Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
The Variants in ADIPOQ are Associated with Maternal Circulating Adipokine Profile in Gestational Diabetes Mellitus
Authors Tangjittipokin W , Narkdontri T , Teerawattanapong N, Thanatummatis B, Wardati F , Sunsaneevithayakul P, Boriboonhirunsarn D
Received 9 November 2022
Accepted for publication 24 January 2023
Published 31 January 2023 Volume 2023:16 Pages 309—319
DOI https://doi.org/10.2147/JMDH.S396238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Watip Tangjittipokin,1,2 Tassanee Narkdontri,1– 3 Nipaporn Teerawattanapong,1– 3 Benyapa Thanatummatis,4 Fauchil Wardati,4 Prasert Sunsaneevithayakul,5 Dittakarn Boriboonhirunsarn5
1Department of Immunology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 2Siriraj Center of Research Excellence for Diabetes and Obesity, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 3Research Division, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 4Graduate Program in Immunology, Department of Immunology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 5Department of Obstetrics and Gynaecology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Watip Tangjittipokin, Department of Immunology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand, Tel +66 2-419-6635, Fax +66 2-418-1636, Email [email protected]
Background: Gestational diabetes mellitus (GDM) is the most common association with hyperglycemia and glucose intolerance during pregnancy. The adipokines play an important to control insulin secretion and glucose. This study aimed to investigate the association between maternal circulating adipokine levels and ADIPOQ gene polymorphism among pregnant women subjects with GDM and normal glucose tolerance (NGT).
Methods: Participants including 229 normal pregnant women and 197 GDM pregnant women were enrolled from 2015 to 2018 at Siriraj hospital. Serum adipokine levels including adiponectin, adipsin/factor D, NGAL/Lipocalin-2, total PAI-1, and resistin were measured by immunoassay. ADIPOQ variations were investigated including − 11377C/G (rs266729), +45T/G (rs2241766), and +276G/T (rs1501299).
Results: Serum adiponectin concentration was also significantly decreased among the GDM who had aged less than 35 years old whereas adipsin levels were significantly lower among the GDM who had aged more than 35 years old. Also, adiponectin and total PAI-1 levels were significantly lower among the GDM who had a BMI of less than 30 kg/m2. The G allele frequency of ADIPOQ +45T/G was significantly different between GDM and controls (p = 0.03). ADIPOQ +45T/G was associated with an increased risk of GDM (odds ratio [OR]: 1.554; 95% confidence interval [CI]: 1.010– 2.390; p=0.045). The − 11377C/G was affected by the level of adiponectin (p = 0.04). The C allele of − 11377C/G SNP declined serum adiponectin levels and may be a risk factor for GDM.
Conclusion: This study revealed that genetics play important roles in circulating adipokines among pregnant women. ADIPOQ polymorphisms had significant associations with adiponectin levels in GDM patients.
Keywords: adiponectin, genetics, adipokine, gestational diabetes mellitus
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Panjaitan has been published for this article.
