Back to Journals » Breast Cancer: Targets and Therapy » Volume 13
Therapeutic Role of Annona muricata Fruit and Bee Venom Against MNU-Induced Breast Cancer in Pregnant Rats and its Complications on the Ovaries
Authors El-Beltagy AEFBM, Elsyyad HIH, Abdelaziz KK, Madany AS, Elghazaly MM
Received 17 February 2021
Accepted for publication 8 April 2021
Published 8 July 2021 Volume 2021:13 Pages 431—445
DOI https://doi.org/10.2147/BCTT.S306971
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Abd El-Fattah BM El-Beltagy,1 Hassan IH Elsyyad,2 Karoline K Abdelaziz,1 Amira S Madany,1 Mohamed M Elghazaly1
1Zoology Department, Faculty of Science, Damanhour University, Damanhour, Egypt; 2Zoology Department, Faculty of Science, Mansoura University, Mansoura, Egypt
Correspondence: Abd El-Fattah BM El-Beltagy Email [email protected]
Aim: To evaluate the potential therapeutic role of Annona muricata (graviola) fruit and bee venom (BV) against N-methylnitrosourea (MNU)-induced breast cancer in pregnant female rats and complications in the ovaries.
Methods: A total of 24 female rats were induced with a single dose of MNU (50 mg/kg body weight). After confirmation of positive tumor marker, female rats were placed with the males for mating. The pregnant rats were randomly divided into four groups (n=6): MNU-induced only (group 1), MNU-induced rats and supplemented with A. muricata 200 mg/kg diet (group 2), MNU-induced and treated with two doses of BV 75 μg/kg (group 3), and MNU-induced and treated with both A. muricata and BV (group 4).
Results: In group 1, the breast tissue of mothers revealed pronounced cellular hyperplasia and histopathological signs. Also, the ovarian tissue of mothers and their offspring displayed deleterious histological changes. In groups 2 and 4, histopathological signs and cellular hyperplasia markedly disappeared in breast tissue. However, the histopathological signs induced by MNU in the ovarian tissue reversed to normal in groups 2– 4. Also in groups 2– 4, levels of serum MMP1, NFκB, and TNFα significantly decreased, and serum caspase 3 significantly increased either in mother rats or their offspring compared to the MNU-alone group. Levels of serum MDA significantly decreased; however, levels of serum antioxidants (CAT and SOD) significantly increased in all groups 2– 4 compared to MNU-alone group.
Conclusion: A. muricata has a more powerful therapeutic role than BV against MNU-induced breast cancer in rats; however, both have a powerful ameliorative role against ovarian histopathological alterations induced by MNU. Such ameliorative effects of A. muricata and BV are mainly attributed to their antioxidant, anti-inflammatory, and antiproliferative constituents.
Keywords: graviola, bee venom, breast cancer, apoptosis, ovaries, offspring
Introduction
Breast Cancer as a Global Challenge
Breast cancer is the commonest type of cancer and the leading cause of cancer mortality among women worldwide.1 A study reported that breast cancer survival is approximately 80% in developed countries; however, this rate does not exceed 40% in developing countries.2 Other studies have revealed that the incidence of breast cancer associated with pregnancy ranges from 1:3,000 to 1:10,000, is mostly indicated at advanced stage, and has a poorer prognosis than in nonpregnant women.3,4
Risk Factors of Breast Cancer
Several causes are implicated in progression of breast cancer, such as early menstruation, late menopause, late age at first birth, obesity, and repeated use of oral contraceptives.5,6 Additionally, some lifestyle factors like excessive alcohol consumption, radiation, obesity, physical inactivity, and exposure to radiation may be implicated in liberation of excessive free radicals, which are highly reactive and cause breast cancer.7,8
Treatment and Prevention of Breast Cancer
Chemotherapy, radiation, and surgery are the commonest therapeutic tools used for treatment of cancer which may be applied alone or in combination with one another.9 In fact, all these treatments have fatal adverse effects on pregnant women and their fetuses.10,11 Accordingly, several studies have been attempted to use natural products from plants and animals as alternatives in the treatment of cancer.12–14 Natural anticancerous products can interfere with the initiation, development, and progression of cancer by modulating various mechanisms, including cellular proliferation, differentiation, apoptosis, angiogenesis, and metastasis.12
Compounds from natural products have recently attracted unique attention from the scientific community for their potent role against inflammation-driven diseases, including cancer. Previous researches, including preclinical, clinical, and epidemiological studies, has indicated that dietary consumption of polyphenols, found at high levels in vegetables and fruit, may prevent the progression of several diseases, including cancer.15 Furthermore, dietary phytochemicals have many built-in advantages over synthetic compounds, due to their proven safety, low cost, and oral bioavailability.16 Researchers have begun to decipher the mode of action of plant-derived agents at the molecular, cellular, and tissue level.17,18
Annona muricata (graviola) is a low fruit–bearing tree that belongs to the family Annonaceae. Graviola trees are widely cultivated in the rainforests of Africa, South America, and Southeast Asia.13 Graviola fruit contains 37 compounds like vitamins, carotenoids, megastigmanes, amides, and cyclopeptides, which have powerful antioxidant, anti-inflammatory and antitumor properties.19–22 Additionally, graviola extracts are used for treatment of several diseases like rheumatism, diabetes, hypertension. and parasitic infection.13,23–25
Another approach that has gained attention is the use of biotoxins like animal venom as cancer therapeutic agents.26,27 Biologically effective compounds from animal venom have long attracted the interest of researchers.28 Bee venom (BV) is a biotoxin compound with a variety of biologically active peptides like melittin, enzymes, amines, and nonpeptides that have powerful pharmaceutical properties.29 Melittin has a potential role in therapy for leukemia, hepatoma, and lung and breast cancer.27 BV can promote cell-cycle arrest and apoptosis in various types of cancer cells,30 and BV possesses antimutagenic and anti-inflammatory properties.31,32
Accordingly the current work was designed mainly to evaluate the possible therapeutic role of graviola fruit and BV against breast cancer and complications in the ovaries of pregnant rats and their offspring.
