Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Therapeutic Effects of Garlic on Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial
Authors Soleimani D, Paknahad Z, Rouhani MH
Received 23 March 2020
Accepted for publication 21 June 2020
Published 7 July 2020 Volume 2020:13 Pages 2389—2397
DOI https://doi.org/10.2147/DMSO.S254555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Davood Soleimani,1 Zamzam Paknahad,2 Mohammad Hossein Rouhani3
1Nutritional Sciences Department, School of Nutrition Sciences and Food Technology, Kermanshah University of Medical Sciences, Kermanshah, Iran; 2Department of Clinical Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran; 3Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
Correspondence: Zamzam Paknahad Tel +98 3137923166
Fax +98 3136681378
Email [email protected]
Background and Aims: Emerging evidence suggests that garlic (Allium sativum L.) and its bioactive components can mitigate hepatic steatosis by the modulation of hepatic lipid metabolism. We aimed to assess the efficacy of the garlic administration on hepatic steatosis in patients with NAFLD.
Patients and Methods: This clinical trial was conducted on adult patients with ultrasound-diagnosed NAFLD. Eligible participants were randomly assigned, with the use of the stratified blocked procedure, to receive 800 mg garlic or placebo for 15 weeks. The primary outcome was the improvement in the hepatic steatosis diagnosed by ultrasound technique after 15 weeks of intervention.
Results: A total of 110 patients underwent randomization, and 98 patients completed the trial. Twenty-four (51.1%) patients in the garlic group achieved improvement in the hepatic steatosis compared to eight (15.7%) patients in the placebo group with the relative risk of 5.6 (95% CI: 2.17 to 14.5; P=0.001), which remained significant after adjusting for baseline value of hepatic steatosis. There were significant reductions in weight and serum ALT, AST, FBS, Hb A1C, total cholesterol, LDL-cholesterol, and TG concentration with the garlic intake compared to placebo (P< 0.05). The results were also significant after adjusting for weight change, energy intake, and physical activity. No serious adverse effects were observed with the garlic intake.
Conclusion: The intake of garlic powder was accompanied by a significant improvement in the hepatic steatosis and comorbidity related to this condition among subjects with NAFLD.
Keywords: Allium sativum, garlic, hepatic steatosis, nonalcoholic fatty liver disease
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome, affecting nearly a quarter of adults worldwide.1,2 This disease involves a broad spectrum of hepatic damage from simple hepatic steatosis to nonalcoholic steatohepatitis (NASH), which can progress to fibrosis or even carcinoma.3 NAFLD has been recently considered as an independent risk factor for cardiovascular events.4 It has been predicted NASH-related cirrhosis to be the main indication for liver transplantation by 2020.5 Despite these facts, there is no licensed pharmacological treatment for NAFLD patients.6
The molecular mechanisms involved in the initiation of NAFLD have not been completely elucidated. Nevertheless, insulin resistance and inflammation exert critical roles in the progression of NAFLD.7 Current therapeutic approach in patients with NAFLD limits to lifestyle modification which is not feasible and poses a major challenge for most patients.8 Emerging evidence suggests that some functional foods and nutriceuticals can exert protective roles in the management of NAFLD by regulating inflammation and hepatic lipid metabolism and modulating gut bacteria flora.9–11
Garlic (Allium sativum L.) is a vegetable belonging to the Liliaceae family. It has been used as a medicinal plant in complementary medicine in many countries and cultures. Nowadays, garlic is considered a functional food with a broad spectrum of organosulfur compounds, such as alliin, allicin, vinyl dithiins, and ajoene, which have antioxidant and anti-inflammatory properties.12,13 Numerous studies have indicated the therapeutic potential of garlic and its derivatives against modifiable cardiovascular risk factors14 and cancer.15 Also, experimental studies show that garlic and its derivatives can mitigate the hepatic steatosis by down-regulating the expression of sterol regulatory element-binding protein-1c (SREBP1c) as a lipogenic gene and up-regulating the expression of peroxisome proliferator-activated receptor α (PPARα) and carnitine palmitoyltransferase-1 (CPT-1) as lipolytic genes.16 However, the therapeutic effect of garlic for hepatic steatosis in NAFLD patients remains unknown. Therefore, the current trial aimed to evaluate the efficacy of garlic supplementation on hepatic steatosis among patients with NAFLD.