Introduction
Diabetes is examined as the main cause of death in most countries and the high percentage of prevalence was 8.8% in 2015.1 Gestational diabetes mellitus (GDM) can be diagnosed using blood glucose levels during pregnancy and is known as hyperglycemia and the incidence rate of type 2 diabetes in mothers is about 1–14% in different populations.2 GDM is defined by early-onset of glucose intolerance during pregnancy and is related to T2D development.3 In a normal pregnancy, it is classified by increasing insulin resistance and insulin secretion by pancreatic β-cells.4 GDM women are an imbalance between insulin resistance and insulin secretion capacity, leading to excess glucose circulates.5 In the past decades, mothers with GDM related the increasing incidence and deleterious results for offspring.6
The pathogenesis of GDM remains unclear but abnormal adipokines may play a role in GDM development.7 It is recognized that the key of the endocrine organ is adipose tissue which plays a crucial role in metabolic regulation and is involved with metabolic syndrome, obesity-related chronic low-grade inflammation, and insulin resistance.8 More specifically, adipokines may promote insulin resistance and metabolic diseases. Kampmann et al reported that GDM was constantly elevated with insulin resistance, adipokines, and glucose tolerance deterioration.9
Adipokines such as adiponectin, resistin, adipsin, plasminogen activator inhibitor-1 (PAI-1), and neutrophil gelatinase-associated lipocalin (NGAL) are secreted by white adipose tissue, which is now recognized to be an active participant in glucose homeostasis.10 Recently, this evidence has become robust suggesting that obesity and inflammation are major components of insulin resistance. One of the mechanisms described in patients with a metabolic syndrome characterized by excess visceral adipose tissue is that long-term exposure to higher adipokine levels leads to a chronic sub-inflammatory state that is involved in the development of insulin resistance.11
Adiponectin is the most abundant adipokine with a role in insulin sensitivity.12 Previous studies have documented that lower adiponectin levels are commonly observed in patients with diabetes and pregnancy.13 Apart from the ADIPOQ genes are encoded on chromosome 3q27. The SNPs of the ADIPOQ gene are associated with metabolic syndrome and diabetes.14 Gestational diabetes is associated with the polymorphism of the ADIPOQ promoter rs266729.15 The TG and GG genotype of ADIPOQ +45T/G (rs2241766) has been reported to be associated with low adiponectin levels in GDM patients.16 Therefore, we were interested to estimate circulating adipokines and molecular genetics of adiponectin may play an important role in the pathogenesis of GDM.
Based on these findings, we aimed to examine maternal serum adipokines during pregnancy both in women with or without GDM, and their associations were assessed between adiponectin, adipsin/factor D, NGAL/Lipocalin-2, total PAI-1, and resistin with maternal pre-pregnancy weight and BMI, compared with healthy pregnant controls. Using the same population, we also investigate 3 SNPs in ADIPOQ genes (−11377C/G, rs266729 in the promoter; +45T/G, rs2241766 in exon 2 and +276G/T, rs1501299 in intron 2 region) between GDM patients and controls.
Methods
Study Population
This cross-sectional study was approved by the Faculty of Medicine Siriraj hospital ethics review board, Mahidol University, Thailand (Si 577/2015) complies with the Declaration of Helsinki. Written informed consent was obtained from all subjects. This study involved 426 subjects (229 normal pregnant women and 197 GDM pregnant women) with no history of the other type of diabetes; type 1 or type 2; were included. All pregnant women subjects had regular follow-up visits and were diagnosed by Siriraj hospital physicians from the outpatient Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University. The screening test for GDM was according to the glucose challenge test (GCT) (measurement of plasma glucose concentration 1 hour after 50 g of oral glucose load). If the screening test was positive (GCT ≥ 140 mg/dl), GDM was diagnosed according to 100g 3-hour OGTT with Carpenter/Coustan criteria. GDM was defined if at least 2 values were equal or exceeded the threshold of 95, 180, 155, and 140 mg/dl for fasting plasma glucose, 1-hour plasma glucose, 2 hours plasma glucose, and 3 hours plasma glucose values respectively.
Blood Sample Collection and Laboratory Measurements
Blood samples were collected from every pregnant woman who had follow-up visits at the outpatient department of obstetrics and gynecology, faculty of medicine Siriraj hospital, Mahidol university. Blood samples for adipokine testing were centrifuged at 1500 rev/min for 15 min at room temperature. The serum was separated and determinations were performed within 1 hour at room temperature. The serum/plasma was stored at −80 ℃ until assayed. Also, the buffy coat was kept at 4 ℃ until assayed.
Adipokine levels were investigated in serum by using MILLIPLEX® MAP human adipokine magnetic bead panel 1, in a 96-well plate (Merck Millipore Corporation, MA, USA). The assay sensitivity was 21 pg/mL for adiponectin, 10 pg/mL for adipsin, 3.5 pg/mL for Lipocalin-2, 5.8 pg/mL for PAI-1, and 4.4 pg/mL for resistin. A total of 5 biomarkers (adiponectin, adipsin/factor D, NGAL/Lipocalin-2, total PAI-1, and resistin) were quantified using Luminex® assays (Luminex Corp., Madison, WI).
