Back to Journals » Clinical Epidemiology » Volume 12
The Validity of Intracerebral Hemorrhage Diagnoses in the Danish Patient Registry and the Danish Stroke Registry
Authors Hald SM , Kring Sloth C, Agger M , Schelde-Olesen MT, Højholt M , Hasle M , Bogetofte H , Olesrud I , Binzer S, Madsen C, Krone W, García Rodríguez LA , Al-Shahi Salman R , Hallas J , Gaist D
Received 13 June 2020
Accepted for publication 27 August 2020
Published 1 December 2020 Volume 2020:12 Pages 1313—1325
DOI https://doi.org/10.2147/CLEP.S267583
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Stine Munk Hald,1,2 Christine Kring Sloth,1 Mikkel Agger,1 Maria Therese Schelde-Olesen,1 Miriam Højholt,1 Mette Hasle,1 Helle Bogetofte,1 Ida Olesrud,1 Stefanie Binzer,3 Charlotte Madsen,1 Willy Krone,4 Luis Alberto García Rodríguez,5 Rustam Al-Shahi Salman,6 Jesper Hallas,7 David Gaist1,2,8
1Department of Neurology, Odense University Hospital, Odense, Denmark; 2Department of Clinical Research, Neurology Research Unit, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; 3Department of Neurology, Lillebaelt Hospital, Kolding, Denmark; 4Department of Radiology, Odense University Hospital, Odense, Denmark; 5Centro Español Investigación Farmacoepidemiológica (CEIFE), Madrid, Spain; 6Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, UK; 7Clinical Pharmacology and Pharmacy, Department of Public Health, University of Southern Denmark, Odense, Denmark; 8Odense Patient Data Explorative Network (OPEN), Odense University Hospital, Odense, Denmark
Correspondence: David Gaist
Department of Neurology, Odense University Hospital, J.B. Winsløwsvej 4, 5000 Odense C, Odense, Denmark
Tel +45 65412485
Fax +45 65413389
Email [email protected]
Purpose: To establish the validity of intracerebral hemorrhage (ICH) diagnoses in the Danish Stroke Registry (DSR) and the Danish National Patient Registry (DNPR).
Patients and Methods: Based on discharge summaries and brain imaging reports, we estimated the positive predictive value (PPV) of a first-ever diagnosis code for ICH (ICD-10, code I61) for all patients in the Region of Southern Denmark (1.2 million) during 2009– 2017 according to either DNPR or DSR. We estimated PPVs for any non-traumatic ICH (a-ICH) and spontaneous ICH (s-ICH) alone (ie, without underlying structural cause). We also calculated the sensitivity of these diagnoses in each of the registers. Finally, we classified the location of verified s-ICH.
Results: A total of 3,956 patients with ICH diagnosis codes were studied (DSR only: 87; DNPR only: 1,513; both registries: 2,356). In the DSR, the PPVs were 86.5% (95% CI=85.1– 87.8) for a-ICH and 81.8% (95% CI=80.2– 83.3) for s-ICH. The PPVs in DNPR (discharge code, primary diagnostic position) were 76.2% (95% CI=74.7– 77.6) for a-ICH and 70.2% (95% CI=68.6– 71.8) for s-ICH. Sensitivity for a-ICH and s-ICH was 76.4% (95% CI=74.8– 78.0) and 78.7% (95% CI=77.1– 80.2) in DSR, and 87.3% (95% CI=86.0– 88.5) and 87.7% (95% CI=86.3– 88.9) in DNPR. The location of verified s-ICH was lobar (39%), deep (33.6%), infratentorial (13.2%), large unclassifiable (11%), isolated intraventricular (1.9%), or unclassifiable due to insufficient information (1.3%).
Conclusion: The validity of a-ICH diagnoses is high in both registries. For s-ICH, PPV was higher in DSR, while sensitivity was higher in DNPR. The location of s-ICH was similar to distributions seen in other populations.
Keywords: stroke, intracerebral hemorrhage, epidemiology, validity, register-based research
Introduction
Administrative registries that routinely collect data at a population level can be useful resources for studies of temporal trends in cerebrovascular disorders,1–5 and in observational research focusing on the cause, treatment, and course of stroke.6–20 Large clinical databases or medical registers anchored in well-defined populations can furthermore be a particularly attractive solution to the challenge of acquiring valid data over extended time-periods for relatively rare disorders such as intracerebral hemorrhage (ICH).10,21–29 However, to be useful data sources for epidemiologic research, registers must first provide data of sufficiently high quality. While validity is important for all types of research based on register information, for studies of trends in the incidence of diseases, knowledge of the degree of sensitivity of the data is also paramount.30,31 In Denmark, two nationwide registers are of particular use for identifying patients with stroke for research purposes: the Danish Stroke Registry (DSR) and the Danish National Patient Registry (DNPR). The sensitivity of ICH diagnoses in these registers is unknown, and to date only a few studies have examined the validity of ICH diagnoses in DSR32 or DNPR.33–36 Supplementary Table S1 summarizes the existing studies.