Patients and Methods
Participants
This placebo-controlled, double-blind, parallel-group, randomized clinical trial focused on adults with ultrasound-diagnosed NAFLD. Participants were recruited from the Metabolic Liver Disease Research Center at Isfahan University of Medical Sciences, Isfahan, Iran. The Research Council and Ethics Committee of the Isfahan University of Medical Sciences approved the study protocol (ID: 393,637) under the Declaration of Helsinki Written informed consent forms were completed by all the participants. This clinical trial was registered at the Iranian Registry of Clinical Trials website (ID: IRCT2014110819853N1).
Patients who were enrolled in the study underwent screening based on inclusion and exclusion criteria. Following inclusion criteria were considered: an age between 20 and 70 years, alanine aminotransferase (ALT) or/and aspartate aminotransferase (AST) above 40 U/L, evidence of hepatic steatosis on ultrasound imaging, having no weight management program during the last three months, the absence of other causes of hepatic steatosis such as alcohol consumption, hypothyroidism, and hepatotoxic drugs or corticosteroids consumption. We also excluded patients if they had a pregnancy, lactation, or any change in their drugs during the study period.
Study Design
This study was a 15-week intervention with garlic supplement or placebo in adults with ultrasound-diagnosed NAFLD. Participants enrolled in the study were assessed for eligibility criteria in the first visit. The randomization procedure was performed using the stratified permuted block method, in which stratification was based on fatty liver grade and sex. The eligible participants were then randomly assigned to one of two groups; a group receiving enteric-coated garlic powder supplement at a dose of 400 mg (equal to 1.5 mg allicin) two times daily and a group receiving garlic-like placebo (microcrystalline cellulose) supplement for 15 weeks. Compliance with assigned treatment in each group was measured by tablet count (tablets are taken/tablets prescribed) throughout the study. Poor compliance was defined as taking less than 80% of prescribed tablets. The treatment assignment was concealed from the patients, investigators, staff, radiologist, and hepatologist throughout the study.
Outcomes
The primary outcome was the improvement in hepatic steatosis diagnosed by ultrasound. The improvement was defined as a reduction in the grade of the hepatic steatosis from the 1st to the 15th week of the intervention. The secondary outcomes were changes in body weight and serum ALT, AST, low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), total cholesterol (TC), triglyceride (TG), fasting blood sugar (FBS), and hemoglobin A1c (HbA1) concentrations.
Liver Assessment
Hepatic steatosis was evaluated with the use of the Esaote ultrasound device with a convex probe of 3.5 MHz (Megahertz) at the 1st and 15th week of the intervention. Ultrasound assessment was carried out using the same equipment and by the same expert radiologist with patients in the fasting state. All ultrasound assessments were performed on the right liver lobe through intercostal spaces with the patient in the supine position and the right arm in maximal abduction. The same expert hepatologist interpreted the liver ultrasound images and were categorized into four grades: grade 0 or normal echogenicity of the liver parenchyma; grade Ι or minimal increase in echogenicity of the liver parenchyma with normal visualization of the diaphragm and intrahepatic vessel borders; grade ΙΙ or moderate increase in echogenicity of the liver parenchyma with minimal impaired visualization of the diaphragm and intrahepatic vessel borders; and grade ΙΙΙ or severe increase in echogenicity of the liver parenchyma with severe impaired visualization of the diaphragm, intrahepatic vessel borders, and posterior right hepatic lobe.17
Anthropometric Assessment
The anthropometric assessment was administered by a well-trained staff. Briefly, body weight and fat mass were measured with light clothes using the bioelectrical impedance analysis (BIA) instrument (Jawon IOI 353, Korea). Patients were asked to avoid taking tea or caffeine and exercising for at least 24 and 6 hours before measuring the BIA, respectively, respectively, based on the methods of using BIA.18 Height was measured using a portable stadiometer (Seca, Hamburg, Germany), without shoes. Body mass index (BMI) was calculated as weight (kilogram) divided by height (meter) squared.