Genotype Analysis
SNPs −11377C/G (rs266729), +45T/G (rs2241766), and +276G/T (rs1501299) of the ADIPOQ gene were genotyped with DNA samples consisting of 197 GDM subjects and 229 controls. Genomic DNA was extracted from the buffy coat by Flexigene® DNA (Qiagen, Valencia, CA, USA). Genomic DNA was amplified with primer-specific genes. PCR primer sequence and annealing temperature are listed in Table 1. Primers were obtained from Integrated DNA Technologies. DNA (125 ng) PCR product was performed by a PCR-restriction fragment length polymorphism (PCR-RFLP) method. The PCR products were digested with HinPlI (−11377C/G) and BspHI (+45T/G) restriction endonuclease (1U, Fermentas, Thermo Scientific, EU) overnight at 37°C, and BsmI (+276G/T) restriction endonuclease (1U, Fermentas, Thermo Scientific, EU) at 65°C. The digested products were separated by 12% polyacrylamide gel electrophoresis and visualized by silver staining.
 |
Table 1 PCR Primer Sequence and Annealing Temperature |
Statistical Analyses
Statistical analyses were performed using SPSS software for window version 18. Continuous variables were given as mean ± standard deviation (SD). Adipokine concentrations and biochemistry variables between GDM and NGT were evaluated for normality using the Kolmogorov–Smirnov test. Clinical characteristics and adipokine levels were compared and tested for significant differences between GDM and control using Student’s t-test (normally distributed data) and Mann–Whitney U-test (non-parametrically distributed data). All haplotype frequency analyses were determined using the SNPStats tool (http://bioinfo.iconcologia.net/SNPstats). Analyses to determine associations between SNPs and GDM were performed under the additive, dominant, and recessive models using logistic regression analysis, with (Pb)/without (Pa) adjustment for age, gestational age at blood collection (weeks), BMI before pregnancy (kg/m2), BMI during pregnancy (kg/m2), systolic blood pressure (mmHg), as covariates. The level of statistical significance for all tests was set at p < 0.05.
Results
Characteristics of Subjects
A total of 426 pregnant participants were analyzed 197 of the participants had GDM, and 229 were NGT as controls. As shown in Table 2, there was a significant difference between the GDM group and the NGT group in terms of age (p < 0.0001) and gestational age (p < 0.0001). Pre-pregnancy BMI and BMI at gestation were greater in the women with GDM than in those NGT (p = 0.004 and p = 0.002, respectively). Systolic and diastolic blood pressure in GDM was significantly elevated when compared with controls (p = 0.048 and p = 0.014, respectively). Also, glucose concentrations of the 50-g GCT, fasting blood sugar, and for each time of the 100-g, OGTT were significantly greater in the GDM than in the non-GDM group (p < 0.0001 for all). Additionally, measurements of serum adipokine levels showed that adipsin and NGAL levels tend to decrease in GDM compared with controls (p = 0.34 and p = 0.23, respectively). In contrast, serum PAI-1 and resistin levels trend to increase in GDM compared with controls (p = 0.35 and p = 0.28, respectively). Among adiponectin levels in the GDM group were significantly lower than in the controls (p < 0.0001).