Methods
Chemicals
N-methylnitrosourea (MNU; molecular weight 103.08 g, N1517-1G) was purchased from Sigma-Aldrich (St Louis, MO, USA).
Annona muricata
Fresh A. muricata fruit was washed, peeled, and cut into tiny pieces, liquified using a blender, and then filtrated to remove fibers and debris. The juice was kept frozen until use in the experiment.
Bee Venom
Dried BV was obtained from the Agriculture Research Center, Cairo, Egypt.
Experimental Animals
For this study, 32 Wistar albino rats (24 female and 8 male) weighing 150–160 g were put in wirebottomed cages in a room at 25°C±1°C with a 12-hour light–dark cycle and relative humidity about 50%. All procedures were performed in accordance with the guidelines of the bioethics committee of Damanhour University, and the university approved the animal experiments.
Induction of Breast Cancer
After acclimatization for 2 weeks, the female rats were injected intraperitoneally with a single dose of MNU (50 mg/kg body weight dissolved in 6.67 mL phosphate citrate–buffered saline).33 After 2 months’ MNU treatment and confirmation of positive tumor markers, the females were placed with the males (3:1) for matting. After 3–4 days and ensuring pregnancy via observation of vaginal plug and vaginal smears, pregnant rats were separated from males and randomly divided into four groups (n=6):
Group 1: MNU-induced female rats (cancer model without treatment).
Group 2: MNU-induced female rats supplemented with A. muricata juice (200 mg/kg) from day 4 of pregnancy till weaning.34
Group 3: MNU-induced female rats subcutaneously injected with two doses of BV (75 µg/kg dissolved in PBS) on days 4 and 16 of gestation.35
Group 4: MNU-induced female rats supplemented with both A. muricata juice and BV at the same previous doses.
At the end of the weaning period, the mothers and their offspring in all groups were killed. Mammary glands and ovaries of mothers and ovaries of their offspring were removed immediately. Blood samples were collected, centrifuged, and kept frozen.
Investigated Parameters
Histological Investigation
The organs (breasts and ovaries) obtained were fixed in 10% neutral buffered formalin. After fixation, the specimens were dehydrated in ascending grades of ethyl alcohol, cleared with xylene, and embedded in paraffin. Sections were stained in Mayer’s H&E.36 Other sections were immunohistochemically stained, then processed for investigation under bright-field light microscopy and photographed.
Immunohistochemical Staining
Paraffin-embedded breast sections (5 μm thick) were cut, put onto positively charged glass slides, rehydrated in descending grades of ethanol, and washed in PBS. The activity of endogenous peroxidase was blocked by immersing the slides in 3% H2O2 with methanol for 40 minutes at 25°C. The breast sections were processed for antigen retrieval by digestion in 0.05% trypsin, then washed in Tris buffered at pH 7.6. Sections were put in a diluted 1:10 monoclonal primary antibody (anti-p53, Dako DO7 clone) for 45 minutes. Slides were then immersed in PBS followed by incubation in the secondary antibody for 20 minutes. Finally, sections were counterstained with Mayer’s H&E, mounted, and photographed under phase-contrast light microscopy.
Sections (6 µm) from ovaries of mothers and their offspring (21 days old) were cut and mounted on lysine-coated slides. After deparaffinization and dehydration, the sections were digested with a mixture of proteinase K (Dako, Carpinteria, CA, USA) and protease (0.1%, Sigma-Aldrich) for 10 minutes. The sections were then incubated with a polyclonal rabbit anticalretinin antibody (1:50, Zymed Laboratories, San Francisco, CA, USA) at 25°C for 30 minutes. After washing with PBS, the sections were incubated with secondary biotinylated goat antimouse/rabbit IgG (Dako) for 20 minutes, followed by peroxidase-conjugated avidin (Dako) at room temperature for 20 minutes. The sections were counterstained with 0.1% H&E and photographed under phase-contrast light microscopy.
Serum Analysis
Two-point ELISA protocol was used to measure serum-matrix MMP1 (Amersham Pharmacia Biotech) in mothers and offspring. Samples were tested in duplicate. Color intensity in each well was measured on a microplate reader (Molecular Devices, SpectraMax Plus).Concentration of MMP1 in serum was calculated by interpolation from the standard curve.37
NFκB levels in the serum samples were measured using a commercial rat NFκB ELISA kit (E-EL-R0673, Elabscience, China).38
Measurement of serum-TNFα levels was done using sandwich ELISA method utilizing 2 monoclonal antibodies charged against separate antigenic determinants on rat TNFα. The kits were provided by Adlitteram Diagnostic Laboratories (San Diego, CA, USA).