We conducted this study with the purpose to provide estimates of the validity of ICH diagnoses in DSR and DNPR and to acquire data on the location of ICH in a large unselected sample of patients with a spontaneous parenchymal hemorrhage.
Patients and Methods
We defined any non-traumatic ICH (a-ICH) as a symptomatic event (new headache, altered level of consciousness, or neurological symptoms), with or without new neurological signs, referable to a focal collection of blood within the brain parenchyma seen on brain imaging with signal characteristics consistent with the time of symptom onset. We defined spontaneous ICH (s-ICH), as ICH not attributable to prior trauma, hemorrhagic transformation of an ischemic stroke, or an alternative explanation (eg, tumor or vascular malformation – but not use of antithrombotic drugs).32,37 The above definition is similar to the World Health Organization (WHO) stroke definition,38 but in addition allows inclusion of patients based on symptoms (eg, severe sudden onset headache), where imaging supports new onset ICH.
Setting and Data Sources
We based this study on data from hospital contacts of residents of the Region of Southern Denmark (RSD; 1.2 million inhabitants), a geographically defined region in Denmark. Patients suspected of a stroke are principally admitted or transferred to one of the four dedicated stroke units at neurology departments in the region, which also hosts a single neurosurgery department.32 All hospitals in Denmark report data in a standardized format to the DNPR.39
The Danish Stroke Registry (DSR), a clinical database, was established in Denmark in 2003 to monitor the quality of care provided to stroke patients. It is mandatory to report standardized detailed information on all acute admissions for stroke at hospitals in Denmark to the DSR.40,41
The regional authorities have copies of all hospital electronic medical records (EMRs) in RSD, which can be used for research purposes, provided consent is obtained from the heads of departments involved in patient care.32
We identified all hospital contacts of residents of RSD recorded in DNPR (data since 2007 made available to us) or DSR (data since 2003, when the register became operational). In DNPR, for the period January 1, 2007 to December 31, 2017 we retrieved information on any hospital contacts (ie, inpatient, outpatient, or emergency department contacts) with International Classification of Diseases version 10 (ICD-10) I61 (intracerebral hemorrhage) as the principal diagnostic code (in Danish, “aktionsdiagnose”) or in any other diagnostic position. In DSR, stroke diagnoses are recorded as “hemorrhagic stroke” (ie, ICH), “ischemic stroke”, or “unspecified”. We retrieved data on all hospital contacts recorded under “hemorrhagic stroke” in DSR for the period January 1, 2003 to December 31, 2017 using the same residency criteria as above. Within each register sample, we limited records to each patient’s first hospital contact within the years 2009–2017, a period where medical records and brain imaging reports were more likely to be available. We focused on first-ever ICH and therefore excluded patients with records of ICH (DNPR inpatient, outpatient or ED – any diagnostic position, or DSR) predating the study period (ie, before January 1, 2009).
Identification of Cases for Validation
We designed this study similar to a previous validation study we performed in the same region for a shorter time-period (2010–2015) and based on a smaller sample of patients with ICH (n=500 patients).32 Motivated by our previous results,32 and aiming to identify reliable algorithms that would enable optimal use of the register data, we designed the present validation study to calculate PPV of the DSR and DNPR when used independently, or in combination. The researchers that performed the assessments described below were blinded with regard to the register source.
We identified information on patients with a possible first-ever diagnosis of ICH as follows. We retrieved data on all patients recorded under ICH diagnoses in the DNPR, or the DSR in 2009–2017, as described above.
To minimize capture of re-admissions for the same ICH event, we identified the first hospital contact during the period. Furthermore, we only included patients that were aged 20+ years at the time of the first hospital contact as DSR only records data on adult patients. We merged the resulting data and classified patients into three mutually exclusive groups: i) recorded in both registries, ii) recorded in DNPR only; and iii) recorded in DSR only. We only classified patients as recorded in both registries if hospital contact dates in the two registries were separated by no more than 7 days, in order to enhance the likelihood of studying the same event.
For patients identified in the DNPR, in addition to the contact on the index date, we also identified all other hospital contacts (ie, admission, outpatient, or emergency department (ED)) with a diagnosis code of ICH that we considered to belong to the same episode of ICH, ie, consecutive contacts separated by a gap of no more than 7 days. Based on this information, we classified patients recorded by DNPR by patterns of contact as follows: a) inpatient with ICH discharge code in primary diagnostic position (regardless of ED/outpatient contacts); b) inpatient with ICH discharge code in diagnostic position other than primary (regardless of ED/outpatient contacts); or c) outpatient only or ED only, any diagnostic position.
Data on all patients, identified as outlined above, were validated based on information from discharge records and brain scan reports. These documents were mainly (63%) provided to us in electronic form by the regional authorities, which hold copies of all hospital EMRs in RSD. All hospitals in RSD use the same EMR system that was gradually implemented in the years 2008–2015. For contacts with no record in the centralized copy of EMRs (mainly records predating the introduction of the EMR system), we requested manually retrieved copies of discharge summaries and brain scan reports from the hospital departments involved. We were granted permission to retrieve data as outlined by all heads of departments involved. In anticipation of the logistic difficulties involved, we did not retrieve information on patients residing in RSD who were only recorded with ICH diagnoses at hospitals located out of the region or abroad (<2% of cases).