 |
Table 2 Participant Demographic, Anthropometric, and Clinical Characteristics |
Comparison of Adipokine Concentrations and Biochemistry Variables by Age, Gestational Age, and at Blood Collection BMI in GDM and NGT Subjects
To explore potential adipokine level changing resulting from variables in GDM, we measured serum adipokine concentrations of GDM patients and controls. The levels of adiponectin were significantly lower in GDM ages less than 30 years (p = 0.005) and age range 30–35 years (p = 0.001) when compared with controls. Also, we found adipsin/factor D levels were significantly lower in GDM over 35 years of age (p = 0.001). During the 12th-24th week of gestational age found that serum adiponectin levels were remarkably lower in GDM patients (p = 0.02). Serum NGAL/lipocalin-2 concentration was significantly lower in GDM by over 24th weeks of gestational age (p = 0.02). While we measured adipokine levels in pregnancy subgroups of BMI, we found that total PAI-1 was significantly reduced in lean women pregnancies when compared to BMI-matched controls (p = 0.04). Serum NGAL/lipocalin-2 concentration was significantly lower in overweight pregnant women with GDM when compared to BMI-matched controls (p = 0.03). We further found that adiponectin in GDM normal weight (25–30 kg/m2) was remarkably lower than BMI-matched controls (p = 0.001) (Table 3).
 |
Table 3 Serum Adipokine Concentrations and Biochemistry Variables by Age, Gestational Age and at Blood Collection BMI in NGT and GDM Subjects |
Genotype Frequencies of −11377C/G (rs266729), +45T/G (rs2241766), and +276G/T (rs1501299) and Haplotype Analysis in GDM and NGT Subjects
The genotypes were investigated by PCR-RFLP. Our results showed significant differences in the major alleles of +45T/G (rs2241766) between GDM and NGT when compared to the minor alleles (p = 0.03) (Table 4). The minor alleles of +45T/G (rs2241766) significantly increased the risk of GDM in pregnant women without adjustment for age, gestational age at blood collection, BMI before pregnancy, BMI during pregnancy, systolic blood pressure, diastolic blood pressure (odds ratio [OR]: 1.359; 95% confidence interval [CI]: 1.010–1.828; pa=0.043) (Table 4). Subsequently, haplotype analysis was performed to assess the combined effect of 3 SNPs in the ADIPOQ gene. The haplotype of three loci in the ADIPOQ gene, −11377C/G (rs266729), +45T/G (rs2241766), and +276G/T (rs1501299) showed different frequencies between cases and controls (Table 5). The global haplotype association p-value was 0.28. The frequencies of the CGT haplotype as a rare haplotype were significantly higher in GDM subjects compared to NGT (p < 0.0001), while the CGG, CTG, CTT, CTG, GTT, and GGG haplotypes were no significant difference between case and controls. However, the CGT haplotype had been found lower frequencies in all participants.
 |
Table 4 Analysis of the Association Between SNPs in ADIPOQ and NGT and GDM |
 |
Table 5 Haplotype Frequencies in NGT Controls and GDM Cases |
Analysis of Association Between SNPs ADIPOQ Gene and GDM
Three SNPs in 197 GDM patients and 229 controls were genotyped. The genotype distributions of SNPs are shown in Table 4. All three SNPs genotypes including −11377C/G (rs266729), +45T/G (rs2241766), and +276G/T (rs1501299) were in Hardy-Weinberg equilibrium (HWE) in GDM and control group (all p > 0.05). Logistic regression analysis was used to examine the association between each SNP and GDM patients in three different models (additive, dominant and recessive model). There was a significant increase in the risk of GDM patients after adjustment for age, gestational age at blood collection, BMI before pregnancy, BMI during pregnancy, systolic blood pressure, diastolic blood pressure (odds ratio [OR]: 1.554; 95% confidence interval [CI]: 1.010–2.390; pb=0.045). No associations between ADIPOQ −11377C/G (rs266729) or +276G/T (rs1501299) and GDM were found in three genetic models.