Dietary and Physical Activity Assessment
Dietary assessment was done by a three-day dietary record, including two weekdays and one weekend day, of each participant at the 1st and 15th week of the intervention. Participants were instructed how to complete three consecutive days of food records and estimate food portion size. A trained nutritionist converted each food item to grams or milliliters per day using household measurements, and then to energy and nutrients intake using the Nutritionist IV software (version 7.0; N-squared Computing, Salam, OR, USA). Physical activity was estimated using the International Physical Activity Questionnaire (IPAQ) short format at the 1st, 7th, and 15th week of the intervention. The intensity of physical activity was calculated based on the IPAQ guideline and was expressed as metabolic equivalent (MET) hours per day.
Biochemical Assessment
The biochemical assessment was conducted on blood samples drawn from the cubital vein after an overnight fast. Blood samples were collected into EDTA containing tubes. The collected blood samples were immediately coagulated and centrifuged at 3000 rpm for 10 minutes at 4°C to separate serum. HbA1c concentration was measured in whole blood sample immediately after collection by immunoturbidimetric assay (Pars Azmoun kit, Tehran, Iran). Serum ALT, AST, TC, LDL-c, HDL-c, TG, and FBS were determined by photometric assay with the use of a biochemistry autoanalyzer (Alfa-Classic; Tajhizat Sanjesh Co., Ltd., Iran) and commercial kits (Pars Azmoun kit, Tehran, Iran).
Statistical Analysis
Statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS) software version 16. The sample size was estimated to be 47 patients in each group to detect at least 20% difference in the primary outcome between two groups with a 90% power at a significance level of 5% based on sample size tables for the exact single-stage Phase II designs19 and previous study.20 The Kolmogorov–Smirnov test was used to determine the normal distribution of the variables. The qualitative variables were compared between two groups with the use of Chi-square test. The independent sample T-Test and Mann–Whitney U-Test were used to carry out within-group comparisons. Between-group comparisons were carried out using the Paired sample T-Test and Wilcoxon Test. The mean change of secondary outcome was adjusted energy intake and physical activity with the use of analysis of covariance (ANCOVA) test. The adjusted odds ratio for the primary outcome was calculated using the binary logistic regression analysis and the Mantel-Haenszel test. We also conducted the intention-to-treat method as a sensitivity analysis for the primary outcome in which discontinued patients imputed as no improvement. A P-value less than 0.05 was considered to be significant.
Results
Two hundred five patients were enrolled in the trial and underwent screening for eligibility. Among these patients, 110 eligible patients underwent the randomization procedure. Twelve patients dropped out during the study because of the change in their telephone number (3 patients), poor compliance (5 patients), or other reasons (Figure 1). The dropout rate was 14.5% in the garlic and 7.2% in the placebo group. There was no significant association between garlic intake and dropout rate (Relative risk: 1.39; 95% CI, 0.89 to 2.18; P=0.22). Participants’ compliance with assigned treatment was reasonable in both groups (garlic: 96 ± 2.2% vs placebo: 95.6 ± 2.4%; P=0.64).
Patients’ baseline characteristics are shown in Table 1. No significant differences were seen in the baseline characteristics between the two groups. Additionally, between-group comparison of patients completing the study resulted in no significant differences at the baseline characteristics. The average dietary intakes during the study are shown in Table 2. The food record showed the distribution of macronutrient was similar in both groups during the study period. The mean physical activity did not significantly differ between the two groups during the study (garlic: 2.59 ± 0.48 MET h/day vs placebo: 2.73 ± 0.42 MET h/day; P = 0.14).