Based on previous experience,32 to ensure coverage of transfers between units (eg, from ED to stroke unit, or from one hospital to another), and of further work-up for secondary causes (eg, follow-up imaging after incident ICH), we requested discharge records and brain imaging study reports for a period spanning 1 week before to 5 months after the hospital contact date of the index event. Nine study physicians – supervised by two neurologists and a specialist in radiology with a special interest in stroke – assessed this information and abstracted data to a structured form. Information collected included ICH diagnosis verified, whether it was s-ICH, and the location of s-ICH.
We classified ICH into “single ICH” or “multiple ICH” (more than one concurrent ICH described in brain scan report). We classified ICH location based on a slightly modified version of the criteria employed in a previous population-based study:37 1) “deep ICH” (single supratentorial deep ICH, or multiple ICHs in solely deep locations); 2) “infratentorial ICH” (single infratentorial ICH, or multiple ICHs in solely infratentorial locations, or infratentorial ICH combined with deep ICH); 3) “large unclassifiable ICH”; 4) “isolated intraventricular ICH”; 5) “insufficient information to classify”; and 6) “lobar ICH” (all other, ie, ICH locations not included in categories 1–5).
Full medical records were retrieved in cases of doubt regarding the diagnosis.
Study data were collected and managed using REDCap electronic data capture tools hosted at Odense Patient data Explorative Network (OPEN).42,43
Statistical Analyses
We computed PPVs of registry diagnoses of ICH for each of the sources (DNPR vs DSR), and for each of the three groups (ie, DNPR & DSR, DNPR only, or DSR only), as proportions of each parameter confirmed by the verification process described above. We computed the sensitivity of the registers when used as independent sources (ie, either DNPR or DSR) as the proportion of number of cases verified by each source divided by the total number of cases verified in either of the registers. We used the Wilson score method to estimate 95% confidence intervals for all proportions.44 We also calculated a chi-square statistic for the trend45 (regression) of sensitivity estimates across the three time periods (2009–2011; 2012–2014; 2015–2017) and age-groups (<65; 65–74; ≥75 years).
All analyses were performed using Stata 16.1 (Stata Corp, TX).
The study was approved by the Danish Data Protection Agency (Approval ID 18/51966) and the Danish Health and Medicines Authority (Approval ID 3–3013-942/1).
Results
A total of 2,590 and 4,176 discharges were recorded under ICH diagnosis codes in DSR and DNPR, respectively. Cross-linkage of the data from the two registries resulted in 4,149 patients, after exclusion of patients younger than 20 years (n=52), and patients who only had been registered with ICH diagnoses at hospitals outside the region (n=78). Exclusion of a further 193 patients, where we could not obtain the medical records, resulted in a total study population of 3,956 patients (Figure 1). In all, 59.6% were recorded in both registries, 2.2% in DSR only, and 38.3% in DNPR only. The percentage of untraced medical record information in the three categories was 4.5% for DSR & DNPR, 7.5% (n=7) for DSR only, and 4.7% for DNPR only.
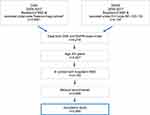 |
Figure 1 Identification of patient records for validation from the DSR and DNPR. |
For patients included in the study, we retrieved both discharge letters and brain scan reports in 93%, discharge letters only in 1%, and brain scan reports only in 6% of cases. In a few cases (n=94), the discharge letter and/or brain scan report were deemed insufficient to reach a diagnosis, in which case we retrieved full medical records or re-evaluated original brain scans.
The diagnosis of a-ICH could not be verified in a total of 1,192 cases (30.1%). Close to half of the patients in this group (n=528) had suffered an ICH that did not fulfill study criteria (eg, ICH due to trauma), and another third of patients had intracranial hemorrhages other than ICH (n=396) (Table 1). The corresponding percentages by registry source (ie, DSR/DNPR inpatient, primary coding position) were a) ICH that did not fulfill study criteria (56.7%/45.8%); and b) intracranial hemorrhage other than ICH (35.4%/23.3%).
 |
Table 1 Underlying Diagnoses in Non-Verified Cases and in Verified Cases Not Classified as Spontaneous ICH |
Among patients with verified a-ICH (n=2,764), an underlying cause was found in 225 patients (most frequently (92%) hemorrhages in brain tumors, or from arteriovenous malformations or cavernous hemangiomas (Table 1)), leaving a total of 2,539 patients with s-ICH.
We compared the earliest date of contact with a code of ICH according to registry data (registry date) with the date of onset of ICH as assessed by the study physician who evaluated the retrieved medical record information. For cases with verified a-ICH, the onset date was within 7 days of the registry date in 93% of cases (the two dates were identical in 82% of cases), while it differed by more than a week in 2.6% of cases only; information on date of onset could not be accurately established based on medical record information in 4.3% of cases, mostly due to vague wording (eg, “symptoms for a few days with subacute ICH changes on brain scan” with no mention of the exact date of onset).
