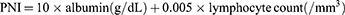Back to Journals » Journal of Inflammation Research » Volume 14
The Value of Prognostic Nutritional Index (PNI) on Newly Diagnosed Diffuse Large B-Cell Lymphoma Patients: A Multicenter Retrospective Study of HHLWG Based on Propensity Score Matched Analysis
Authors Shen Z, Wang F, He C, Li D, Nie S, Bian Z , Yao M, Xue Y, Wang Y, Gu W, Zhu T, Shi Y, Zhang H, Huang S, Miao Y, Sang W
Received 23 September 2021
Accepted for publication 14 October 2021
Published 27 October 2021 Volume 2021:14 Pages 5513—5522
DOI https://doi.org/10.2147/JIR.S340822
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ziyuan Shen,1,* Fei Wang,2,* Chenlu He,1,* Dashan Li,3 Shanlin Nie,3 Zhenzhen Bian,3 Mingkang Yao,4 Yuhao Xue,5 Ying Wang,6 Weiying Gu,2 Taigang Zhu,7 Yuye Shi,5 Hao Zhang,4 Shuiping Huang,1,8 Yuqing Miao,9 Wei Sang3 On behalf of the Huaihai Lymphoma Working Group
1Department of Epidemiology and Biostatistics, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Hematology, The First People’s Hospital of Changzhou, Changzhou, Jiangsu, People’s Republic of China; 3Department of Hematology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 4Department of Hematology, Affiliated Hospital of Jining Medical University, Jining, Shandong, People’s Republic of China; 5Department of Hematology, The First People’s Hospital of Huaian, Huaian, Jiangsu, People’s Republic of China; 6Department of Personnel, Suqian First Hospital, Suqian, Jiangsu, People’s Republic of China; 7Department of Hematology, The General Hospital of Wanbei Coal-Electric Group, Suzhou, People’s Republic of China; 8Center for Medical Statistics and Data Analysis, Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 9Department of Hematology, Yancheng First People’s Hospital, Yancheng, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wei Sang
Department of Hematology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Tel +8613645207648
Email [email protected]
Yuqing Miao
Department of Hematology, Yancheng First People’s Hospital, Yancheng, Jiangsu, People’s Republic of China
Tel +8613851342032
Email [email protected]
Introduction: Immunonutritional status is associated with the survival of DLBCL. This multicenter retrospective study aimed to explore the prognostic value of Prognostic Nutrition Index (PNI) in DLBCL patients by using propensity score matched analysis (PSM).
Methods: A total of 990 DLBCL cases were recruited from 5 centers of Huaihai Lymphoma Working Group (HHLWG). A 1:1 PSM analysis was performed using the nearest-neighbor method, with a caliper size of 0.02. Cox regression analysis was used to examine factors associated with survival.
Results: The median age at diagnosis was 62 years and 52.5% were males, with the 3-y overall survival of 65.1%. According to the MaxStat analysis, 44 was the optimal cut-off point of PNI. After PSM analysis, a total of 282 patients in PNI < 44 group could be propensity matched to PNI ≥ 44 patients, creating a group of 564 patients. Multivariable analysis revealed that PNI, age, central nervous system involvement and International Prognostic Index (IPI) were independent prognostic factors for DLBCL. Kaplan–Meier analysis indicated that patients with low PNI in Ann Arbor Stage (III/VI), ECOG (< 2), IPI (LR+LIR), GCB, and BCL-2 negative groups had a poor prognosis.
Discussion: PNI could accurately stratify the prognosis of DLBCL after PSM analysis.
Keywords: diffuse large B-cell lymphoma, prognostic nutrition index, propensity score matched, prognosis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin’s lymphoma with high heterogeneity and aggressiveness. Although 60–70% patients can be cured by the current standard of care in the frontline setting, the majority of the remaining patients will experience refractory and relapse. Clinical variables based prognostic systems, such as International Prognostic Index (IPI)1 and NCCN-IPI,2 can achieve prognostic stratification of DLBCL patients. However, these indices do not take the nutritional status of patients into account.
