Back to Journals » Cancer Management and Research » Volume 12
The Value of Geriatric Nutritional Risk Index in Evaluating Postoperative Complication Risk and Long-Term Prognosis in Elderly Colorectal Cancer Patients
Authors Tang S, Xie H, Kuang J, Gao F , Gan J , Ou H
Received 15 October 2019
Accepted for publication 11 December 2019
Published 9 January 2020 Volume 2020:12 Pages 165—175
DOI https://doi.org/10.2147/CMAR.S234688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Shuangyi Tang,1,* Hailun Xie,2,* Jiaan Kuang,2 Feng Gao,2 Jialiang Gan,2 Hesheng Ou3
1Department of Pharmacy, The First Affiliated Hospital, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 2Department of Colorectal and Anal Surgery, The First Affiliated Hospital, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 3Pharmaceutical College, Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jialiang Gan
Department of Colorectal and Anal Surgery, The First Affiliated Hospital, Guangxi Medical University, No. 6 Shuangyong Road, Nanning 530021, Guangxi, People’s Republic of China
Tel +86- 771- 5356529
Email [email protected]
Hesheng Ou
Pharmaceutical College of Guangxi Medical University, No. 22 Shuangyong Road, Nanning 530021, Guangxi, People’s Republic of China
Tel +86- 771- 5358272
Email [email protected]
Purpose: The geriatric nutritional risk index (GNRI) has been reported as a useful tool for predicting the prognosis of many diseases; however, there is currently little research on the relationship between GNRI and outcomes in elderly colorectal cancer (CRC) patients. This study aimed to explore the value of GNRI in evaluating postoperative complication risk and long-term prognosis in elderly CRC patients.
Patients and Methods: The medical records of 230 CRC patients aged≥65 years who underwent surgery between January 2012 and December 2014 were retrospectively analyzed. Patients were divided into abnormal and normal GNRI groups by modified binary classification. Logistic regression analysis was used to evaluate the correlation between GNRI and complication risk. The Kaplan–Meier method with log-rank test was used to construct survival curves. The Cox proportional hazard model was used for univariate, multivariate and subgroup survival analyses to assess the relationship between GNRI and long-term prognosis.
Results: Multivariate logistic regression analysis showed that GNRI (p = 0.009, HR 2.280, 95% CI: 1.224–4.247) was an independent risk factor for postoperative complications in elderly CRC patients. Kaplan–Meier survival curves revealed that the abnormal GNRI group had significantly lower disease-free survival (DFS; p = 0.005) and overall survival (OS; p=0.007) than the normal GNRI group had, especially in TNM I stage. In multivariate survival analysis, GNRI was an independent prognostic factor for DFS (p = 0.003, HR 1.842, 95% CI: 1.229–2.760) and OS (p = 0.003, HR 1.852, 95% CI: 1.231–2.787).
Conclusion: GNRI is a simple and effective tool for predicting the risk of postoperative complications and the long-term prognosis of postoperative elderly CRC patients and can provide a scientific basis for early nutrition interventions in elderly CRC patients.
Keywords: colorectal cancer, geriatric nutritional risk index, prognosis, complication
Introduction
Colorectal cancer (CRC) is the third most frequent malignancy and the second leading cause of cancer-related death worldwide, and its incidence ranks fourth among men and third among women. In 2018, there were >1.8 million new cases and 881,000 deaths estimated worldwide.1 In China, CRC is the fifth most usual malignancy, and its mortality ranks fourth, and the incidence continues to increase.2
Surgical resection is still a mainstay of curative treatment for CRC.3 However, malnutrition may increase the risk of surgery and prolong hospital stays and has been confirmed to significantly increase postoperative mortality in elderly patients.4 Malnutrition is often found in cancer patients and is not only associated with postoperative complications, but also with long-term prognosis.5,6 A large body of research has reported malnutrition in >80% of patients with gastrointestinal malignancies.7,8 Therefore, further studies are needed to determine prognostic indicators to assess better the risk of postoperative complications and long-term prognosis in elderly CRC patients.