For the majority of patients with a-ICH, the initial scan was a computed tomography (CT) of the brain (CTC) (94%), while for 4% of patients the initial scan was CT-angiography (CTA), or brain magnetic resonance imaging (MRI) (1.2%). In 0.8% of a-ICH patients the type of brain scan was unknown as the brain scan report was not traced and the discharge letter, although referring to the brain imaging result, did not specify the type of scan performed. Initial brain scan reports of patients with a-ICH frequently described intraventricular spread of the hematoma (37%), a known poor prognostic sign, and signs indicative of increased intracranial pressure (deviation of midline structures 32%, presence of hydrocephalus 10%, herniation 3.2%).
Among patients with s-ICH, further imaging work-up was performed in 49.5% of cases (n=1,256); in 24% of cases in the form of brain MRI, CTA, or both. The percentage of patients with s-ICH that had been further investigated with imaging was higher in younger patients (age ≤55 years: 64%; age 56+years: 48%).
Our definition of ICH differed slightly from the WHO definition, in that we also included patients with non-focal symptoms or symptoms lasting less than 24 hours if brain imaging revealed acute parenchymal hemorrhage. Cases verified according to our criteria would also have been classified as such according to WHO criteria in 96.7% of cases for a-ICH and 96.4% of cases for s-ICH.
Positive Predictive Values of ICH Diagnosis Code
In DSR, the PPV for a-ICH diagnosis was 86.5% (95% CI=85.1–87.8) and for s-ICH 81.8% (95% CI=80.2–83.3). The corresponding values for DNPR were higher for inpatient codes with ICH in the primary diagnostic position, compared with inpatient codes with ICH in the coding position other than primary for both a-ICH (76.2%; 95% CI=74.7–77.6 vs 49.5%; 95% CI=45.6–53.4) and s-ICH (70.2%; 95% CI=68.6–71.8 vs 43.7%; 95% CI=39.9–47.6) (Table 2). In analyses where we combined data from the two sources, the highest PPV was observed among patients concurrently recorded in DSR and DNPR–inpatient primary diagnostic position (a-ICH: 88.6%; 95% CI=87.1–89.9 and s-ICH: 83.7%; 95% CI=82.1–85.2) and the lowest PPV among patients only recorded in DNPR with ICH codes for outpatient or ED contacts (7.4%; 95% CI=3.2–16.1 for both a-ICH and s-ICH) (Table 3). Year of admission, age, and sex had little impact on PPV of a-ICH and s-ICH in DSR or DNPR–inpatient primary diagnostic position (Table 4).
 |
Table 2 Positive Predictive Value of Admission Codes for ICH in Danish National Patient Registry (DNPR) and Danish Stroke Registry (DSR) |
 |
Table 3 Number of Patients Identified and Number of Verified Diagnoses of ICH by Type of Contact in Danish National Patient Registry (DNPR) and by Concurrent Recording in Danish Stroke Registry (DSR) |
 |
Table 4 PPV and Sensitivity of Inpatient First-Ever Codes for ICH in the Danish Stroke Registry and the Danish National Patient Registry Stratified by Year, Age, and Sex |
Sensitivity of ICH Diagnosis Code
The sensitivity in DSR for a-ICH was 76.4% (95% CI=74.8–78.0) and for s-ICH 78.7% (95% CI=77.1–80.2); for DNPR–inpatient primary diagnostic position the sensitivity for a-ICH was 87.3% (95% CI=86.0–88.5) and for s-ICH 87.7% (95% CI=86.3–88.9). The sensitivity in DSR declined over time for both a-ICH and s-ICH (eg, s-ICH: 2009–2011: 85.6%, 95% CI=83.0–87.9 vs 2012–2014: 78.2%; 95% CI=75.2–80.9% vs 2015–2017: 73.3%; 95% CI=70.4–76.0). Sensitivity in DSR was higher in older patients for both a-ICH and s-ICH (eg, s-ICH: <64 years: 74.5%; 95% CI=71.1–77.7 vs 65–74 years: 79.2%; 95% CI=76.0–82.1 vs 75+ years 80.6%; 95% CI=78.3–82.7) (Table 4). When stratified across both age (<75 years vs ≥75 years) and sex strata, sensitivity remained high for DNPR; for DSR, sensitivity estimates were highest for men aged 75+ years (91% in 2009–2011; 80% in 2012–2014; 82% in 2015–2017) and lowest for women younger than 75 years (82% in 2009–2011; 72% in 2012–2014; 64% in 2015–2017), the overall pattern being compatible with a decline in sensitivity over time across all strata (Table 5).