Malnutrition is a common problem in cancer patients, occurring in up to 80% of patients with advanced stage.3 It will affect the response to treatment and lead to poor quality of life in cancer patients.4 Assessment of systemic nutritional status was refined by the introduction of the prognostic nutrition index (PNI), a continuous variable based on serum albumin concentration and total lymphocyte count in peripheral blood.5 PNI was originally designed to assess perioperative immunonutritional status and surgical risk in patients undergoing gastrointestinal surgery.6,7 Several studies have confirmed that PNI has prognostic value in a variety of malignant tumors.6,8,9 Yao et al suggested that PNI was a significant indicator of extranodal natural killer/T cell lymphoma.10 Besides, there were a few reports exploring the prognostic role of PNI in DLBCL with limitations of small sample size and single-center data.11–13
Confounding factors always weaken the accuracy and objectivity of retrospective studies. Propensity score analysis (PSM) is commonly used to overcome selection bias, adjust the confounding factors, improve the comparability between groups by increasing the evidence level in retrospective studies, and mimic randomization on observed covariates.14–18
In this retrospective study, based on multicenter data of DLBCL from Huaihai Lymphoma Working Group (HHLWG) in China, we firstly carried out this PSM analysis to evaluate the prognostic value of PNI in DLBCL patients.
Materials and Methods
Patients
A total of 990 newly diagnosed DLBCL patients from 2008 to 2021 were recruited, and all pathological biopsies were double blinded and reviewed by at least two pathologists. Exclusion criteria: 1) patients with other malignant tumors; 2) patients with special types of lymphoma (primary central nervous system lymphoma, primary mediastinal DLBCL, transformed DLBCL); 3) patients with other immune diseases; 4) diseases that affect the lymphocyte level and albumin level of patients (such as hepatitis, cirrhosis, chronic kidney disease). During admission, the following variables were considered: age, gender, lactate dehydrogenase (LDH), hemoglobin (HB), platelet (PLT), albumin (Alb), lymphocyte count (LYC), red blood cell count (RBC), Ki-67, presence of bulky disease, B symptoms, β2‐microglobulin (β2-MG), ECOG PS and Ann Arbor stage.
Follow-Up and Endpoints
Follow-up was conducted by consulting inpatient medical records and making phone calls. We followed up all the patients until July 28, 2021 or the death of patients. Overall survival (OS) was calculated as the interval between the time of diagnosis and death from any cause or the last follow-up. The survival status of all patients was confirmed with death records or a telephone call to next of kin (if patient died during the follow-up) or to the patients themselves. Study approval was obtained from the independent Ethics Committees of each participating center in HHLWG and met with the Helsinki Declaration.
Assessment of PNI
PNI, based on serum albumin and lymphocytes, is a scoring system that reflects the nutritional status and immune status of patients. It is calculated using the formula:7
Statistical Analysis
Data were presented as numbers (percentages) for categorical variables and median (interquartile range, IQR) for all continuous variables. Differences in clinical factors were analyzed by using χ2 test. PNI was transformed into a categorical variable by MaxStat analysis (titled as Maximally Selected Rank Statistics). The Cox proportional hazard model was used to analyze the univariate association between prognostic factors and OS. All variables with P < 0.05 in univariable analysis were kept in the multivariate analysis by using backward selection for the best predictor set, and Akaike Information Criteria (AIC) was used to evaluate the model. All the statistical tests were two-sided, and the statistical significance was set at P < 0.05.
A 1:1 propensity score-matched (PSM) analysis was performed using the nearest-neighbor method, with a caliper size of 0.02 to identify the impact of PNI on DLBCL. To reduce selection bias and confounding factors, the propensity score was calculated using logistic regression analysis. The standardized mean difference (SMD) was measured to determine the balance between the two groups before and after PSM. The SMD cut-off value was <0.1, which was regarded as indicating a sufficient balance between the two groups. After 1:1 propensity score matching, continuous variables were compared using Mann–Whitney U-test, and categorical variables were compared using χ2 test. All statistical analyses were performed by SPSS statistics for Windows, Version 22.0 (Armonk, NY: IBM corp.), R software (version 4.0.3; http://www.Rproject.org) and Stata version 15.0.