Clinical and Genotype Frequencies of SNPs ADIPOQ Gene in GDM and NGT Subjects
To compare serum adipokine concentrations and biochemistry variables between subcategories of major allele and minor alleles in 3 SNPs ADIPOQ gene, the 50g GCT levels were significantly decreased in major alleles than minor alleles (p = 0.01) in SNPs at −11377C/G (rs266729) in GDM patients. Furthermore, analysis of glucose concentrations after 1 hr by OGTT, the results showed that glucose levels were significantly elevated in major alleles compared with minor alleles in GDM subjects (p = 0.02). Interestingly, the levels of adiponectin were significantly reduced in major alleles (−11377C/G, rs266729) when compared with minor alleles in GDM (p = 0.04). Moreover, fasting blood glucose was significantly decreased at SNPs +276G/T (rs1501299) major alleles compared to minor alleles in GDM (p = 0.02) (Table 6). Levels of adipsin were significantly elevated in major alleles compared to minor alleles in SNPs +276G/T (rs1501299) in NGT subjects (p = 0.005) (Table 7).
 |
Table 6 Serum Adipokine Concentrations and Biochemistry Variables by ADIPOQ Single Nucleotide Polymorphism Genotype in GDM Subjects |
 |
Table 7 Serum Adipokine Concentrations and Biochemistry Variables by ADIPOQ Single Nucleotide Polymorphism Genotype in NGT Controls |
Discussion
GDM is a pregnancy-related complication. It shows the risk factors of both poor maternal and poor newborn health.17 The most symptoms of GDM are premature birth, premature rupture of membranes, gestational hypertension, pre-eclampsia, cesarean section, and macrosomia.18 It is well known that insulin resistance (IR), which causes a change of increased maternal adipose tissues and anti-insulin in the placenta.19 Previous studies of adipokines show that they have been associated with insulin resistance related to pregnancy in women with a history of GDM.20 However, no previous evidence has investigated a cohort of these five adipokines and their relationship to glucose level with advancing pregnancy within a single sample of women. To our knowledge, there have been few published reports on simultaneously determining genetic factors, and maternal concentrations of adipokines in pregnancy subsequently developing GDM.7 Type 2 diabetes (T2D) after pregnancy has also been linked to GDM as a risk factor.3 The meta-analysis of T2D variants was relevant in various tissue such as adipose subcutaneous tissue, adipose visceral omentum, liver, and pancreas.21
Adiponectin is a protein released from adipocytes, which plays a role in the pathogenesis of GDM.22 In this current study, serum adiponectin levels were lower in GDM subjects compared to NGT subjects. Pala et al reported similar findings in their study.23 Adipsin is secreted in abundance in adipose tissue. It activates glucose transport through an insulin mechanism.24 This finding identified adipsin as a circulating factor linking fat cells to beta-cell function, more specifically, adipsin potentiates insulin secretion.25 Resistin is a hormone secreted from adipose tissue. It belongs to the family of cysteine-rich, c-terminal proteins, and actively opposes insulin action in peripheral tissues.26 Some evidence has approved lower resistin levels in GDM than in NGT with a further decline after childbirth.27 NGAL or lipocalin-2 is a potential mediator involved in the inflammatory marker in insulin resistance, high blood glucose, and obesity.28 PAI-1 is the regulator of the fibrinolytic system. It is produced by the endothelium but is also secreted by adipose tissue, liver, lung, and muscle.29 Increased PAI-1 levels in plasma accompany symptoms of metabolic syndromes, such as glucose intolerance and insulin resistance.30 However, this study did not find significant differences in adipsin, NGAL/lipocalin-2, PAI-1, and resistin levels between GDM and NGT.