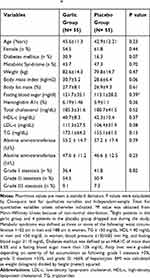 |
Table 1 Between-Group Comparisons of Demographic and Clinical Characteristics of Patients Who Underwent Randomization Procedure# |
 |
Table 2 Comparison of Energy and Main Nutrient Intakes Between Garlic and Placebo Group During the Intervention Period |
The adjusted mean changes in secondary outcomes are shown in Table 3. The within-group comparisons showed that the garlic intake resulted in a significant reduction in weight, FBS, HbA1c, ALT, AST, TG, LDL-c, and total cholesterol (P<0.05). In the placebo group, a significant reduction was observed in weight, whereas FBS and HbA1c concentrations were significantly increased at the end of the study. The reduction in the above variables was statistically significant in the garlic group compared to the placebo group, even after the adjustment for energy intake and physical activity.
 |
Table 3 Energy and Physical Activity-Adjusted Mean Change in Metabolic Factors and Serum Liver Enzymes from Baseline and After 15 Weeks of Study |
The changes in the ultrasound findings of hepatic steatosis are illustrated in Figure 2. At the end of 15th week of the intervention, hepatic steatosis was increased from grade I to II in 1 patient (7.1%); had no change from grade I in 11 patients (78.6), from grade II in 10 patients (34.5%), from grade III in 1 patient (25%); and was decreased from grade I in 2 patients (14.3%), from II to I in 19 patients (65.5%), from III to II in 1 patient (25%), and from III to I in 2 patients (50%) who received garlic. In the placebo group, hepatic steatosis was increased from grade I to II in 5 patients (21.7%); had no change from grade I in 16 patients (69.6%), from grade II in 20 patients (80%), from grade III in 2 patients (66.6%); and was decreased from grade I in 2 patients (8.7%), from II to I in 5 patients (20%), and from III to II in 1 patient (33.3%).
Totally, twenty-four patients (51.1%, P=0.001) in the garlic group and eight patients (15.7%, P=0.45) in the placebo group had at least one degree of improvement in hepatic steatosis which was significantly higher in the garlic group than the placebo group (P=0.001). The odds of improvement in hepatic steatosis with garlic was 5.6 (95% CI: 2.17 to 14.5; P=0.001) which remained significant after adjusting for baseline value of hepatic steatosis (adjusted odds ratio: 5.25; 95% CI: 1.95 to 14.11; P<0.001) and energy intake, physical activity, and weight loss (adjusted odds ratio: 5.5; 95% CI: 2.15 to 14.2; P<0.001). The intention-to-treat analysis indicated that 43.6% patients with a garlic treatment and 14.5% patients with placebo treatment had an improvement in hepatic steatosis (Relative risk: 3; 95% CI, 1.5 to 6.1; P=0.001). Similar results revealed that the discontinued patients did not significantly affect the primary outcome of our study.
No serious adverse events were reported by patients across the study. Interestingly, only one patient who received garlic treatment reported back pain, which was transient and deemed unrelated to garlic intake.
Discussion
The current clinical trial indicated that intake of garlic powder at 800 mg/day for 15 weeks improved hepatic steatosis, liver enzymes, lipid profiles, and fasting blood sugar in patients with NAFLD. The beneficial effects of garlic supplement on cardiovascular disease risk factors have been shown in previous studies, whereas no data exists regarding hepatic steatosis among NAFLD patients. To our knowledge, the current study is the first clinical trial that has highly focused on the above issue.