Results
Characteristics of DLBCL Patients
The characteristics of 990 DLBCL patients were detailed in Table 1. The median follow-up time was 46.133 (95% CI: 43.759–48.508) months and the median OS was 140.033 (95% CI: 70.614–209.453) months. At the end of follow-up, a total of 358 (36.1%) deaths occurred. The median age at diagnosis was 62 years (range: 10–91), with 520 (52.5%) males and 431 (43.5%) patients were younger than 60 years. A total of 261 (26.3%) patients with B symptoms and 117 (11.8%) patients were with bulky disease. The 5-y of patients were 65.1% (Figure 1). In this study, patients received regimens of CHOP-like (n = 261), R-CHOP/R-CHOP-like (n = 601) and R-based intensive regimens (n = 128). Twenty-five patients received BTK inhibitor (BTKi), forty-four patients received methotrexate (MTX), and thirty-five patients received autologous hematopoietic stem cell transplantation (Auto-HSCT). Kaplan–Meier analysis indicated that there was no significant difference in therapeutic regimens in global comparison (P > 0.05). Further subgroup analysis of R-based intensive regimens also showed no difference.
 |
Table 1 Associations Between PNI and Clinicopathological Factors Before and After PSM |
 |
Figure 1 Overall survival of 990 DLBCL patients. |
The Optimal Cut-off Point for PNI Based on MaxStat
According to the maximal chi-square method, 44 was the optimal cut-off point that distinguished between two prognostic groups most effectively (P < 0.05, χ2 = 7.28, Figure 2). Applying this result, the new definition of PNI < 44 was poor nutritional status group, and PNI ≥ 44 was normal nutritional status group.
 |
Figure 2 The optimal cut-off point for PNI by using MaxStat. |
Characteristics of DLBCL Patients After PSM
In this study, the Pseudo R2 before matching was about 0.09, which decreased to less than 0.01 after matching, indicating that the overall equilibrium test could pass. After PSM, a total of 282 patients in PNI < 44 group could be propensity matched to PNI ≥ 44 patients, creating a group of 564 patients. The two groups were remarkably balanced after matching based on 8 confounding factors, as each confounding factor was no longer be significantly different (P > 0.05, Table 1). The absolute standard deviation of the two matched groups were <10% for all the covariates (Figure 3).
 |
Figure 3 Impact of PNI on DLBCL after PSM. |
After PSM, the median OS of DLBCL patients was 34 months (95% CI: 15.042–52.824) in low PNI group and 61 months (95% CI: 31.795–90.205) in high PNI group. Ages of patients ranged from 14 to 91 years with a median age of 65 years, of whom 244 (43.2%) patients were female. A total of 121 (27.1%) patients (21.4%) patients were with CNS involvement and 87 (15.4%) patients were with bulky disease. The characteristics of patients after PSM were detailed in Table 1.
The predictive value of the PNI < 44 group for the prognosis of DLBCL was shown in Figure 4. Compared with PNI ≥ 44, PNI < 44 was associated with poorer survival of DLBCL in unadjusted (HR = 0.432, 95% CI: 0.350–0.533; P < 0.001), multivariate-adjusted (HR = 0.769, 95% CI: 0.603–0.982; P = 0.035), and propensity score matched analyses (HR = 0.751, 95% CI: 0.590–0.956; P = 0.020, Figure 4).
 |
Figure 4 Predictive value of the PNI for the prognosis of DLBCL (*P < 0.05). |
Univariable and Multivariable Analysis of DLBCL Patients
After PSM, a univariable analysis revealed that low PNI was an adverse factor for the survival of patients (HR = 0.783, P = 0.047, Table 2). In addition, age, CNS involvement and IPI appeared to be stronger predictors (P < 0.001). However, BCL-2, BCL-6, and cell of origin were not independent prognostic factors of DLBCL (P > 0.05). Multivariable analysis revealed that PNI remained an independent prognostic factor for DLBCL (HR = 0.751, P = 0.020). Compared to patients with low PNI (PNI < 44), patients with PNI ≥ 44 had a high 5-y OS (51.1% vs 44.8%).