Furthermore, few studies investigated the relationships between adiponectin levels determined early in pregnancy and GDM. Low adiponectin levels were associated with an increased risk of GDM.31 Contradictory evidence can be partly explained by experimental designs, confounding factors, and the cutoff of impaired glucose regulation in pregnancy.31 Adiponectin concentrations in the circulation are associated with triacylglycerol and HDL levels.23 Besides, we examined the blood glucose and circulating adiponectin as early as 14 weeks of GDM pregnancy, compared with those who avoid GDM recurrence.20
Maternal adipokine profiles and a glucose tolerance assay will be used in early pregnancy to prognosis women at high risk of GDM recurrence, as maternal adiponectin may function for fetal growth and birth weight.32 Based on adipokine functions, further studies on the role of these adipokines are important to understand in the pathogenesis of insulin resistance and GDM and may help to identify biomarkers of GDM prediction or prognosis. The current study showed that adiponectin concentration was significantly decreased in GDM after subcategorized by age, gestational age, and blood collection BMI. Adiponectin concentrations were lower in GDM at age<30, 30–35 years old, 12th-24th week of gestational age, and 25–30 kg/m2 of gestational BMI when compared to NGT. It has been similar to the prospective cohort study, adiponectin was negatively correlated with age, glucose, and BMI, and positively correlated with gestational age at delivery.33
The relationship between ADIPOQ polymorphism and GDM has been the subject of many recent studies. The present study revealed that the GDM had a higher distribution of +45T/G (rs2241766) G allele frequency than the NGT group. It was considered that the adiponectin SNP +45T/G (rs2241766) might be associated with GDM, and the G allele might be the ultimate risk factor for GDM in Thai women. Our finding was confirmed with the previous document that Han women with GDM, G allele at SNP +45T/G (rs2241766) might be correlated with declined plasma adiponectin concentrations and inverse clinical outcomes.34 In agreement with our findings, GDM resembles an early stage of type 2 diabetes, which was associated with low adiponectin levels and the ADIPOQ gene. SNP −11377C/G (rs266729) demonstrated the prevalence of Korean T2D.35 Our results have shown that adiponectin levels significantly declined in GDM with SNP −11377 C/G (rs266729) C allele. Nomani et al found that an elevated level of adiponectin was associated with the G allele of SNPs −11377 C/G (rs266729) in the Iran population.36 It might indicate the association between SNP −11377 C/G (rs266729) in the promoter region of the adiponectin gene and the regulation of adiponectin expression. However, there was no association between SNP −11377C/G and GDM in Asian, South American, and European populations which may be due to the difference between the ethnic group.37 In this study, fasting blood glucose levels were significantly higher in GDM patients with +276G/T (rs1501299) minor alleles compared to those with major alleles. Obese subjects with the T allele of ADIPOQ +276G/T (rs1501299) had higher fasting glucose levels on the Mediterranean hypocaloric diet.38 Moreover, our results demonstrated that maternal age, pre-pregnancy BMI, and increasing weight were not predictive factors for GDM. Our sample size may be small conducted with less power to predict glucose levels in GDM.
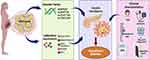
In conclusion, our studies illustrated that adiponectin concentrations were remarkably decreased in GDM pregnant women than in NGT. Adiponectin levels were controlled by ADIPOQ gene polymorphisms. GDM patients with minor alleles of ADIPOQ −11377C/G (rs266729) had higher levels of adiponectin. ADIPOQ +45T/G (rs2241766) was associated with an increased risk of GDM in Thai pregnant women (Figure 1). These results were implicated in biomarker risk prediction in early diagnosis and preventing high glucose during gestation.
Acknowledgments
This research project was supported by Siriraj Research Grant for Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University to WT (grant no. R015934014). The authors gratefully acknowledge Miss Maria Asad-Dehghani, Department of Obstetrics and Gynecology, Faculty of Medicine Siriraj Hospital, Mahidol University for assistance with the data collection procedure.
Disclosure
The authors declare no conflict of interest.