The current therapeutic approach for NAFLD treatment is lifestyle modification, in which weight management is the most important factor.21 In the current trial, the consumption of garlic powder was accompanied by a slight reduction in body weight, which remained unchanged after the adjustment for energy intake and physical activity. Consistent with our findings, the administration of garlic extract influenced body weight in postmenopausal women.22 It is suggested that the weight-reducing effect of garlic has been attributed to its thermogenic effect through adenosine monophosphate-activated protein kinase activation.23
The current study showed that garlic treatment has a significant reduction in fasting blood sugar as well as HbA1C. In accordance with our findings, a meta-analysis indicated that the garlic powder intake results in a significant lowering effect of fasting blood sugar by 17.3 mg/dL.24 The anti-diabetic effects of garlic might be due to its ability to increase insulin sensitivity25 and serum insulin levels26 and decrease in glucose absorption within the lumen of the gastrointestinal tract.27
Hyperlipidemia is one of the most common comorbidities related to NAFLD, increasing the risk of cardiovascular disease and premature death.4 Our results showed that the intake of garlic powder leads to a positive effect on LDL-c and total cholesterol concentrations. A recent meta-analysis indicated that garlic reduces LDL-c and total cholesterol, whereas its effect on HDL-c depends on the type of garlic.28 Similarly, in another meta-analysis, garlic powder intake has resulted in a significant reduction in LDL-c by 15.8 mg/dL and total cholesterol by 8.1 mg/dL, without changing the serum HDL-c.24 Although ample evidence has shown the consistency in serum cholesterol parameters with garlic treatment, there are some controversies in serum triglycerides.29 The subgroup analysis of the previous meta-analysis has shown that the aforesaid issue may be due to the difference in the type of garlic, duration of the study, baseline serum triglycerides of the study population, and dosage.28 The results obtained in our study showed a significant reduction in serum triglycerides with the intake of garlic. The current findings were supported by a similar study in which the administration of garlic powder at 800 mg/day for 4 months reduced TG by 38 mg/dL.30 The hypolipidemic property of garlic has been linked to the down-regulation of hepatic lipogenic and cholesterogenic genes and the enhancement of bile acid excretion from the gastrointestinal tract.31,32
The most important finding of the current study was a significant improvement in hepatic steatosis by the administration of garlic powder. Along with the improving hepatic steatosis, a significant reduction was observed in serum ALT and AST concentrations. Also, the improvement in the hepatic steatosis and liver enzymes was independent of weight loss, energy intake, and physical activity. In support of our findings, there are possible underlying mechanisms that could explain how garlic exerts a direct protective effect against hepatic fat content and hepatic injury.
Garlic contains a broad spectrum of organosulfur compounds, several enzymes such as allinase, and various minerals. Alliin is the main organosulfur compounds of garlic which converts to allicin by allinase enzyme following damage to the garlic bulb. However, allicin is well-known organosulfur compounds of garlic which has antioxidant and anti-inflammatory activities through scavenging hydroxyl radicals, inhibiting production of superoxide anion and nitric oxide, down-regulating the expression of NF-κB (Nuclear factor-κB), and suppressing JNK (Jun N-terminal Kinase) Pathway.33,34 Ajoene is another main and stable organosulfur compound of garlic which is mainly generated from allicin through sulfoxide rearrangement. It has been shown that ajoene might up-regulate the expression of nuclear factor erythroid-2-related factor 2 (Nrf2), a main transcription factor for genes involved in the biosynthesis of detoxification enzymes and glutathione.35 According to a study on animal models, treatment with garlic and its derivatives also can down-regulate the lipogenic gene expression such as fatty acid synthase and acetyl coenzyme A (CoA) carboxylase through SREBP1c, as well as up-regulation of the lipolytic gene expression such as PPAR α and CPT-1.16,36-38 Therefore, it seems that therapeutic effect of garlic on hepatic steatosis might derive from cumulative from multiple pathways.
The current clinical trial is limited by the absence of histological assessment. However, since liver biopsy is an invasive procedure with sampling error and serious adverse events, we used ultrasound technique as an appropriate method for monitoring of NAFLD.39 Also, the current trial is a small number of patients having severe grade of fatty liver which can affect its generalizability to this group.
Several factors are considered as the main strengths of this clinical trial as follows: the well-matching performance because of using stratified blocked randomization, the evaluation of energy intake and physical activity, the similar distribution of macronutrients in both groups during the study, and high compliance rate with the assigned treatment. Also, a posteriori power analysis for the proportion of patients with improvement in steatosis in both groups showed our study had the power of above 80% at type I error of 0.05.
Conclusion
Our findings showed that the use of enteric-coated garlic powder for 15 weeks at 800 mg per day can reduce the hepatic steatosis and common comorbidity related to it in patients with NAFLD. In future studies, it is necessary to assess the combined effect of garlic and lifestyle modification on the hepatic steatosis or even on features of NASH and clarify its mechanism (s) of action on liver fat content.