 |
Table 5 Sensitivity of Inpatient First-Ever Codes for ICH in the Danish Stroke Registry and the Danish National Patient Registry Stratified by Year, Age, and Sex |
Patients with Untraceable Medical Records
We excluded 193 patients with untraceable medical records (untraced cases) from the above analyses. The proportion of untraced cases declined during the study period (2009–2011: 116 [60%]; 2012–2014: 57 [30%]; 2015–2017: 20 [10%]) (Table 6). The proportion of untraced cases among inpatients in DSR and DNPR also varied by time period, while similar in analyses by age and sex (Table 7). The majority of untraced cases before 2015 had received ICH diagnosis codes as inpatients and were recorded in both DSR and DNPR, while in the last 3-year part of the study period, the small number of untraced cases primarily comprised hospital contacts other than inpatients (Table 7). The higher number of untraced cases before 2015 was primarily due to paper-based medical records (ie, predating the EMR system) no longer being available in the archives of some hospitals in the catchment area.
 |
Table 6 Distribution of Patients with Untraceable Medical Records by Year and Contact Recorded in Danish National Patient Registry (DNPR) and/Or Danish Stroke Registry (DSR) |
 |
Table 7 Distribution of Patients with Untraceable Medical Records by Source, Year, Age, and Sex |
Location of s-ICH
Among the 2,539 cases of s-ICH, 2,430 had a single ICH and 109 multiple concurrent ICH (Table 8). The location of the hemorrhage in s-ICH (single or multiple) was lobar (39.0%), deep (33.6%), infratentorial (13.2%), large unclassifiable (11.0%), or isolated intraventricular hemorrhage (1.9%); we could not classify the location in 1.3% of s-ICH due to insufficient information (1.2% of single ICH and 4.6% of multiple ICH cases).
 |
Table 8 Location of Spontaneous Intracerebral Hemorrhage |
Discussion
In this large study with data from an entire Danish region for the years 2009–2017, we found a high validity of a-ICH diagnosis in DSR and DNPR–inpatient primary coding position using each of these registries as a single data source. For s-ICH, validity was also high in DSR, but was lower in DNPR. PPVs of patients recorded in both registries were higher, compared with PPVs based on patients only recorded in one of the registries. The sensitivity of DNPR was higher than that of DSR, with the latter exhibiting some degree of variation by age of patients (ie, higher sensitivity in older patients) and time-period (ie, lower sensitivity in later compared with earlier part of study period).
Four studies have reported data on the validity of ICH diagnoses in DNPR (Supplementary Table S1).32–34,36 Studies predating year 2000 reported PPVs of 66%33 and 74%,34 respectively, while a study36 concerning data from 1993 to 2009 reported a PPV of 76%. Finally, a study32 by our group with data from the same region as the present study for the years 2010–2015 reported a PPV of 75% for s-ICH in DNPR. The reported PPVs from previous studies by other groups presumably also represent s-ICH, as stroke was defined according to WHO criteria in these studies (although only one study36 explicitly stated that traumatic ICH was excluded). We conclude that the PPV of 70% in this study, although somewhat lower, is in line with previous reports on the validity of s-ICH in DNPR. We report a PPV of 81% for s-ICH in DSR, which is similar to the result of our previous study (PPV 85%) in the same catchment area. We previously reported higher PPVs for a-ICH in both DNPR (88%) and DSR (94%), compared with the results of the present study (DNPR: 76%; DSR: 87%). In our previous study, we only studied a sample of patients with ICH diagnoses and only validated patients with available EMR records. This may have resulted in overrepresentation of patients treated at the university hospital in the catchment area, as this hospital had the largest patient volume and, in addition, was the first hospital in RSD to switch to the EMR system. We also note that our estimates of a-ICH in the present study would have been even higher, had we included certain cases of ICH that were excluded by design (eg, ICH due to trauma, hemorrhagic transformation, iatrogenic ICH).
We report novel data on the sensitivity of ICH diagnoses in DSR and DNPR. As DSR is primarily based on reporting from stroke units in Denmark, patients only admitted to non-stroke units could potentially be missed. Our results regarding sensitivity of DSR for s-ICH (79%) were therefore reassuring, although we did observe some variation in sensitivity over time and across age-groups. While the cause of this variation is unknown to us, these findings emphasize the need for periodic validation of the data. DNPR inpatient data with primary diagnostic position codes are frequently used in Danish studies of cerebrovascular disorders, as this approach is believed to result in outcomes with higher validity than data with broader criteria (eg, inclusion of inpatients regardless of diagnostic position of code).4,6,46–50 Our findings support this strategy with regard to s-ICH. We also note that the high sensitivity of s-ICH based on inpatient primary diagnostic position codes was stable in the 9-year study period and across age-groups.
The distribution of location of hemorrhage in patients with s-ICH was as expected from the literature.37,51,52 However, we did not provide results regarding mixed-location ICH (ie, lobar and deep), as we excluded patients with a history of ICH; also, among the included patients we only had information on concurrent multiple ICHs at our disposal. When assessing for mixed-location ICH, a more appropriate method would have been to evaluate patients with sufficiently sensitive brain imaging studies (eg, MRI with SWI or T2* sequences) for signs of previous hemorrhage.53 However, in our study, such an approach would probably provide a low yield, as MRI with SWI/T2*, although available upon request at all hospitals throughout the study period, only became part of the routine ICH work-up late in the study period (after 2015) and only in some of the hospitals in RSD (Nina Nguyen, consultant radiologist, personal communication).