Caspase 3 levels were measured in serum with a solid-phase enzyme-linked immunosorbent quantitative sandwich using human caspase 3 (Elisa Blue Gene Biotech, Shanghai, China). Intra- and interassay coefficients of variation were <5.6% and <7.9%, respectively. The detection limit for the assay was 0.1 ng/mL.39
Levels of lipid-peroxidation by-product (MDA) and catalytic action of catalase (CAT) were estimated spectrophotometrically. MDA was assessed using thiobarbituric acid assays based on the liberation of color complex due to thiobarbituric acid reaction with MDA. CAT activity was determined with assays based on the rate of hydrogen peroxide-ammonium molybdate complex formation.40 Superoxide dismutase (SOD) was measured according to the method of Sun et al.41
Statistical Analysis
Data are expressed as means ± SE. P<0.05 on one-way ANOVAwas considered significant.
Results
Histological Observations
Breast
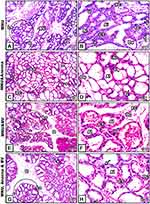
Breast sections of MNU-induced female rats showed degenerative breast lobules and pronounced cellular hyperplasia in the epithelial lining of alveoli and the ducts and ductules. Moreover, some alveoli and ducts showed clusters of hypertrophied cells with scattered vacuoles. Also, the connective tissue showed dendritic blood vessels and aggregated fat cells (Figure 1A and B). Following supplementation with A. muricata juice the sections revealed remarkable improvement in architecture and the cells of alveoli and ducts appeared intact with well-organized epithelial lining, in spite of some cellular hyperplasia still found in some ducts (Figure 1C and D). In contrast, supplementation with BV did not ameliorate the degenerative changes induced by MNU in breast alveoli or ducts (Figure 1E and F). However, cosupplementation with A. muricata fruit and BV revealed obvious ameliorative effects against the deleterious histological alterations induced by MNU (Figure 1G and H).
Ovaries of Mothers
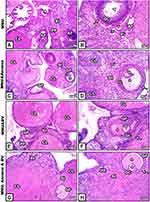
In MNU-induced female rats, the ovarian sections showed atretic or shrinkage follicles with lytic oocytes. Also, ovarian stroma appeared disintegrated, with scattered cytoplasmic vacuoles (Figure 2A and B). On the other hand, following supplementation with graviola fruit and/or BV, ovarian sections displayed obvious recovery in histological structure, and Graafian follicles appeared regular, filled with antra surrounded by thecal sheaths, and contain oocytes. In addition, corpus lutea and ovarian stromata appeared concomitant with normal histological appearance of standard ovarian structure (Figure 2C–H).
Ovaries of 21-Day-Old Offspring
Ovarian sections from MNU-exposed offspring revealed shrinkage of the entire ovary, increased number of atretic primary follicles, and disintegrated germinal epithelium (Figure 3A). On the other hand, the ovarian sections from offspring of rats supplemented with A. muricata and/or BV displayed remarkable restoration of damaged histological structures induced by MNU, with very little atretic follicles in some areas of the sections (Figure 3B–D).
Immunohistochemical Observations (p53 and Calretinin)
Immunohistochemical Observations of p53 in Breast
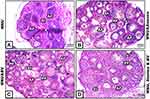
A strong positive immunoreaction for p53 protein appeared prominent in the nuclei and cytoplasm of most breast alveoli and duct cells of MNU-induced mothers; however, the breast connective tissue showed negative reactions (Figure 4A). A moderate positive reaction for p53 was observed in the breast tissue of mother rats supplemented with graviola fruit juice. Such reactions were restricted to the cytoplasm of breast alveoli cells, though very weak reactions were noticed in the lining epithelia of ducts (Figure 4B). Breast tissue of mothers supplemented with BV displayed very weak immunoreactivity for p53 especially in breast-duct cells; however alveoli showed negative reactions (Figure 4C). In mothers supplemented with A. muricata and BV, the degree of p53 immunoreactivty was apparently negative all over the breast tissue, with very few scattered cells appearing weakly stained in some areas of section (Figure 4D).
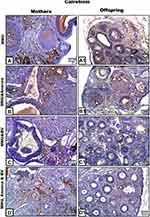
Calretinin in Ovarian Tissue of Mothers
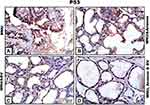
Ovarian sections from MNU-induced mothers rats displayed strong positive reactions for calretinin protein. This reaction was more prominent in thecal cells and antra of Graafian follicles, as well as in ovarian stromata (Figure 5A). In graviola-supplemented mother rats, some tissue of ovarian stroma and little cells of follicles appeared moderately stained with calretinin antibody; however, other tissue appeared negatively stained (Figure 5B). In BV-treated female rats, ovarian tissue appeared negatively stained for calretinin protein, with the exception of little areas in ovarian stroma, which displayed weakly stained cells (Figure 5C). In A. muricata and BV–supplemented mothers, ovarian stroma showed moderately calretinin-stained cells; however, follicular cells appeared negatively stained (Figure 5D).
Calretinin in Ovarian Tissue of 21-Day-Old Offspring
Ovarian sections from MNU exposed offspring displayed moderate reactions for calretinin, especially in stromata and cells of primary follicles (Figure 5A1). On the other hand, calretinin reactivity appeared very weak in the same ovarian follicles and negative in ovarian stroma for offspring whose mothers had been supplemented with A. muricata juice or BV (Figure 5B1 and C1), respectively. Furthermore, the ovarian tissue from offspring whose mothers had been supplemented with A. muricata juice and BV displayed prominent negative or very weak reactions for calretinin (Figure 5D1).