References
1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
2. Farrar D. Hyperglycemia in pregnancy: prevalence, impact, and management challenges. Int J Womens Health. 2016;8:519–527. doi:10.2147/IJWH.S102117
3. Alejandro EU, Mamerto TP, Chung G, et al. Gestational diabetes mellitus: a harbinger of the vicious cycle of diabetes. Int J Mol Sci. 2020;21:14. doi:10.3390/ijms21145003
4. Moyce BL, Dolinsky VW. Maternal β-cell adaptations in pregnancy and placental signalling: implications for gestational diabetes. Int J Mol Sci. 2018;19:11. doi:10.3390/ijms19113467
5. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;37(Supplement_1):S81–S90. doi:10.2337/dc14-S081
6. Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z. Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int J Mol Sci. 2021;22:6. doi:10.3390/ijms22062965
7. Gutaj P, Sibiak R, Jankowski M, et al. The role of the adipokines in the most common gestational complications. Int J Mol Sci. 2020;21:24. doi:10.3390/ijms21249408
8. Dilworth L, Facey A, Omoruyi F. Diabetes mellitus and its metabolic complications: the role of adipose tissues. Int J Mol Sci. 2021;22:14. doi:10.3390/ijms22147644
9. Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res. 2019;2019:5320156. doi:10.1155/2019/5320156
10. Garg MK, Dutta MK, Mahalle N. Adipokines (adiponectin and plasminogen activator inhibitor-1) in metabolic syndrome. Indian J Endocrinol Metab. 2012;16(1):116–123. doi:10.4103/2230-8210.91206
11. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13(4):851–863. doi:10.5114/aoms.2016.58928
12. Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iran J Basic Med Sci. 2015;18(5):430–442.
13. Hedderson MM, Darbinian J, Havel PJ, et al. Low prepregnancy adiponectin concentrations are associated with a marked increase in risk for development of gestational diabetes mellitus. Diabetes Care. 2013;36(12):3930–3937. doi:10.2337/dc13-0389
14. Howlader M, Sultana MI, Akter F, Hossain MM. Adiponectin gene polymorphisms associated with diabetes mellitus: a descriptive review. Heliyon. 2021;7(8):e07851. doi:10.1016/j.heliyon.2021.e07851
15. Pawlik A, Teler J, Maciejewska A, Sawczuk M, Safranow K, Dziedziejko V. Adiponectin and leptin gene polymorphisms in women with gestational diabetes mellitus. J Assist Reprod Genet. 2017;34(4):511–516. doi:10.1007/s10815-016-0866-2
16. Low CF, Mohd Tohit ER, Chong PP, Idris F. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet. 2011;283(6):1255–1260. doi:10.1007/s00404-010-1548-4
17. Kouhkan A, Najafi L, Malek M, et al. Gestational diabetes mellitus: major risk factors and pregnancy-related outcomes: a cohort study. Int J Reprod Biomed. 2021;19(9):827–836. doi:10.18502/ijrm.v19i9.9715
18. Miao M, Dai M, Zhang Y, Sun F, Guo X, Sun G. Influence of maternal overweight, obesity and gestational weight gain on the perinatal outcomes in women with gestational diabetes mellitus. Sci Rep. 2017;7(1):305. doi:10.1038/s41598-017-00441-z
19. Tumurbaatar B, Poole AT, Olson G, et al. Adipose tissue insulin resistance in gestational diabetes. Metab Syndr Relat Disord. 2017;15(2):86–92. doi:10.1089/met.2016.0124
20. Guelfi KJ, Ong MJ, Li S, et al. Maternal circulating adipokine profile and insulin resistance in women at high risk of developing gestational diabetes mellitus. Metabolism. 2017;75:54–60. doi:10.1016/j.metabol.2017.08.003
21. Xue A, Wu Y, Zhu Z, et al. Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat Commun. 2018;9(1):2941. doi:10.1038/s41467-018-04951-w
22. Meller M, Qiu C, Vadachkoria S, Abetew DF, Luthy DA, Williams MA. Changes in placental adipocytokine gene expression associated with gestational diabetes mellitus. Physiol Res. 2006;55(5):501–512. doi:10.33549/physiolres.930830