Our study has some strengths. We used nationwide registries where simple and accurate cross-linkage was facilitated by the unique and permanent civil registration number of residents of Denmark. The majority of previous studies (Supplementary Table S1) were based on relatively small samples of patients with ICH occurring among participants in various cohort studies conducted in urban areas of Denmark.33,34,36 In two of these studies,33,36 patients diagnosed with stroke were on average younger than seen in recent population-based studies of stroke. In this study, we included all patients with ICH diagnosis codes seen at any hospital department according to two sources (DSR and DNPR) and regardless of type of hospital contact (inpatient, outpatient, or ED), or diagnostic code position in DNPR. By securing data for these potential cases of ICH in an entire region for a 9-year period we ensured a large sample diagnosed through standards corresponding to current clinical practice and in all probability representative of the whole spectrum of this frequently devastating disorder. Therefore, the results of this study are likely better with regard to generalizability as compared with previous studies. Studies based on Danish registries are frequently published in international peer-reviewed journals and therefore presumably have a large impact on research all over the world. The information provided by this manuscript can contribute to the correct interpretation of such register-based studies concerning ICH.
Our study has a number of limitations. First, we used discharge letters and brain scan reports to verify the diagnoses, a previously validated method.32 It could be argued that a more optimal approach would have been to evaluate the entire medical record of each patient and re-evaluate the original brain imaging studies. We evaluated this more extensive and logistically challenging approach in a small sample of patients (n=100) in a previous study, and found it produced highly similar results to those achieved with the more limited data approach used in the present study, both with regard to a-ICH/s-ICH status and ICH location.32 Second, although we used multiple sources to identify all adult patients with ICH, it is likely that some patients were not captured by our hospital-based method. We did not identify patients with ICH that died before reaching hospital. As the rate of autopsy in Denmark is quite low,54 supplementing our data with information from the Cause-of-Death Registry would most likely not improve our estimates to any measurable degree with regard to this source of bias. Our data sources did not include information on patients suspected of suffering an ICH that were not referred to hospital for evaluation. However, in Denmark, patients suspected of stroke, regardless of age, are considered medical emergencies and are therefore almost invariably promptly referred/transferred to hospital for evaluation. Based on our clinical experience, we therefore believe that the magnitude of this selection bias is relatively small. Third, some patients with ICH may have been incorrectly coded under non-ICH diagnoses. Previous Danish studies reported a very low degree of misclassification of ICH coded as other types of stroke or intracranial hemorrhage.6,12,32,55 Our calculation of sensitivity is based on the assumption that patients with true ICH would have an ICH code in at least one of the studied data sources. We do not know how often physicians diagnosing ICH accidentally use non-cerebrovascular disorder codes but judging by data on the reverse situation in our study (ie, patients with other diagnoses coded as ICH), this scenario is rare. Fourth, our more inclusive approach with regard to diagnostic criteria is debatable. We used the WHO criteria for stroke, but in addition accepted inclusion of patients based on symptoms (eg, severe sudden onset headache), where imaging supported new onset ICH. Our more inclusive approach could potentially hamper comparison with previous studies based exclusively on WHO criteria. However, as more than 96% of cases included in this study also fulfilled the WHO criteria, we believe that use of our criteria, while more accurately reflecting current clinical practice, had little impact on the comparability of our results with those of previous studies. Our criteria also diverged from the original, strictly clinical WHO criteria in that we only included patients with available neuroimaging results. However, routine use of neuroimaging in cases suspected of stroke is widely available at all hospitals in Denmark. Also, neuroimaging results made it possible to classify the location of ICH, a practice encouraged in updated criteria for population-based studies of ICH.56 Fifth, the generalizability of our study results at a national level can be questioned, as we only included data from one of the five regions in Denmark. However, patients with stroke are treated in accordance to the national guidelines in all of Denmark57 and RSD is representative of the Danish population with regard to demographic characteristics, healthcare utilization, and medication use.58 Finally, we excluded patients with untraced medical records, which may have influenced our estimates of validity of ICH coding. However, as patients from this group comprised less than 5% of the entire sample, and additional analyses made it most likely that PPV was high among untraced cases, we find it unlikely that this factor had other than a minor impact on our results.
Conclusion
In our study, based on all identifiable consecutive cases of first-time ever ICH in a defined Danish population – irrespective of clinical severity and mode of hospital contact – we found that the validity of a-ICH diagnoses was high in both DSR and DNPR. With regard to s-ICH, data in DSR were more valid, but less complete than DNPR data. Our results can provide guidance for the future use of these data sources for research (eg, cohort and case-control studies, time-series, before-and-after studies) of ICH and clinical practice (eg, audit purposes, evaluation of services).
Acknowledgments
The authors wish to thank Jan Helldén, Department of Business Intelligence, RSD for contributing to the collection of the data. The authors also wish to thank the staff in the Danish Clinical Quality Program – National Clinical Registries (RKKP) and the Danish Stroke Registry for their work in data collection and delivery.