Serum MMP1
Serum-MMP1 levels appeared significantly lower in graviola- and/or BV-treated mothers (P<0.001); however; offspring showed highly significant increases (P<0.001) with graviola and no significant changes with BV alone or in combination with A. muricata (P>0.05) compared to the MNU-only group (Figure 6A).
Serum NFκB
Treatment with A. muricata juice and/or BV showed a highly significant decrease (P<0.001) in serum-NFκB levels of mothers compared to the MNU-only group. Offspring of mothers treated with A. muricata juice alone or incombination with BV showed a low significant decrease (P<0.05) in the serum-NFκB levels, while offspring of the BV-treated group showed a moderately significant decrease (P<0.01) NFκB compared to the MNU-only group (Figure 6B).
Serum TNFα
Treatment with graviola juice and/or BV showed a highly significant decrease (P<0.001) in serum-TNFα of mothers and offspring compared to the MNU-only group (Figure 6C).
Caspase 3
Serum caspase 3 in A. muricata juice– or BV-treated mothers showed a small significant increase (P<0.05), while their offspring showed a moderately significant increase (P<0.01) compared to the MNU-only group. However, treatment with both A. muricata and BV showed no significant difference (P>0.05) in serum caspase 3 of mothers, while their offspring showed a small significant increase (P<0.05; Figure 6D).
Serum MDA
In graviola juice– and/or BV-supplemented mother rats and their offspring, serum MDA levels were significantly lowered (P<0.001) compared to the MNU-only group (Figure 7A).
Changes in Serum Antioxidants
In A. muricata juice/BV-supplemented groups, the serum CAT in mothers showed a highly significant increase (P<0.001), but offspring showed no significant difference (P>0.05) with A. muricata and a highly significant increase with BV (P<0.001) compared to the MNU-only group. Cosupplementation with A. muricata and BV showed a moderately significant increase in CAT (P<0.01) in both mothers and offspring (Figure 7B).
A highly significant increase (P<0.001) in serum SOD was recorded in mothers and offspring who received graviola juice and/or BV compared to the MNU-only group (Figure 7C).
Discussion
In the current work, breast tissue of MNU treated female rats displayed severe histopathological signs and pronounced cellular hyperplasia. Supplementation of A. muricata fruit juice alone or in combination with BV to MNU-only mothers successfully restored the histopathological alterations and cellular hyperplasia of breast tissue induced by MNU. However, supplementation of BV alone did not ameliorate these alterations. Studies have revealed that MNU has an experimental evidence in induction of breast cancer.42,43 It has been confirmed that the histopathological signs and carcinogenic effects caused by MNU are basically attributable to methylation of DNA in the target cells.43 Ting et al found that MNU can induce cellular hyperplasia in breast tissue via acceleration of mitotic pathways in the epithelial lining of breast alveoli, as well as in ducts and ductules.44 Additionally, MNU can cause histopathological alterations in breast tissue through induction of oxidative stress, followed by pronounced decline in serum antioxidants, especially CAT and SOD.45
The ameliorative role of A. muricata against breast cancer in this study is consistent with previous research on breast cancer cell lines.46 Gomes et al reported that the vital phytochemical constituents of A. muricata, ie, alkaloids, saponins, flavonoids, tannins, phenols, and phytosterols, inhibited the propagation of breast cancer cells through activation of apoptotic pathways.47 Annonaceous acetogenins also have a potential role in inhibition of cancer cells.48
Several reports have found that BV and/or its component melittin have a potential therapeutic role against breast cancer, prostate cancer, hepatocellular carcinoma, and ovarian cancer cell lines via activation of apoptosis and necrosis.49–53 The results of the present do not align with thee. This may be attributable to differences in doses and environments.
The tumor-suppressing p53 protein is involved in the progression of a number of biological processes, such as cell-cycle arrest, apoptosis, senescence, and aging. Activation of p53 as a reaction to cancer can remove tumor cells through apoptosis or senescence. In the current work, the immunoreactivity of p53 was gradually reduced in the breast tissue of mothers that supplemented with A. muricata fruit extract and/or BV. The data on p53 expression in epithelial cells of ducts and alveoli in mammary tissue parallel Yoshizawa et al who reported that increased p53 activity in breast cancer tissue was considered as responsive mechanism to resist the hyperproliferative effect of MNU.54 The decreased activities of p53 in the tumor cells in breasts following treatment with graviola juice are also in line with previous studies.55,56 The authors suggest that annonaceous acetogenins can induce apoptosis through activation of the proapoptotic proteins caspase 3 and 7 and upregulation of Bax synthesis followed by downregulation of the antiapoptotic BCL2 and inactivation of the TP53 gene. In contrast to our results, A. muricata fruit can promote cell arrest in cancer cells through activation of p53 at the beginning of the early stage of tumor induction.57 This finding does not agree with our results, where this occurred at the end stage of tumor induction.
The inhibitory effect of BV on the immunoreactivity of p53 in breast tissue may be attributed to the BV-derived peptide apamin.58 The interaction between p53 and apamin is well documented to have an antiproliferative effect in some cancer cells via activation of apoptosis and elimination of tumor cells.59 The apoptotic role of A. muricata and BV was also confirmed in this study by significant elevation in serum caspase 3.