23. Pala HG, Ozalp Y, Yener AS, Gerceklioglu G, Uysal S, Onvural A. Adiponectin levels in gestational diabetes mellitus and in pregnant women without glucose intolerance. Adv Clin Exp Med. 2015;24(1):85–92. doi:10.17219/acem/38141
24. Wang P, Keijer J, Bunschoten A, Bouwman F, Renes J, Mariman E. Insulin modulates the secretion of proteins from mature 3T3-L1 adipocytes: a role for transcriptional regulation of processing. Diabetologia. 2006;49(10):2453–2462. doi:10.1007/s00125-006-0321-5
25. Lo JC, Ljubicic S, Leibiger B, et al. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158(1):41–53. doi:10.1016/j.cell.2014.06.005
26. Vitoratos N, Deliveliotou A, Dimitrakaki A, et al. Maternal serum resistin concentrations in gestational diabetes mellitus and normal pregnancies. J Obstet Gynaecol Res. 2011;37(2):112–118. doi:10.1111/j.1447-0756.2010.01327.x
27. Megia A, Vendrell J, Gutierrez C, et al. Insulin sensitivity and resistin levels in gestational diabetes mellitus and after parturition. Eur J Endocrinol. 2008;158(2):173–178. doi:10.1530/EJE-07-0671
28. Lou Y, Wu C, Wu M, Xie C, Ren L. The changes of neutrophil gelatinase-associated lipocalin in plasma and its expression in adipose tissue in pregnant women with gestational diabetes. Diabetes Res Clin Pract. 2014;104(1):136–142. doi:10.1016/j.diabres.2014.01.014
29. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5):e72–91. doi:10.1111/j.1755-5922.2010.00171.x
30. Nawaz SS, Siddiqui K. Plasminogen activator inhibitor-1 mediate downregulation of adiponectin in type 2 diabetes patients with metabolic syndrome. Cytokine X. 2022;4(1):100064. doi:10.1016/j.cytox.2022.100064
31. Lacroix M, Battista MC, Doyon M, et al. Lower adiponectin levels at first trimester of pregnancy are associated with increased insulin resistance and higher risk of developing gestational diabetes mellitus. Diabetes Care. 2013;36(6):1577–1583. doi:10.2337/dc12-1731
32. Mallardo M, Ferraro S, Daniele A, Nigro E. GDM-complicated pregnancies: focus on adipokines. Mol Biol Rep. 2021;48(12):8171–8180. doi:10.1007/s11033-021-06785-0
33. Doruk M, Uğur M, Oruç AS, Demirel N, Yildiz Y. Serum adiponectin in gestational diabetes and its relation to pregnancy outcome. J Obstet Gynaecol. 2014;34(6):471–475. doi:10.3109/01443615.2014.902430
34. Han Y, Zheng YL, Fan YP, Liu MH, Lu XY, Tao Q. Association of adiponectin gene polymorphism 45TG with gestational diabetes mellitus diagnosed on the new IADPSG criteria, plasma adiponectin levels and adverse pregnancy outcomes. Clin Exp Med. 2015;15(1):47–53. doi:10.1007/s10238-014-0275-8
35. Choi J-H, Min NY, Park SK, et al. Dual matrilineal geographic distribution of Korean type 2 diabetes mellitus-associated −11377 G adiponectin allele. Mol Med Rep. 2014;10(6):2993–3002. doi:10.3892/mmr.2014.2639
36. Nomani H, Hesami O, Vaisi-Raygani A, et al. Association between the −11377 C/G and −11391 G/A polymorphisms of adiponectin gene and adiponectin levels with susceptibility to type 1 and type 2 diabetes mellitus in population from the west of Iran, correlation with lipid profile. J Cell Biochem. 2019;120(3):3574–3582. doi:10.1002/jcb.27634
37. Huang LT, Wu SL, Liao X, Ma SJ, Tan HZ. Adiponectin gene polymorphisms and risk of gestational diabetes mellitus: a meta-analysis. World J Clin Cases. 2019;7(5):572–584. doi:10.12998/wjcc.v7.i5.572
38. de Luis DA, Izaola O, Primo D, et al. Role of rs1501299 variant in the adiponectin gene on total adiponectin levels, insulin resistance and weight loss after a Mediterranean hypocaloric diet. Diabetes Res Clin Pract. 2019;148:262–267. doi:10.1016/j.diabres.2017.11.007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.