The geriatric nutritional risk index (GNRI) was originally established by Bouillanne et al9 to assess nutritional risk in elderly people. GNRI is an adaptation of the NRI of Buzby et al10 and it is a simple nutrition screening tool for assessing the nutritional risks for elderly patients.11,12 GNRI is widely used in nutritional assessment of elderly patients with chronic liver failure, chronic obstructive pulmonary disease, and cardiovascular disease.13–15 Recently, many studies have found that GNRI can be used in the prognostic assessment of various malignant tumors, including renal cell carcinoma,16,17 esophageal squamous cell carcinoma,18 hepatocellular carcinoma,19 and pathological stage I non-small cell lung cancer.20 However, there is currently little research on the relationship between GNRI and outcomes in elderly CRC patients. Thus, this study aimed to explore the value of GNRI in evaluating postoperative complication risk and long-term prognosis in elderly CRC patients.
Materials and Methods
Study Population
A total of 230 CRC patients aged≥65 years who experienced surgery in the Department of Colorectal and Anal Surgery, the First Affiliated Hospital of Guangxi Medical University between January 2012 and December 2014 were retrospectively analyzed. The inclusion criteria were as follows: 1) pathological diagnosis of the primary lesion was CRC adenocarcinoma; 2) receiving radical resection; 3) age≥65 years; and 4) complete clinicopathological characteristics and follow-up data. The exclusion criteria were as follows: 1) uncertain primary tumor site; 2) patients undergoing palliative surgery; 3) missing clinical data and patients lost to follow-up. The current study protocol was permitted by the Hospital Ethics Committee of the First Affiliated Hospital of Guangxi Medical University, Guangxi, China: 2019 (KY-E-100), and patients were informed in detail about the study and verbal consents were obtained during the follow-up. This study strictly followed all regulations of the Declaration of Helsinki, and all data were maintained with confidentiality.
Data Collection
The clinicopathological characteristics we collected included gender, age, serum albumin, prognostic nutritional index (PNI), body mass index (BMI), tumor–node–metastasis (TNM) stage, pathology tumor (pT) stage, pathology node (pN) stage, preoperative metastasis, tumor differentiation, perineural/vascular invasion, tumor location, pathological types, surgical approach, preoperative serum carcinoembryonic antigen (CEA). The postoperative outcomes we collected included operative mortality, postoperative complications, overall survival (OS) and disease-free survival (DFS).
Definition of GNRI and Other Parameters
Serum albumin, serum CEA levels were measured by preoperative fasting venous blood (4 mL) collected 3–5 days before surgery. Albumin <35 g/L was defined as hypoproteinemia, serum CEA level <5 ng/mL was defined as normal. According to the World Health Organization’s BMI standard, a BMI less than 18.5 is underweight, and a BMI greater than or equal to 24 is overweight. PNI = albumin level (g/L) + 5×lymphocyte count (109/L), PNI < 45 was considered low, and PNI≥45 was considered high. The GNRI formula was: GNRI = 1.487×serum albumin concentrations (g/L) + 41.7×preoperative body weight/ideal body weight. The ideal body weight was calculated according to the modified Broca’s index, male: (height-100) ×0.9; female: (height-100) ×0.85. When the preoperative body weight of patients exceeded the ideal body weight, the preoperative body weight/ideal body weight (kg) was set at 1. The weight and height of each patient were assessed and averaged by two medical professionals. Based on previous research,9,19 patients were classified according to the following cut-offs: high risk (GNRI < 82), moderate risk (82–92), low risk (92–98), and normal level (≥98). The postoperative complication grades were assessed using the Clavien–Dindo complication classification system.21
Survival Follow-Up
CRC patients were re-examined every 3 months within 2 years after surgery and then every 3–6 months thereafter. The final follow-up visit occurred in August 1, 2019. Postoperative follow-up examination consisted of blood tests, checking the tumor biomarkers, imaging diagnosis (X-ray photography, positron emission tomography, and computed tomography), and colonoscopy. DFS was defined as the interval from cancer resection to recurrence, death or last follow-up. OS was defined as the interval from cancer resection to death or last follow-up.