In the current work, severe complications were recorded in the histogenesis in ovaries of mothers and their offspring, and the ovarian sections of MNU-induced mother rats displayed several atretic follicles, lytic oocytes, and disintegrated ovarian stromata with scattered cytoplasmic vacuoles. Furthermore, ovarian sections from offspring revealed shrinkage of the entire ovary, multiple atretic primary follicles, and remarkable disintegration of the germinal epithelium. Ovarian histopathological signs induced by MNU were markedly ameliorated following supplementation with A. muricata juice and/or BV. Similar observations have been recorded in the ovarian sections of MNU- and 7,12-dimethylbenzα-anthracene (DMBA)–induced mother rats and MNU-induced offspring.44,60 Syed et al declared that systemic MNU and DMBA appeared to contribute not only to breast oncogenesis but also to the initiation of ovarian preneoplasia.61 Theey also added that MNU is implicated in the proliferative effect of estrogen receptors, which is a major cause of initiation of ovarian neoplastic changes. Similarly, Stewart et al reported that local ovarian DMBA induced more ovarian histopathological lesions.62
Studies have revealed that annonaceous acetogenins are a vital source of enzymatic antioxidants like CAT and SOD and nonenzymatic antioxidants, incluing vitamin C and E.63,64 These antioxidants play a vital role in the restoration of ovarian histopathological signs induced by MNU.
The ameliorative role of BV against the deleterious histological alterations induced by MNU in the ovaries of mother rats and their offspring may be attributed to the metformin constituent of BV, which play an essential role in suppression of serum NFκB elevated by MNU.65
Generally, oxidative stress induced by MNU is implicated in production of free radicals, which results in significant increases in lipid peroxidation and consequently significant decreases in antioxidant enzymes. This is in accordance with our results. In the current work, supplementation of A. muricata extract and/or BV was able to increase the levels of serum SOD and CAT that had been decreased by MNU. Previous reports have revealed that graviola megastigmanes and cyclopeptides and essential oils play a potential role in inhibition of oxidative stress and elevation of antioxidants.66,67 Other studies have shown that the antioxidant effects of A. muricata may be attributed to phytochemical constituents, such as luteolin, quercetin, epicatechin gallate, and emodin,63,68 and vitamin C and carotenoids.69
Melittin, apamin, and adolapin constituents of BV have pronounced antioxidant, anti-inflammatory, and antiproliferative effects.70,71 Hegazi reported that BV therapy is a potent antioxidant that leads to decreased the levels of ROS.72 Other studies have revealed that BV can inhibit production of superoxide anions by human neutrophils result in decreased MDA and elevated antioxidant enzymes.73,74
Previous reports have been discovered that calretinin within the mesothelial cells and the ovarian tissue.67,75,76 Calretinin reactivity may also be necessary for the diagnosis of ovarian tumors.77,78 In the current work, a strong positive calretinin immunoreaction was noticed in ovarian tissue of mothers, while their offspring displayed moderate calretinin expression in their ovarian follicles; however, this expression was weak to moderate following supplementation with A. muricata and/or BV. BV was able to inhibit the expression of calretinin activity if used alone, but when used with A. muricata extract, calretinin appeared moderately expressed in ovarian tissue. Overexpression of calretinin in ovarian tissue of MNU-induced mother rats parallels previous reports.77,79 In comparison to mothers, the moderate immunoexpression of calretinin in the ovarian follicles of offspring may be attributable to small leakage of MNU via the placenta to the fetus during gestation. The negative expression of calretinin in the ovarian tissue of MNU-induced mother ratssupplemented with BV is mainly due to the direct cytotoxic effects of melittin, which manifests in decreased TNFα secretion.80,81 The moderate or weak expression of calretinin in the ovarian tissue of A. muricata–supplemented rats was confirmed by Pieme et al, who reported that acetogenins, alkaloids, and terpenoids of A. muricata can inhibit calretinin activity.55
Proinflammatory cytokines like TNFα play an important role in tissue damage.82,83 As such, in the current study, the ameliorative role of A. muricata juice might be attributable to its anti-inflammatory properties84 and attenuation of TNFα expression.82,83 The results of the present work are in accordance with previous studies in that a significant decrease in serum TNFα and significant increase in serum of caspase 3 were noticed after supplementation with A. muricata juice and/or BV.
The present study found that treatment with BV significantly decreased elevated levels of NFκB caused by MNU. This result indicates the cell-protective effect of BV.85 Other studies have found that BV has a potent anti-inflammatory role through the direct inhibition of NFκB and key inflammatory mediators like TNFα.29,80,81,86 This was confirmed by histological examination of mothers’ breasts and ovaries of both mothers and offspring. These findings explain the antiameliorative effect of BV, in line with the results in this study.
In conclusion, A. muricata fruit has a more powerful therapeutic role than BV against MNU-induced breast cancer in rats. However, both have a powerful ameliorative role against ovarian histopathological alterations induced by MNU. Such ameliorative effects of A. muricata and BV are mainly attributed to their antioxidant, anti-inflammatory, and antiproliferative constituents. This conclusion was drawn from the histological and biochemical results of this study.
Acknowledgment
The authors would like to express their deepest gratitude and appreciation to the team of the Central Lab of Physiology in the Faculty of Science, Damanhour University, for facilitating the overall conditions to measure parameters in this research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work did not receive any specific funding.
