirimanne S. Investigating antithyroglobulin antibody as a prognostic marker for differentiated thyroid cancer: a meta-analysis and systematic review. Thyroid. 2020;30(11):1601–1612. doi:10.1089/thy.2019.0368
24. Wassner AJ, Della Vecchia M, Jarolim P, Feldman HA, Huang SA. Prevalence and significance of thyroglobulin antibodies in pediatric thyroid cancer. J Clin Endocrinol Metab. 2017;102(9):3146–3153. doi:10.1210/jc.2017-00286
25. Karatzas T, Vasileiadis I, Zapanti E, Charitoudis G, Karakostas E, Boutzios G. Thyroglobulin antibodies as a potential predictive marker of papillary thyroid carcinoma in patients with indeterminate cytology. Am J Surg. 2016;212(5):946–952. doi:10.1016/j.amjsurg.2015.12.030
26. Giagourta I, Evangelopoulou C, Papaioannou G, et al. Autoimmune thyroiditis in benign and malignant thyroid nodules: 16-year results. Head Neck. 2014;36(4):531–535. doi:10.1002/hed.23331
27. Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335(2):99–107. doi:10.1056/NEJM199607113350206
28. Jara SM, Carson KA, Pai SI, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. Surgery. 2013;154(6):1272–1280. doi:10.1016/j.surg.2013.07.021
29. Scheffel RS, Dora JM, Maia AL. BRAF mutations in thyroid cancer. Curr Opin Oncol. 2022;34(1):9–18. doi:10.1097/CCO.0000000000000797
30. Semsar-Kazerooni K, Morand GB, Payne AE, et al. Mutational status may supersede tumor size in predicting the presence of aggressive pathologic features in well differentiated thyroid cancer. J Otolaryngol Head Neck Surg. 2022;51(1):9. doi:10.1186/s40463-022-00559-9
31. Kim SK, Lee JH, Woo JW, et al. BRAF V600E mutation: differential impact on central lymph node metastasis by tumor size in papillary thyroid carcinoma. Head Neck. 2016;38:E1203–E1209. doi:10.1002/hed.24192
32. Tao Y, Wang F, Shen X, et al. BRAF V600E status sharply differentiates lymph node metastasis-associated mortality risk in papillary thyroid cancer. J Clin Endocrinol Metab. 2021;106(11):3228–3238. doi:10.1210/clinem/dgab286
33. Wahid MHA, Almudhafar RH. Comparative BRAF V600E immunohistochemical expression in differentiated thyroid tumors with papillary features. J Med Life. 2022;15(4):520–525. doi:10.25122/jml-2021-0415
34. Gao J, Ma XP, Deng FS, Jiang L, Jia WD, Li M. Associations of the BRAF V600E Mutation and PAQR3 protein expression with papillary thyroid carcinoma clinicopathological features. Pathol Oncol Res. 2020;26(3):1833–1841. doi:10.1007/s12253-019-00779-x
35. Danilovic DL, Lima EU, Domingues RB, Brandão LG, Hoff AO, Marui S. Pre-operative role of BRAF in the guidance of the surgical approach and prognosis of differentiated thyroid carcinoma. Eur J Endocrinol. 2014;170(4):619–625. doi:10.1530/EJE-13-0944
36. Dong SY, Zeng RC, Jin LP, et al. BRAF(V600E) mutation is not associated with central lymph node metastasis in all patients with papillary thyroid cancer: different histological subtypes and preoperative lymph node status should be taken into account. Oncol Lett. 2017;14(4):4122–4134. doi:10.3892/ol.2017.6694
37. Zhou SL, Guo YP, Zhang L, et al. Predicting factors of central lymph node metastasis and BRAF(V600E) mutation in Chinese population with papillary thyroid carcinoma. World J Surg Oncol. 2021;19(1):211. doi:10.1186/s12957-021-02326-y
38. Akın Ş, Yazgan Aksoy D, Akın S, Kılıç M, Yetişir F, Bayraktar M. Prediction of central lymph node metastasis in patients with thyroid papillary microcarcinoma. Turk J Med Sci. 2017;47(6):1723–1727. doi:10.3906/sag-1702-99
39. Shi Y, Yang Z, Heng Y, Ju H, Pan Y, Zhang Y. Clinicopathological findings associated with cervical lymph node metastasis in papillary thyroid microcarcinoma: a retrospective study in China. Cancer Control. 2022;29:10732748221084926. doi:10.1177/10732748221084926
40. Yang Z, Heng Y, Qiu W, Tao L, Cai W. Cervical lymph node metastasis differences in patients with unilateral or bilateral papillary thyroid microcarcinoma: a multi-center analysis. J Clin Med. 2022;11(16):4929. doi:10.3390/jcm11164929
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