Statistical Analysis
The chi-square test or Fisher’s exact test was applied to explore differences in categorical variables. Logistic regression analysis was applied to assess factors associated with postoperative complications. The Kaplan–Meier method with log-rank test was used to construct survival curves. Univariate, multivariate and subgroup multivariate Cox regression analyses were applied to identify variables associated with DFS and OS. The nomogram for predicting complication risk was founded by logistic regression analysis. The nomograms for predicting DFS and OS were founded by Cox regression analysis. Two-sided p < 0.05 was significant. All data were analyzed by IBM SPSS 24.0 (IBM Corp, Armonk, NY) and R software Version 3.5.3 (version 3.5.3; www.r-project.org).
Results
Clinicopathological Factors
Our study included 230 patients (154 males and 76 females), with a mean age of 70.6 ± 5.4 years and age range 65–92 years. The median follow-up time for OS was 61 months (range 1–80 months). According to the American Joint Committee on Cancer (AJCC) eighth version TNM classification, 58 patients (25.2%) were classified as stage I, 80 (34.8%) as stage II, 76 (33.0%) as stage III, and 16 (7%) as stage IV. 124 patients underwent open surgery and 106 patients underwent laparoscopic surgery.
Associations of GNRI Quartiles with Clinicopathological Factors
Correlations of GNRI with various clinicopathological factors included gender, age, albumin, BMI, PNI, pT stage, pN stage, TNM stage, perineural/vascular invasion, tumor location, pathological type, differentiation, and CEA level. GNRI was significantly correlated with gender (p < 0.001), albumin (p < 0.001), BMI (p < 0.001), and PNI (p < 0.001) (Table 1).
 |
Table 1 Associations of GNRI Quartiles with Clinicopathological Factors |
Modified Categories of GNRI in Elderly CRC Patients
We performed Cox regression analysis using the categorical variables. In the multivariate Cox regression analysis of DFS, the survival rate of normal GNRI patients was significantly higher than that of high-risk GNRI patients (p = 0.014, HR 0.390, 95% CI: 0.184–0.829), while the survival rate of moderate- and low-risk GNRI patients was not significantly improved compared with that of high-risk GNRI patients (p = 0.392 and 0.315, respectively). In the multivariate analysis of OS, the survival rate of normal GNRI patients was significantly higher than that of high-risk GNRI patients (p = 0.009, HR 0.368, 95% CI: 0.173–0.782), while the survival rate of moderate- and low-risk GNRI patients was not significantly improved compared with that of high-risk GNRI patients (p = 0.317 and 0.245, respectively). Based on this analysis, we established the dichotomous GNRI variable, defining normal level group as normal GNRI group, and combining high-, moderate- and low-risk groups into the abnormal GNRI group (Table 2).
 |
Table 2 Modified Categories of GNRI in Elderly CRC Patients |
Risk Factors for Postoperative Complications in CRC Patients
In total, 61 (26.5%) patients had postoperative complications, including postoperative bowel obstruction (15 cases), anastomotic leak (3 cases), pulmonary complications (12 cases), wound problems (18 cases), death (1 case), and other complications (12 cases). Clavien–Dindo classification showed: 30 cases (13.0%) with grade I complications, 22 (9.6%) with grade II, 3 (1.3%) with grade III, 5 (2.2%) with grade IV, and 1 (0.4%) with grade V. Incidence of postoperative complications was significantly higher in the abnormal GNRI group (17.4% vs 9.1%, p = 0.003) (Table 3). In the univariate logistic regression analysis, GNRI (p = 0.003), tumor location (p =0.026), and surgical approach (p = 0.007) were associated with postoperative complications. Variables with p <0.05 in univariate logistic regression analysis were further assessed with the multivariate logistic regression analysis, showing that only GNRI (p = 0.009, HR 2.280, 95% CI: 1.224–4.247) and surgical approach (p = 0.040, HR 1.965, 95% CI: 1.031–3.746) were independent risk factors for postoperative complications in elderly CRC patients (Table 4).