Data Sharing Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Acknowledgment
We are grateful of all participants and assistants in our research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare no conflicts of interest.
References
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi:10.1002/hep.28431
2. Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes. 2018;11:533. doi:10.2147/DMSO.S146339
3. Yilmaz Y. Is non‐alcoholic fatty liver disease a spectrum, or are steatosis and non‐alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36(9):815–823. doi:10.1111/apt.12046
4. Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65(2):425–443. doi:10.1016/j.jhep.2016.04.005
5. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi:10.1053/j.gastro.2011.06.061
6. Sanyal AJ, Friedman SL, McCullough AJ, Dimick‐Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: findings and recommendations from an American association for the study of liver diseases–US food and drug administration joint workshop. Hepatology. 2015;61(4):1392–1405. doi:10.1002/hep.27678
7. Yu J, Marsh S, Hu J, Feng W, Wu C. The pathogenesis of nonalcoholic fatty liver disease: interplay between diet, gut microbiota, and genetic background. Gastroenterol Res Pract. 2016;2016:1–13. doi:10.1155/2016/2862173
8. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–378. e365. doi:10.1053/j.gastro.2015.04.005
9. Zheng X, Zhao M-G, Jiang C-H, et al. Triterpenic acids-enriched fraction from Cyclocarya paliurus attenuates insulin resistance and hepatic steatosis via PI3K/Akt/GSK3β pathway. Phytomedicine. 2020;66:153130. doi:10.1016/j.phymed.2019.153130
10. Gamede M, Mabuza L, Ngubane P, Khathi A. Plant-derived oleanolic acid ameliorates markers associated with non-alcoholic fatty liver disease in a diet-induced pre-diabetes rat model. Diabetes Metab Syndr Obes. 2019;12:1953. doi:10.2147/DMSO.S218626
11. Soleimani D, Ranjbar G, Rezvani R, Goshayeshi L, Razmpour F, Nematy M. Dietary patterns in relation to hepatic fibrosis among patients with nonalcoholic fatty liver disease. Diabetes Metab Syndr Obes. 2019;12:315. doi:10.2147/DMSO.S198744
12. Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136(3):716S–725S. doi:10.1093/jn/136.3.716S
13. Kumar R, Chhatwal S, Arora S, et al. Antihyperglycemic, antihyperlipidemic, anti-inflammatory and adenosine deaminase–lowering effects of garlic in patients with type 2 diabetes mellitus with obesity. Diabetes Metab Syndr Obes. 2013;6:49. doi:10.2147/DMSO.S38888
14. Schwingshackl L, Missbach B, Hoffmann G. An umbrella review of garlic intake and risk of cardiovascular disease. Phytomedicine. 2016;23(11):1127–1133. doi:10.1016/j.phymed.2015.10.015
15. Nicastro HL, Ross SA, Milner JA. Garlic and onions: their cancer prevention properties. Cancer Prev Res. 2015;8(3):181–189. doi:10.1158/1940-6207.CAPR-14-0172
16. Lai Y-S, Chen W-C, Ho C-T, et al. Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J Agric Food Chem. 2014;62(25):5897–5906. doi:10.1021/jf500803c
17. Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–750. doi:10.1053/gast.2002.35354
18. Kyle UG, Bosaeus I, De Lorenzo AD, et al. Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr. 2004;23(5):1226–1243. doi:10.1016/j.clnu.2004.06.004
19. A’hern R. Sample size tables for exact single‐stage phase II designs. Stat Med. 2001;20(6):859–866. doi:10.1002/sim.721
20. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675–1685. doi:10.1056/NEJMoa0907929
21. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4):829–846. doi:10.1016/j.jhep.2017.05.016