 |
Table 2 Univariable and Multivariable Analysis After 1:1 Ratio PSM |
Prognostic Values of PNI with Clinicopathologic Factors in DLBCL
Of 564 patients after PSM, 303 cases were positive for BCL-2, and 257 cases were positive for BCL-6. Sixty-four (11.3%) patients were with high Ki-67 score (≥0.9) and 259 (45.9%) were non-GCB. PNI in Ann Arbor Stage (III/VI), ECOG (<2), IPI (LR+LIR), GCB, and BCL-2 negative groups could re-stratify the survival of patients (Figure 5). While PNI in Ann Arbor Stage (I/II), ECOG (≥2), IPI (HLR+HR), non-GCB, BCL-2 positive group, BCL-6, Ki-67, and MYC groups could not predict the survival of patients.
Discussion
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin’s lymphoma with highly heterogeneous. In this retrospective multicenter study, we analyzed the value of PNI on DLBCL. To our knowledge, this is the first study evaluating the prognostic effect of PNI on DLBCL with propensity score matching analysis, and we proved that PNI was an independent factor for patients with DLBCL.
Nutritional status is closely related to the prognosis of lymphoma patients.19–21 Nutritional insufficiency may influence poor survival outcomes of DLBCL patients.22 Kanemasa et al confirmed that geriatric nutritional risk index could identify a population of poor-risk DLBCL patients by NCCN-IPI.23 The controlling nutritional status was identified as an independent prognostic factor for OS of DLBCL patients.24,25 PNI, a feasible tool for assessing the relationship between immunonutritional status and prognosis of patients has been widely used in acute heart failure, esophageal cancers and lymphoma.10,26–28 In this study, we confirmed that PNI was an independent factor for predicting the survival of DLBCL, and we calculated the optimal cut-off point of PNI. According to the maximal chi-square method, 44 was the optimal cut-off point that distinguished between two prognostic groups most effectively, which was close to the results of previous studies.11,12,29,30 However, these studies did not take confounding factors into consideration, which might misevaluate the prognostic value on DLBCL. In our study, using propensity score matched analysis, other clinical characteristics of patients were well adjusted, and no differences were confirmed between low PNI and high PNI groups. This reduces the interference of confounders on survival outcome to some extent. Multivariate analysis showed that PNI, age, CNS involvement and IPI were independent prognostic factors for OS. Patients in HIR/HR groups of IPI had poor survival, which was consistent with one of our previous studies.31
DLBCL is highly heterogeneous in pathological features and elements of heterogeneity are associated with the prognosis of patients. Therefore, we evaluated the values of PNI on different groups of cell-of-origin, Ann Arbor Stage, ECOG, IPI and other pathological factors. The results confirmed that PNI could not re-stratify BCL-2 positive and non-GCB groups, while PNI could re-stratify BCL-2 negative, Ann Arbor Stage (III/VI) and IPI (LR+LIR) groups. The 5-y OS for malnourished patients was 56.3% in GCB group and 55.9% in ECOG (<2) group.
In conclusion, by using propensity score matched analysis, we confirmed that adjusted PNI was an effective prognostic factor for DLBCL patients. However, due to the inherent flaw of the retrospective design, more prospective studies need to be conducted to confirm this result in the future.
Ethics Approval and Informed Consent
Study approval was obtained from the independent Ethics Committees of each center in HHLWG and met with the Helsinki Declaration. Ethics committees approved the patients or next of kin being contacted by telephone. Written, informed consent was waived, since this study used retrospective data obtained only from hospital medical records.