Disclosure
The authors declare that they have no conflict of interests in this work.
References
1. Abou Zaid OA, H. A-M, A. E, A. O. Biochemical study on the effect of cetyl trimethyl ammonium bromide surfactant coated spirulina zinc oxide nanocomposite as antimammary carcinogenesis in rats. Benha Vet Med J. 2015;29(1):170–182. doi:10.21608/bvmj.2015.31815
2. Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9:730–756.
3. Keinan‑Boker L, Lerner‑Geva L, Kaufman B, Meirow D. Pregnancy‑associated breast cancer. ISR Med Assoc J. 2008;10:722‑7.
4. Hahn KM, Johnson PH, Gordon N. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer. 2006;107:1219‑26.
5. Mc Pherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321(7261):624–628.
6. Shah R, Rosso K, Nathanson SD. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J Clin Oncol. 2014;5(3):283–298.
7. Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21(3):361–370.
8. Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793.
9. Shinoura N, Yamada R, Okamoto K, Nakamura O, Shitara N. Local recurrence of metastatic brain tumor after stereotactic radiosurgery or surgery plus radiation. J Neuro-Oncol. 2002;60:71–77.
10. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi:10.1249/MSS.0b013e3181e0c112
11. Sjövall K, Strömbeck G, Löfgren A, Bendahl P, Gunnars B. Adjuvant radiotherapy of women with breast cancer – information, support and side-effects. Eur J Oncol Nurs. 2010;14(2):147–153. doi:10.1016/j.ejon.2009.09.002
12. Gupta S, Kim J, Prasad S, Aggarwal B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29(3):405–434.
13. Moghadamtousi S, Fadaeinasab M, Nikzad S, et al. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16(7):15625–15658. doi:10.3390/ijms160715625
14. Ko J-H, Sethi G, Um J, et al. The role of resveratrol in cancer therapy. Int J Mo Sci. 2017;18(12):2589.
15. Ko J-H, Sethi G, Um J, et al. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18(12):36.
16. Gullett N, Amin A, Bayraktar S. Cancer prevention with natural compounds. Semin Oncol. 2010;37(3):258–281.
17. Camargo E, Bandeira M, de Oliveira A. Diagnosis of public programs focused on herbal medicines in Brazil. Nat Prod Commun. 2011;6(7):1001–1002.
18. Komlaga G, Agyare C, Dickson R. Medicinal plants and finished marketed herbal products used in the treatment of malaria in the Ashanti region, Ghana. J Ethnopharmacol. 2015;172:333–346.
19. George VC, Kumar DR, Suresh PK, Kumar RA. Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J Food Sci Technol. 2014;52(4):2328–2335.
20. Wélé A, Ndoye I, Badiane M. Fatty acid ans essential oil composition of the seed oil of five Annona species. Nigerian J Nat Prod Med. 2005;8(1):62–65.
21. Kuete V, Dzotam J, Voukeng I, Fankam A, Efferth T. Cytotoxicity of methanol extracts of Annona muricata, Passiflora edulis and nine other Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Springerplus. 2016;5(1):1–12.
22. Sun S, Liu J, Zhou N, Zhu W, Dou Q, Zhou K. Isolation of three new annonaceous acetogenins from graviola fruit (Annona muricata) and their antiproliferation on human prostate cancer cell PC-3. Bioorg Med Chem Lett. 2016;26(17):4382–4385.
23. Mishra S, Ahmad S, Kumar N, Sharma B. Annona muricata (the cancer killer): a review. Global J Pharmace Res. 2013;2(1):1613–1618.
24. Brussell DE. A medicinal plant collection from Montserrat, West Indies. Econ Bot. 2004;58:203–220.
25. Kamaraj C, Rahuman AA. Efficacy of anthelmintic properties of medicinal plant extracts against Haemonchus contortus. Res Vet Sci. 2011;91(3):400–404.
26. Diaz-Garcia A, Morier-Diaz L, Frion-Herrera Y, et al. In vitro anticancer effect of venom from Cuban scorpion Rhopalurus junceus against a panel of human cancer cell lines. J Venom Res. 2013;4:5–12.
27. Premratanachai P, Chanchao C. Review of the anticancer activities of bee products. Asian Pac J Trop Biomed. 2014;4(5):337–344.
28. Silva J, Monge-Fuentes V, Gomes F, et al. Pharmacological alternatives for the treatment of neurodegenerative disorders: wasp and bee venoms and their components as new neuroactive tools. Toxins. 2015;7(8):3179–3209.
29. Son DJ, Lee JW, Lee YH, et al. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115(2):246–270.
30. Kohno M, Horibe T, Ohara K, Ito S, Kawakami K. The membrane-lytic peptides K8L9 and melittin enter cancer cells via receptor endocytosis following subcytotoxic exposure. Chem Biol. 2014;21(11):1522–1532.
31. Varanda EA, Monti R, Tavares DC. Inhibitory effect of propolis and bee venom on the mutagenicity of some direct- and indirect-acting mutagens. Teratog Carcinog Mutagen. 1999;19(6):403–413.
32. Nam KW, Je KH, Lee JH. Inhibition of COX-2 activity and proinflammatory cytokines (TNF-alpha and IL-1beta) production by water-soluble sub-fractionated parts from bee (Apis mellifera) venom. Arch Pharm Res. 2003;26(5):383–388.