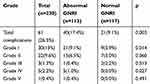 |
Table 3 Details of Postoperative Complications According to the Clavien–Dindo Classification |
 |
Table 4 Univariate and Multivariate Logistic Regression Analysis of Postoperative Complication in Elderly CRC Patients |
Kaplan–Meier Curve of GNRI in Elderly CRC Patients
In the Kaplan–Meier analysis, patients in the normal GNRI group experienced significantly higher DFS and OS, than those in the abnormal GNRI group (Figure 1). Further stratified analysis showed that in TNM stage I patients, there were significant differences between the normal and abnormal GNRI groups in both DFS and OS, but there were no significant differences in TNM II, III and IV stage patients (Figure 2).
Survival Analysis of GNRI in Elderly CRC Patients
In univariate analysis, patients in the normal GNRI group had significantly longer DFS and OS than those in the abnormal GNRI group ((p = 0.010, HR 1.681, 95% CI: 1.133–2.496); (p=0.007, HR 1.319, 95% CI: 1.196–2.712), respectively). In multivariate analysis, adjusted for other clinicopathological factors, the DFS rate was significantly higher in the normal GNRI group than in the abnormal GNRI group (p = 0.003, HR 1.842, 95% CI: 1.229–2.760). Similar results were also found in OS (p = 0.003, HR 1.852, 95% CI: 1.231–2.787) (Table 5).
 |
Table 5 Univariate and Multivariate Survival Analysis of Clinicopathological Characteristics in Elderly CRC Patients |
We also examined whether GNRI was altered by any subgroup variables of clinicopathological factors. We found that GNRI in male patients, patients aged <70 years, and patients with normal albumin, normal BMI, high PNI, T1–2 stage, pN0 stage, rectal cancer, no preoperative metastasis, positive and negative perineural/vascular invasion, protruding tumor type, poor tumor differentiation, laparoscopy surgery and normal CEA had significant significance through multivariate subgroup analysis in DFS (Figure 3A). While had significant significance in male, <70 years, normal ALB, normal BMI, high PNI, T1-2 stage, pN0 stage, rectal cancer, no preoperative metastasis, negative perineural/vascular invasion, poor differentiation, laparoscopy surgery and normal CEA had significant significance through multivariate subgroup analysis in OS (Figure 3B).
Development of Nomograms
A nomogram was constructed to assess the risk of postoperative complications of elderly CRC patients. After adjustment with the logistic regression model, only GNRI and surgical approach were included in the complication risk model (Figure 4). The points against each factor can be counted, and the complication risk could also be predicted. The nomograms were used to assess the correlation of GNRI and prognosis in elderly CRC patients. After adjustment with the Cox regression analysis, only GNRI, pN stage, and preoperative metastasis were included in the DFS model, and GNRI, differentiation, pN stage, and preoperative metastasis were included in the OS model. The points against each factor can be counted, and the 1- to 5-year DFS and OS could also be predicted (Figure 5A and B).
 |
Figure 4 The nomogram of complication risk model in elderly CRC patients. Abbreviation: GNRI, geriatric nutritional risk index. |
Discussion
Gastrointestinal malignancy, especially CRC, is often accompanied by malnutrition, which is due to the decrease in oral intake and increase in consumption caused by gastrointestinal obstruction, chronic intestinal bleeding and cancer pain.5,21,22 Elderly patients are more prone to cancer-related death due to malnutrition and deterioration of physical function. Many studies have shown that malnutrition makes it more difficult to recover from diseases, trauma and surgery. Deterioration of nutritional status can inhibit tumor immunity, leading to an increase of postoperative complications and tumor spread.23,24 Therefore, early identification of nutritional high-risk status is crucial to improve the prognosis of elderly CRC patients.