Acknowledgment
Thanks to the Huaihai Lymphoma Working Group (HHLWG) for its participation in this study. HHLWG was a non-governmental group established in November 2017 and included 18 medical centers in Huaihai Economic Zone of China. The 18 medical centers are listed as follows:
1. Department of Hematology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221002, China
2. Department of Hematology, Qilu Hospital of Shandong University, Jinan, Shandong, 250012, China
3. Department of Hematology, The Affiliated Hospital of Jining Medical University, Jining, Shandong, 272000, China
4. Department of Hematology, Taian Central Hospital, Taian, Shandong, 271000, China
5. Department of Hematology, The First People’s Hospital of Changzhou, Changzhou, Jiangsu, 213003, China
6. Department of Hematology, Yancheng First People’s Hospital, Yancheng, Jiangsu, 224001, China
7. Department of Hematology, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, China
8. Department of Hematology, The First People’s Hospital of Huaian, Huaian, Jiangsu, 223300, China
9. Department of Hematology, The First People’s Hospital of Bozhou, Bozhou, Anhui, 236800, China
10. Department of Hematology, The First People’s Hospital of Lianyungang, Lianyungang, Jiangsu, 222002, China
11. Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, China
12. Department of Hematology, The Second Affiliated Hospital of Soochow University, Soochow, Jiangsu, 215000, China
13. Department of Hematology, The People’s Hospital of Suqian, Suqian, Jiangsu, 223800, China
14. Department of Hematology, The Henan cancer Hospital, Zhengzhou, Henan, 450008, China
15. Department of Hematology, Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, 233004, China
16. Department of Hematology, Wanbei Coal Power Group General Hospital, Suzhou, Anhui Province, Suzhou,234000,China
17. Department of Hematology, The Affiliated Shuyang Hospital of Xuzhou Medical University/Shuyang People’s Hospital, Suqian, 223600, China
18. Department of Hematology, Fengxian People’s Hospital, Xuzhou, 221700, Jiangsu, China
Funding
This study was funded by Natural Science Foundation of Jiangsu Province, Grant/Award Number: BK20171181; Jiangsu Key Research and Development Project of Social Development, Grant/Award Number: BE2019638; Young Medical Talents of Jiangsu Science and Education Health Project, Grant/Award Number: QNRC2016791.
Disclosure
The authors declare no conflicts of interest.
References
1. Shipp MA. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi:10.1056/NEJM199309303291402
2. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. doi:10.1182/blood-2013-09-524108
3. Tisdale MJ. Cachexia in cancer patients. Nat Rev Cancer. 2002;2(11):862–871. doi:10.1038/nrc927
4. Gupta D, Lis CG, Granick J, Grutsch JF, Vashi PG, Lammersfeld CA. Malnutrition was associated with poor quality of life in colorectal cancer: a retrospective analysis. J Clin Epidemiol. 2006;59(7):704–709. doi:10.1016/j.jclinepi.2005.08.020
5. Yang L, Xia L, Wang Y, et al. Low prognostic nutritional index (PNI) predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. PLoS One. 2016;11(7):e0158853. doi:10.1371/journal.pone.0158853
6. Lien YC, Hsieh CC, Wu YC, et al. Preoperative serum albumin level is a prognostic indicator for adenocarcinoma of the gastric cardia. J Gastrointest Surg. 2004;8(8):1041–1048. doi:10.1016/j.gassur.2004.09.033
7. Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi:10.1016/0002-9610(80)90246-9
8. Fanetti G, Polesel J, Fratta E, et al. Prognostic nutritional index predicts toxicity in head and neck cancer patients treated with definitive radiotherapy in association with chemotherapy. Nutrients. 2021;13(4):1277. doi:10.3390/nu13041277
9. Liu N, Jiang A, Zheng X, et al. Prognostic nutritional index identifies risk of early progression and survival outcomes in advanced non-small cell lung cancer patients treated with PD-1 inhibitors. J Cancer. 2021;12(10):2960–2967. doi:10.7150/jca.55936
10. Yao N, Hou Q, Zhang S, et al. Prognostic nutritional index, another prognostic factor for extranodal natural killer/T cell lymphoma, nasal type. Front Oncol. 2020;10:877. doi:10.3389/fonc.2020.00877