Author Contributions
SMH and DG conceived the study, performed the analyses, and wrote the first draft. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This work is part of a PhD fellowship (SMH) supported by the University of Southern Denmark and the Region of Southern Denmark. The project also received funding from Odense University Hospital (A2926; 70-A3187; 49-A2483) and the A. P. Møller and Chastine Mc-Kinney Møller Foundation (18-L-0214).
Disclosure
The activities of DG are supported by a grant from Odense University Hospital.
Luis Alberto García Rodríguez reports grants from Bayer AG, personal fees from Bayer AG, outside the submitted work.
Rustam Al-Shahi Salman reports grants from British Heart Foundation, grants from The Stroke Association, outside the submitted work.
The authors report no other potential conflicts of interest in this work.
References
1. Fischer T, Johnsen SP, Pedersen L, Gaist D, Sørensen HT, Rothman KJ Seasonal variation in hospitalization and case fatality of subarachnoid hemorrhage - a nationwide danish study on 9367 patients. Neuroepidemiology. 2005;24(1–2):32–37. doi:10.1159/000081047
2. Biotti D, Jacquin A, Boutarbouch M, et al. Trends in case-fatality rates in hospitalized nontraumatic subarachnoid hemorrhage: results of a population-based study in Dijon, France, from 1985 to 2006. Neurosurgery. 2010;66(6):1039–1043. doi:10.1227/01.NEU.0000369512.58898.99
3. Schmidt M, Jacobsen JB, Johnsen SP, Bøtker HE, Sørensen HT Eighteen-year trends in stroke mortality and the prognostic influence of comorbidity. Neurology. 2014;82(4):340–350. doi:10.1212/WNL.0000000000000062
4. Gaist D, García Rodríguez L, Hellfritzsch M, et al. Association of antithrombotic drug use with subdural hematoma risk. JAMA. 2017;317:836–846. doi:10.1001/jama.2017.0639
5. Aked J, Delavaran H, Norrving B, Lindgren A Temporal trends of stroke epidemiology in southern Sweden: a population-based study on stroke incidence and early case-fatality. Neuroepidemiology. 2018;50(3–4):174–182. doi:10.1159/000487948
6. Gaist D, Vaeth M, Tsiropoulos I, et al. Risk of subarachnoid haemorrhage in first degree relatives of patients with subarachnoid haemorrhage: follow up study based on national registries in Denmark. BMJ. 2000;320(7228):141–145.
7. Bak S, Gaist D, Sindrup SH, Skytthe A, Christensen K Genetic liability in stroke: a long-term follow-up study of Danish twins. Stroke. 2002;33(3):769–774. doi:10.1161/hs0302.103619
8. Bak S, Andersen M, Tsiropoulos I, et al. Risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nested case-control study. Stroke. 2003;34(2):379–386.
9. Mackey J, Kleindorfer D, Sucharew H, et al. Population-based study of wake-up strokes. Neurology. 2011;76(19):1662–1667. doi:10.1212/WNL.0b013e318219fb30
10. García-Rodríguez LA, Gaist D, Morton J, Cookson C, González-Pérez A. Antithrombotic drugs and risk of hemorrhagic stroke in the general population. Neurology. 2013;81(6):566–574. doi:10.1212/WNL.0b013e31829e6ffa
11. González-Pérez A, Gaist D, Wallander M-A, McFeat G, García-Rodríguez LA. Mortality after hemorrhagic stroke: data from general practice (The Health Improvement Network). Neurology. 2013;81(6):559–565. doi:10.1212/WNL.0b013e31829e6eff
12. Østergaard K, Pottegård A, Hallas J, Bak S, dePont Christensen R, Gaist D. Discontinuation of antiplatelet treatment and risk of recurrent stroke and all-cause death: a cohort study. Neuroepidemiology. 2014;43(1):57–64. doi:10.1159/000365732
13. Schmidt M, Hováth-Puhó E, Christiansen CF, Petersen KL, Bøtker HE, Sørensen HT. Preadmission use of nonaspirin nonsteroidal anti-inflammatory drugs and 30-day stroke mortality. Neurology. 2014;83(22):2013–2022. doi:10.1212/WNL.0000000000001024
14. Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GYH. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
15. Sundbøll J, Horváth-Puhó E, Schmidt M, et al. Preadmission use of glucocorticoids and 30-day mortality after stroke. Stroke. 2016;47(3):829–835. doi:10.1161/STROKEAHA.115.012231
16. Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GYH, Larsen TB. Effectiveness and safety of reduced dose non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2017;356:j510.