33. Steinetz BG, Gordon T, Lasano S, et al. The parity-related protection against breast cancer is compromised by cigarette smoke during rat pregnancy: observations on tumorigenesis and immunological defenses of the neonate. Carcinogenesis. 2006;27(6):1146–1152.
34. Dai Y, Hogan S, Schmelz E, et al. Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr Cancer. 2011;63(5):795–801.
35. Oršolić N, Šver L, Verstovsek S, Terzić S, Bašić I. Inhibition of mammary carcinoma cell proliferation in vitro and tumor growth in vivo by bee venom. Toxicon. 2003;41(7):861–870.
36. Bancroft JD, Gamble M. Theory and Practice of Histological Techniques.
37. Daniels J, Schultz G, Blalock T, et al. Mediation of transforming growth factor-1-stimulated matrix contraction by fibroblasts. A role for connective tissue growth factor in contractilescarring. Am J Pathol. 2003;163(5):2043–2052.
38. Usta A, Dede S. The effect of thymoquinone on nuclear factor Kappa B Levels and Oxidative DNA Damage on experimental diabetic rats. Pharmacogn Mag. 2017;13(l 3):458–461.
39. Lorente L, Martín M, Argueso M, et al. Serum caspase-3 levels and mortality are associated in patients with severe traumatic brain injury. BMC Neurol. 2015;15(228):1–6.
40. Peluso M, Munnia A, Risso GM, et al. Breast fine-needle aspiration malondialdehyde deoxyguanosine adduct in breast cancer. Free Radic Res. 2011;45(4):477–482.
41. Sun Y, Oberley LW, Li YA. Simple method for clinical assay of superoxide dismutase. ClinChem. 1988;34:497–500.
42. Tsubura A, Lai Y, Miki H, et al. Animal Models of N-Methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials. vivo. 2011;25(1):11–22.
43. Kour M, Anantharaya V, Bhat K, Chakraborti S, Kodavanji B. Effect of vitex agnus extract on MNU induced mammary tumor of sprague dawley rats. Young Pharm. 2017;9(3):367–370.
44. Ting AY, Kimler BF, Fabian CJ, Petroff BK. Characterization of a preclinical model of simultaneous breast and ovarian cancer progression. Carcinogenesis. 2007;28(1):130–135.
45. Ragab OA, Ahmed AS, El-sonbaty SM, Aboel-ftouh AE. Effect of probiotic fermented soy milk and gamma radiation on nitrosourea-induced mammary carcinogenesis. Benha Vet Med J. 2013;25(2):
46. Gavamukulya Y, Abou-Elella F, Wamunyokoli F, Ael-Shemy H. Phytochemical screening, anti-oxidant activity and in vitro anticancer potential of ethanolic and water leaves extracts of Annona muricata (Graviola). Asian Pac J Trop Med. 2014;7:355–363.
47. Gomes J, de Sousa Araújo T, NobreV CD. Antiproliferative activity, antioxidant capacity and tannin content in plants of semi-arid northeastern Brazil. Molecules. 2010;15(12):8534–8542.
48. Tundis R, Xiao J, Loizzo MR. Annona species (Annonaceae): a rich source of potential antitumor agents. Ann N Y Acad Sci. 2017;1398(1):30–36.
49. Ip SW, Liao SS, Lin SY. The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. Vivo. 2008;22(2):237–245.
50. Park MH, Choi MS, Kwak DH. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-kappaB. Prostate. 2011;71(8):801–812.
51. Li B, Gu W, Zhang C, et al. Growth arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Onkologie. 2006;29(8–9):367–371.
52. Jo M, Park MH, Kollipara PS, et al. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol Appl Pharmacol. 2012;258(1):72–81.
53. Putz T, Ramoner R, Gander H, et al. Antitumor action and immune activation through cooperation of bee venom secretory phospholipase A2 and phosphatidylinositol-(3,4)-bisphosphate cancer immunology. Cancer Immunol Immunother. 2006;55(11):1374–1383.
54. Yoshizawa K, Kuwata M, Kawanaka A, et al. N-Methyl-N-nitrosourea-induced retinal degeneration in mice is independent of the p53 gene. Mol Vis. 2009;15:2919–2925.
55. Pieme C, Kumar S, Dongmo M. Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement Altern Med. 2014;14(1):516–526.
56. Liu N, Yang H, Wang P. Functional proteomic analysis revels that the ethanol extract of Annona muricata L. induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J Ethnopharmacol. 2016;189:210–217.
57. Agu K, Okolie N, Falodun A, Engel-Lutz N. In vitro anticancer assessments of Annona muricata fractions and in vitro antioxidant profile of fractions and isolated acetogenin (15-acetyl guanacone). J Cancer Res Pract. 2018;5:53–66.
58. Li C, Pazgier M, Liu M, Lu WY, Lu W. Apamin as a template for structure-based rational design of potent peptide activators of p53. Angew Chem Int Ed Engl. 2009;48(46):8712–8715.
59. Bykov VJ, Issaeva N, Shilov A, et al. Restoration of the tumor suppressor functions to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8(3):282–288.
60. Okamura H, Katabuchi H. Detailed morphology of human ovarian surface epithelium focusing on its metaplastic and neoplastic capability. Ital J Anat Embryol. 2001;106(2):263–276.
61. Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61(18):6768–6776.