11. Go SI, Park S, Kang MH, Kim HG, Kim HR, Lee GW. Clinical impact of prognostic nutritional index in diffuse large B cell lymphoma. Ann Hematol. 2019;98(2):401–411. doi:10.1007/s00277-018-3540-1
12. Yu W, Guo Q, Wang Z, et al. Clinical significance of prognostic nutritional index for patients with diffuse large B-cell lymphoma. Nutr Cancer. 2019;71(4):569–574. doi:10.1080/01635581.2018.1540718
13. Zhou Q, Wei Y, Huang F, et al. Low prognostic nutritional index predicts poor outcome in diffuse large B-cell lymphoma treated with R-CHOP. Int J Hematol. 2016;104(4):485–490. doi:10.1007/s12185-016-2052-9
14. Kim DH, Pieper CF, Ahmed A, Colon-Emeric CS. Use and interpretation of propensity scores in aging research: a guide for clinical researchers. J Am Geriatr Soc. 2016;64(10):2065–2073. doi:10.1111/jgs.14253
15. Reiffel JA. Propensity-score matching: optimal, adequate, or incomplete? J Atr Fibrillation. 2018;11(4):2130. doi:10.4022/jafib.2130
16. Su CW, Fang KC, Lee RC, et al. Association between esophagogastric varices in hepatocellular carcinoma and poor prognosis after transarterial chemoembolization: a propensity score matching analysis. J Formos Med Assoc. 2020;119(2):610–620. doi:10.1016/j.jfma.2019.09.003
17. Baek S, Park SH, Won E, Park YR, Kim HJ. Propensity score matching: a conceptual review for radiology researchers. Korean J Radiol. 2015;16(2):286–296. doi:10.3348/kjr.2015.16.2.286
18. He C, Wang W, Chen Q, et al. Factors associated with stroke among patients with type 2 diabetes mellitus in China: a propensity score matched study. Acta Diabetol. 2021;58(11):1513–1523. doi:10.1007/s00592-021-01758-y
19. Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23(4):322–330. doi:10.1097/CCO.0b013e3283479c66
20. Ravasco P. Nutrition in cancer patients. J Clin Med. 2019;8(8):1211. doi:10.3390/jcm8081211
21. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical value of nutritional status in cancer: what is its impact and how it affects disease progression and prognosis? Nutr Cancer. 2017;69(8):1151–1176. doi:10.1080/01635581.2017.1367947
22. Park S, Han B, Cho JW, et al. Effect of nutritional status on survival outcome of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. Nutr Cancer. 2014;66(2):225–233. doi:10.1080/01635581.2014.867065
23. Kanemasa Y, Shimoyama T, Sasaki Y, Hishima T, Omuro Y. Geriatric nutritional risk index as a prognostic factor in patients with diffuse large B cell lymphoma. Ann Hematol. 2018;97(6):999–1007. doi:10.1007/s00277-018-3273-1
24. Nagata A, Kanemasa Y, Sasaki Y, et al. Clinical impact of controlling nutritional status score on the prognosis of patients with diffuse large B-cell lymphoma. Hematol Oncol. 2020;38(3):309–317. doi:10.1002/hon.2732
25. Baysal M, Bas V, Demirci U, et al. The utility of CONUT score in diffuse large B cell lymphoma patients. Niger J Clin Pract. 2021;24(8):1194–1199.
26. Cheng YL, Sung SH, Cheng HM, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6(6). doi:10.1161/JAHA.116.004876
27. Okadome K, Baba Y, Yagi T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271(4):693–700. doi:10.1097/SLA.0000000000002985
28. Paydas S, Lacin S, Dogan M, et al. Easier and more explanatory indices by integrating leukocyte lymphocyte ratio (LLR) and prognostic nutritional index (PNI) to IPS systems in cases with classical Hodgkin lymphoma. Leuk Res. 2021;107:106586. doi:10.1016/j.leukres.2021.106586
29. He J, Yin H, Xia Y, et al. Prognostic nutritional index, a novel biomarker which predicts worse prognosis in diffuse large B cell lymphoma. Leuk Res. 2021;110:106664. doi:10.1016/j.leukres.2021.106664
30. Perisa V, Zibar L, Knezovic A, Perisa I, Sincic-Petricevic J, Aurer I. Prognostic nutritional index as a predictor of prognosis in patients with diffuse large B cell lymphoma. Wien Klin Wochenschr. 2017;129(11–12):411–419. doi:10.1007/s00508-016-1077-7
31. Sang W, Zhou H, Qin Y, et al. Risk stratification model based on VEGF and International Prognostic Index accurately identifies low-risk diffuse large B-cell lymphoma patients in the rituximab era. Int J Hematol. 2021;114(2):189–198. doi:10.1007/s12185-021-03145-3
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.