22. Seo DY, Lee SR, Kim HK, et al. Independent beneficial effects of aged garlic extract intake with regular exercise on cardiovascular risk in postmenopausal women. Nutr Res Pract. 2012;6(3):226–231. doi:10.4162/nrp.2012.6.3.226
23. Lee M-S, Kim I-H, Kim C-T, Kim Y. Reduction of body weight by dietary garlic is associated with an increase in uncoupling protein mRNA expression and activation of AMP-activated protein kinase in diet-induced obese mice. J Nutr. 2011;141(11):1947–1953. doi:10.3945/jn.111.146050
24. Kwak JS, Kim JY, Paek JE, et al. Garlic powder intake and cardiovascular risk factors: a meta-analysis of randomized controlled clinical trials. Nutr Res Pract. 2014;8(6):644–654. doi:10.4162/nrp.2014.8.6.644
25. Padiya R, Banerjee SK. Garlic as an anti-diabetic agent: recent progress and patent reviews. Recent Pat Food Nutr Agric. 2013;5(2):105–127. doi:10.2174/18761429113059990002
26. Augusti K, Sheela C. Antiperoxide effect of S-allyl cysteine sulfoxide, an insulin secretagogue, in diabetic rats. Experientia. 1996;52(2):115–119. doi:10.1007/BF01923354
27. Younas J, Hussain F. In vitro antidiabetic evaluation of allium sativum L. In J Chem Biochem Sci. 2014;5:22–25.
28. Ried K, Toben C, Fakler P. Effect of garlic on serum lipids: an updated meta-analysis. Nutr Rev. 2013;71(5):282–299. doi:10.1111/nure.12012
29. Alder R, Lookinland S, Berry JA, Williams M. A systematic review of the effectiveness of garlic as an anti‐hyperlipidemic agent. J Am Acad Nurse Pract. 2003;15(3):120–129. doi:10.1111/j.1745-7599.2003.tb00268.x
30. Mader F. Treatment of hyperlipidaemia with garlic-powder tablets. Evidence from the German association of general practitioners’ multicentric placebo-controlled double-blind study. Arzneimittel-Forschung. 1990;40(10):1111–1116.
31. Chang MLW, Johnson MA. Effect of garlic on carbohydrate metabolism and lipid synthesis in rats. J Nutr. 1980;110(5):931–936. doi:10.1093/jn/110.5.931
32. Yeh -Y-Y, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131(3):989S–993S. doi:10.1093/jn/131.3.989S
33. Li C, Lun W, Zhao X, et al. Allicin alleviates inflammation of trinitrobenzenesulfonic acid-induced rats and suppresses P38 and JNK pathways in Caco-2 cells. Mediators Inflamm. 2015;2015:1–11. doi:10.1155/2015/434692
34. Rahman MS. Allicin and other functional active components in garlic: health benefits and bioavailability. Int J Food Prop. 2007;10(2):245–268. doi:10.1080/10942910601113327
35. Kay HY, Won Yang J, Kim TH, et al. Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes. J Nutr. 2010;140(7):1211–1219. doi:10.3945/jn.110.121277
36. Panyod S, Wu W-K, Ho C-T, et al. Diet supplementation with allicin protects against alcoholic fatty liver disease in mice by improving anti-inflammation and antioxidative functions. J Agric Food Chem. 2016;64(38):7104–7113. doi:10.1021/acs.jafc.6b02763
37. Shi L, Lin Q, Li X, et al. Alliin, a garlic organosulfur compound, ameliorates gut inflammation through MAPK‐NF‐κB/AP‐1/STAT‐1 inactivation and PPAR‐γ activation. Mol Nutr Food Res. 2017;61(9):1601013. doi:10.1002/mnfr.201601013
38. Han CY, Ki SH, Kim YW, et al. Ajoene, a stable garlic by-product, inhibits high fat diet-induced hepatic steatosis and oxidative injury through LKB1-dependent AMPK activation. Antioxid Redox Signal. 2011;14(2):187–202. doi:10.1089/ars.2010.3190
39. Masuzaki R, Tateishi R, Yoshida H, et al. Comparison of liver biopsy and transient elastography based on clinical relevance. Can J Gastroenterol Hepatol. 2008;22(9):753–757.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