62. Stewart SL, Querec TD, Ochman AR, et al. Characterization of a carcinogenesis rat model of ovarian preneoplasia and neoplasia. Cancer Res. 2014;64(22):8177–8183.
63. George V, Kumar D, Suresh P, Kumar R. Antioxidant, DNA protective efficacy and HPLC analysis of Annona muricata (soursop) extracts. J Food Sci Tech. 2015;52(4):2328–2335.
64. Vijayameena C, Sabhashini G, Loganayagi M, Ramesh B. Phytochemical screening ans assessment of antibacterial activity for the bioactive compounds in. Annona Muricata Int J Curr Microbiol App Sci. 2013;2(1):1–8.
65. Diamanti-Kandarakis E, Paterakis T, Alexandraki K, et al. Indices of low-grade chronic inflammation in polycystic ovary syndrome and the beneficial effect of metformin. Hum Reprod. 2006;21(6):1426–1431.
66. Matsushige A, Matsunami K, Kotake Y, Otsuka H, Ohta S. Three new megastigmanes from the leaves of Annona muricata. J Nat Med. 2012;66(2):284–291.
67. Adewole SO, Ojewole JA. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid prof iles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afri J Tradit Complement Altenr Med. 2008;6(1):30–41.
68. Muthu S, Durairaj B. Evaluation of antioxidant and free radical scavenging activity of Annona muricata. Eur J Exp Biol. 2015;5(3):39–45.
69. Usunomena U, Paulinus ON. Phytochemical analysis and mineral composition of Annonamuricata leaves. IJRERD. 2015;1(1):38–42.
70. Müller UR. (2011). Hymenoptera venom proteins and peptides for diagnosis and treatment of venom allergic patients. Inflamm Allergy Drug Targets. 2011;10(5):420–428.
71. Chowdhury FR, Bari MS, Shafi AM, et al. Acute Kidney Injury Following Rhabdomyolysis due to Multiple Wasp Stings (Vespa affinis). Asian Pac JMed Toxic. 2014;3:41–43.
72. Hegazi AG. Medical importance of bee products. Uludag Bee J. 2014;12:136–146.
73. Stanley DS, Jean-Louis S, Charles M, Francine G, Emil S. Bee venom inhibits superoxide production by human neutrophils. Inflammation. 1984;8(4):385–391.
74. Abdel-Rahman M, Elebiary AS, Hafez SS, Mohammed HE, Abdel Moneimm AE. Therapeutic activity of bee-stings therapy in rheumatoid arthritis causes inflammation and oxidative stress in female patients. Int J Res Ayurveda Pharm. 2013;4(3):316–321.
75. Bertschy S, Genton CY, Gotzos V. Selective immunocytochemical localization of calretinin in the human ovary. Histochem Cell Biol. 1998;109(1):59–66.
76. Deavers MT, Malpica A, Liu J, Broaddus R, Silva EG. Ovarian Sex Cord-Stromal Tumors: an Immunohistochemical Study Including a Comparison of Calretinin and Inhibin. Mod Pathol. 2003;16(6):584–590.
77. Cao QJ, Jones JG, Li M. Expression of calretinin in human ovary, testis, and ovarian sex cord-stromal tumors. Int J Gyneco Pathol. 2001;20(4):346–352.
78. Movahedi-Lankarani S, Kurman RJ. Calretinin, a more sensitive but less specific marker than α-inhibin for ovarian sex cord-stromal neoplasms: an immunohistochemical study of 215 cases. Am J Surg Pathol. 2002;26(1):1477–1483.
79. Mc Cluggage WG, Maxwell P. Immunohistochemical staining for calretinin is useful in the diagnosis of ovarian sex cord-stromal tumours. Histopathology. 2001;38(5):403–408.
80. Kwon YB, Kim HW, Ham TW, et al. The anti-inflammatory effect of bee venom stimulation in a mouse air pouch model is mediated by adrenal medullary activity. J Neuroendocrinol. 2003;15(1):93–96.
81. Moon DO, Park SY, Lee KJ, et al. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. 2007;7(8):1092–1101.
82. Chan P, Ah R, Mh K. Anti-arthritic activities of Annona muricataL Leaves extract on complete Freund’s adjuvant (CFA)-induced arthritis in rats. Planta Med. 2010;76:166.
83. Hamid RA, Foong CP, Ahmad Z, Hussain MK. Antinociceptive and anti-ulcerogenic activities of the ethanolic extract of Annona muricata leaf. Rev Bras Farmacogn Braz J Pharmacogn. 2012;22(3):630–641.
84. Ishola IO, Awodele O, Olusayero AM, Ochieng CO. Mechanisms of analgesic and anti-inflammatory properties of AnnonamuricataLinn. (Annonaceae) fruit extract in rodents. J Med Food. 2014;17(12):1375–1382.
85. Darwish SF, El balky WM, Arafa HM, El-Demerdash E. Targeting TNF-and NF-kB activation by bee venom: role in suppressing adjuvant induced arthritis and methotrexate hepatotoxicity in rats. Bee Venom Acupuncture and Methotrexate Interaction. PLoS One. 2013;8(11):1–13.
86. Park HJ, Lee SH, Son DJ, et al. Antiarthritic effect of bee venom: inhibition of inflammation mediator generation by suppression of NF-kappa B through interaction with the p50 subunit. Arthritis Rheum. 2004;50(11):3504–3515.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.