GNRI combines three important nutritional indicators, including serum albumin levels and body weight and height. Serum albumin is one of the most common factors used to assess malnutrition. Some studies25,26 have suggested that albumin is an index that indirectly reflects inflammation, it affects the catabolism of liver cells through pro-inflammatory cytokines, including interleukin-1 and tumor necrosis factor-α. These inflammatory factors play a significant role in tumor cell proliferation and invasion, and tumor neovascularization. Roxburgh and McMillan27 found that hypoalbuminemia may be an association with impaired immune response. Gupta and Lis28 and Hu et al29 also showed that a decrease in serum protein in CRC patients contributed significantly to poor postoperative outcome. Height and weight are also commonly used to assess an individual’s nutritional status. Renfro et al30,31 pointed out that low BMI may increase the risk of disease progression and death in CRC patients, which may be related to the negative effects of cancer cachexia. Due to the influence of cancer malignancy, the BMI of CRC patients may be lower than that of the normal population. GNRI has additional information on ideal weight, which can more objectively reflect the weight change of CRC patients. GNRI combined with serum albumin level and changes in body weight can better predict nutrition-related death than serum albumin or BMI can.
Since GNRI can be easily calculated from conventionally clinical laboratory data, it has gradually been applied to the assessment of nutrition, complications and prognosis in various chronic diseases, including various malignant tumors. However, few studies have verified the clinical application of GNRI for elderly CRC patients. In the present study, we explored the potential value of GNRI as a postoperative complication or prognostic factor in elderly CRC patients. In previous studies,9,32 GNRI was based on four classifications. We performed Cox regression analysis and Kaplan–Meier survival curve analysis on GNRI and found that a cutoff value of GNRI=98 could better distinguish CRC patients with poor prognosis. Thus, we modified the GNRI classification criteria, which makes it more convenient and suitable to evaluate elderly CRC patients.
Malnutrition is associated with postoperative intestinal spasm, delayed wound healing, postoperative pulmonary infection, and a significant increase in morbidity and mortality the elderly patients. In the present study, about 26.5% of the elderly patients experienced varying degrees of postoperative complications, and most could be resolved by medication. The risk of postoperative complications was confirmed to be associated with abnormal GNRI, and multivariate logistic regression analysis showed that GNRI is an independent risk factor for postoperative complications. We also found that preoperative GNRI levels in CRC patients could play an important role in assessing long-term outcome. Kaplan–Meier survival analysis showed that the abnormal GNRI group had a lower postoperative survival rate than the normal GNRI group had, whether in DFS or OS. In the Cox regression analysis, although GNRI, pT stage, preoperative metastasis, perineural/vascular invasion, tumor differentiation and CEA were associated with prognosis in elderly CRC patients, only GNRI, pN stage, and preoperative metastasis were independent risk factors for DFS, and GNRI, differentiation, pN stage, and preoperative metastasis were independent risk factors for OS. The TNM staging system is widely used to predict the prognosis of CRC patients, but the prognosis of patients with the same stage is often different, so it is necessary to find other factors that can further distinguish the prognosis in the same TNM stage. Based on the different TNM stages, we further performed a stratified analysis of GNRI. The results showed that only in TNM stage I could GNRI be used to screen patients with poor prognosis. There are many factors that affect the prognosis of CRC patients, such as tumor invasion, metastasis, nutrition, and immunity. For elderly CRC patients, nutrition-related factors may be another important factor affecting prognosis in addition to TNM staging. The reason why GNRI is not significantly different in advanced tumors may be in the early stages of tumors, nutrition-related factors have a large influence and other factors have a small influence, as the tumor progresses, tumor invasion, metastasis and other factors gradually play a dominant role. In the subgroup multivariate survival analysis, GNRI is significant in most subgroups, demonstrating that GNRI can be widely used in elderly CRC patients.
For easier assessment of the risk of postoperative complications in elderly CRC patients, we established a complication risk nomogram by logistic regression. By applying this model, we could predict the complication risk, and patients with lower scores had better nutritional status than those with higher scores. For easier assessment of the risk of postoperative adverse long-term prognosis in elderly CRC patients, we established two survival risk nomograms by the Cox regression analysis model. By applying the models, we could predict the 1- to 5-year survival, and patients with lower scores had better survival than those with higher scores.
In elderly CRC patients with abnormal GNRI, approximately 26.55% had normal BMI and albumin. Although these patients appeared to be sufficiently healthy based on their appearance and preoperative examination and could undergo surgical resection, the true malnutrition status and acceptable organ function were often ignored. In multivariate subgroup analysis, GNRI had implications for assessing prognosis in the normal BMI and albumin subgroups. This indicates that preoperative GNRI could be useful to identify patients with true malnutrition. PNI, a nutrition-related indicator, is considered a good tool for assessing the prognosis of CRC patients, but in this study, we found that PNI was not statistically significant in both survival analysis and complications-related analysis. We believe that GNRI is more appropriate than other nutrition-related tools in assessing the prognostic value of elderly CRC patients.
Our study had some limitations. It was a retrospective study from a single center, with a small sample. Further research with multicenter prospective studies and larger samples is needed. Other nutrition-related indicators, such as Glasgow Prognostic Score (GPS) and Controlling Nutritional Status (CONUT), have also been confirmed to be related to the prognosis of CRC patients. However, due to the lack of these clinical data in this study, it is currently impossible to compare the effectiveness of GNRI with them, and we plan to conduct these useful explorations in the future.
Conclusion
This study demonstrated that GNRI is a simple and effective tool for predicting the risk of complications and long-term prognosis of elderly CRC patients, and can provide a scientific basis for early nutrition intervention in elderly CRC patients.
Acknowledgments
This research was supported by the 2019 Innovation Project of Guangxi Graduate Education (JGY2019052).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
3. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369. doi:10.6004/jnccn.2018.0021
4. Chen CCH, Schilling LS, Lyder CH. A concept analysis of malnutrition in the elderly. J Adv Nurs. 2001;36(1):131–142. doi:10.1046/j.1365-2648.2001.01950.x
5. Schwegler I, Von Holzen A, Gutzwiller JP, Schlumpf R, Mühlebach S, Stanga Z. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. Br J Surg. 2010;97(1):92–97. doi:10.1002/bjs.6805
6. Alifano M, Mansuet-Lupo A, Lococo F, et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS One. 2014;9(9):e106914. doi:10.1371/journal.pone.0106914
7. Paccagnella A, Morassutti I, Rosti G. Nutritional intervention for improving treatment tolerance in cancer patients. Curr Opin Oncol. 2011;23(4):322–330. doi:10.1097/CCO.0b013e3283479c66
8. Muscaritoli M, Anker S, Argiles J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG)“cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr. 2010;29(2):154–159. doi:10.1016/j.clnu.2009.12.004
9. Bouillanne O, Morineau G, Dupont C, et al. Geriatric nutritional risk index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777–783. doi:10.1093/ajcn/82.4.777
10. Buzby G, Knox L, Crosby L, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988;47(2):366–381. doi:10.1093/ajcn/47.2.366
11. Abd-El-Gawad WM, Abou-Hashem RM, El Maraghy MO, Amin GE. The validity of geriatric nutrition risk index: simple tool for prediction of nutritional-related complication of hospitalized elderly patients. Comparison with mini nutritional assessment. Clin Nutr. 2014;33(6):1108–1116. doi:10.1016/j.clnu.2013.12.005
12. Cereda E, Klersy C, Pedrolli C, et al. The geriatric nutritional risk index predicts hospital length of stay and in-hospital weight loss in elderly patients. Clin Nutr. 2015;34(1):74–78. doi:10.1016/j.clnu.2014.01.017
13. Wada H, Dohi T, Miyauchi K, et al. Prognostic impact of the geriatric nutritional risk index on long-term outcomes in patients who underwent percutaneous coronary intervention. Am J Cardiol. 2017;119(11):1740–1745. doi:10.1016/j.amjcard.2017.02.051
14. Taniguchi E, Kawaguchi T, Otsuka M, et al. Nutritional assessments for ordinary medical care in patients with chronic liver disease. Hepatol Res. 2013;43(2):192–199. doi:10.1111/hepr.2013.43.issue-2
15. Matsumura T, Mitani Y, Oki Y, et al. Comparison of geriatric nutritional risk index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung. 2015;44(6):534–538. doi:10.1016/j.hrtlng.2015.08.004
16. Miyake H, Tei H, Fujisawa M. Geriatric nutrition risk index is an important predictor of cancer-specific survival, but not recurrence-free survival, in patients undergoing surgical resection for non-metastatic renal cell carcinoma. Curr Urol. 2016;10(1):26–31. doi:10.1159/000447147
17. Gu W, Zhang G, Sun L, et al. Nutritional screening is strongly associated with overall survival in patients treated with targeted agents for metastatic renal cell carcinoma. J Cachexia Sarcopenia Muscle. 2015;6(3):222–230. doi:10.1002/jcsm.12025
18. Yamana I, Takeno S, Shimaoka H, et al. Geriatric nutritional risk index as a prognostic factor in patients with esophageal squamous cell carcinoma–retrospective cohort study. Int J Surg. 2018;56:44–48. doi:10.1016/j.ijsu.2018.03.052
19. Li L, Wang H, Yang J, et al. Geriatric nutritional risk index predicts prognosis after hepatectomy in elderly patients with hepatitis B virus-related hepatocellular carcinoma. Sci Rep. 2018;8(1):12561. doi:10.1038/s41598-018-30906-8
20. Shoji F, Matsubara T, Kozuma Y, et al. Preoperative geriatric nutritional risk index: a predictive and prognostic factor in patients with pathological stage I non-small cell lung cancer. Surg Oncol. 2017;26(4):483–488. doi:10.1016/j.suronc.2017.09.006
21. Kwag S-J, Kim J-G, Kang W-K, Lee J-K, Oh S-T. The nutritional risk is a independent factor for postoperative morbidity in surgery for colorectal cancer. Ann Surg Treat Res. 2014;86(4):206–211. doi:10.4174/astr.2014.86.4.206
22. Wie G-A, Cho Y-A, Kim S-Y, Kim S-M, Bae J-M, Joung H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010;26(3):263–268. doi:10.1016/j.nut.2009.04.013
23. Barao K, Abe Vicente Cavagnari M, Silva Fucuta P, Manoukian Forones N. Association between nutrition status and survival in elderly patients with colorectal cancer. Nutr Clin Pract. 2017;32(5):658–663. doi:10.1177/0884533617706894
24. Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi:10.1016/j.clnu.2007.10.007
25. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361. doi:10.1038/nrc2628
26. Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15(6):362. doi:10.1038/nri3834
27. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 2010;6(1):149–163. doi:10.2217/fon.09.136
28. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9(1):69. doi:10.1186/1475-2891-9-69
29. Hu W-H, Eisenstein S, Parry L, Ramamoorthy S. Preoperative malnutrition with mild hypoalbuminemia associated with postoperative mortality and morbidity of colorectal cancer: a propensity score matching study. Nutr J. 2019;18(1):33. doi:10.1186/s12937-019-0458-y
30. Renfro LA, Loupakis F, Adams RA, et al. Body mass index is prognostic in metastatic colorectal cancer: pooled analysis of patients from first-line clinical trials in the ARCAD database. J Clin Oncol. 2016;34(2):144. doi:10.1200/JCO.2015.61.6441
31. Jeong SH, Kim P, Yi SW, Kim YJ, Baeg MK, Yi JJ. Body mass index and gastrointestinal cancer mortality in Korean adults: a prospective cohort study. J Gastroenterol Hepatol. 2018;33(9):1582–1589. doi:10.1111/jgh.14115
32. Wang H, Hai S, Zhou Y, Liu P, Dong B-R. The geriatric nutritional risk index predicts mortality in nonagenarians and centenarians receiving home care. Asia Pac J Clin Nutr. 2018;27(1):78.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