17. Mortensen JK, Johnsen SP, Andersen G. Prescription and predictors of post-stroke antidepressant treatment: a population-based study. Acta Neurol Scand. 2018;138(3):235–244. doi:10.1111/ane.12947
18. Seminog OO, Scarborough P, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute stroke in England: linked national database study of 795 869 adults. BMJ. 2019;365:l1778. doi:10.1136/bmj.l1778
19. Ekker MS, Verhoeven JI, Vaartjes I, Jolink WMT, Klijn CJM, de Leeuw F-E. Association of stroke among adults aged 18 to 49 years with long-term mortality. JAMA. 2019;321(21):2113–2123. doi:10.1001/jama.2019.6560
20. Weber R, Krogias C, Eyding J, et al. Age and sex differences in ischemic stroke treatment in a nationwide analysis of 1.11 million hospitalized cases. Stroke. 2019;50(12):3494–3502. doi:10.1161/STROKEAHA.119.026723
21. Béjot Y, Cordonnier C, Durier J, Aboa-Eboulé C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain. 2013;136(Pt 2):658–664. doi:10.1093/brain/aws349
22. Gaist D, Wallander M-A, González-Pérez A, García-Rodríguez LA. Incidence of hemorrhagic stroke in the general population: validation of data from The Health Improvement Network. Pharmacoepidemiol Drug Saf. 2013;22(2):176–182. doi:10.1002/pds.3391
23. Gaist D, González-Pérez A, Ashina M, Rodríguez LAG. Migraine and risk of hemorrhagic stroke: a study based on data from general practice. J Headache Pain. 2014;15:74. doi:10.1186/1129-2377-15-74
24. Carlsson M, Wilsgaard T, Johnsen SH, et al. Temporal trends in incidence and case fatality of intracerebral hemorrhage: The Tromsø Study 1995–2012. Cerebrovasc Dis Extra. 2016;6(2):40–49. doi:10.1159/000447719
25. Gulati S, Solheim O, Carlsen SM, et al. Risk of intracranial hemorrhage (RICH) in users of oral antithrombotic drugs: nationwide pharmacoepidemiological study. PLoS One. 2018;13(8):e0202575. doi:10.1371/journal.pone.0202575
26. Øie LR, Madsbu MA, Solheim O, et al. Functional outcome and survival following spontaneous intracerebral hemorrhage: a retrospective population-based study. Brain Behav. 2018;8(10):e01113. doi:10.1002/brb3.1113
27. Carlsson M, Wilsgaard T, Johnsen SH, et al. The impact of risk factor trends on intracerebral hemorrhage incidence over the last two decades-The Tromsø Study. Int J Stroke. 2019;14(1):61–68. doi:10.1177/1747493018789996
28. Overvad TF, Andersen SD, Larsen TB, et al. Incidence and prognostic factors for recurrence of intracerebral hemorrhage in patients with and without atrial fibrillation: a cohort study. Thromb Res. 2020;191:1–8. doi:10.1016/j.thromres.2020.03.024
29. Ribe AR, Vestergaard CH, Vestergaard M, et al. Statins and risk of intracerebral hemorrhage in individuals with a history of stroke. Stroke. 2020;51(4):1111–1119. doi:10.1161/STROKEAHA.119.027301
30. Sacco S, Pistoia F, Carolei A. Stroke tracked by administrative coding data: is it fair? Stroke. 2013;44(7):1766–1768. doi:10.1161/STROKEAHA.113.001742
31. Li L, Binney LE, Luengo-Fernandez R, Silver LE, Rothwell PM. Oxford vascular study. temporal trends in the accuracy of hospital diagnostic coding for identifying acute stroke: a population-based study. Eur Stroke J. 2020;5(1):26–35. doi:10.1177/2396987319881017
32. Hald SM, Kring Sloth C, Hey SM, et al. Intracerebral hemorrhage: positive predictive value of diagnosis codes in two nationwide Danish registries. Clin Epidemiol. 2018;10:941–948. doi:10.2147/CLEP.S167576
33. Johnsen SP, Overvad K, Sørensen HT, Tjønneland A, Husted SE. Predictive value of stroke and transient ischemic attack discharge diagnoses in the Danish national registry of patients. J Clin Epidemiol. 2002;55(6):602–607. doi:10.1016/S0895-4356(02)00391-8
34. Krarup L-H, Boysen G, Janjua H, Prescott E, Truelsen T. Validity of stroke diagnoses in a national register of patients. Neuroepidemiology. 2007;28(3):150–154. doi:10.1159/000102143
35. Wildenschild C, Mehnert F, Thomsen RW, et al. Registration of acute stroke: validity in the Danish stroke registry and the danish national registry of patients. Clin Epidemiol. 2014;6:27–36. doi:10.2147/CLEP.S50449
36. Lühdorf P, Overvad K, Schmidt EB, Johnsen SP, Bach FW. Predictive value of stroke discharge diagnoses in the Danish national patient register. Scand J Public Health. 2017;45(6):630–636. doi:10.1177/1403494817716582
37. Samarasekera N, Fonville A, Lerpiniere C, et al. Influence of intracerebral hemorrhage location on incidence, characteristics, and outcome: population-based study. Stroke. 2015;46(2):361–368. doi:10.1161/STROKEAHA.114.007953
38. Marshall, J. Cerebrovascular diseases: prevention, treatment, and rehabilitation. Report of a WHO meeting. World Health Organ Tech Rep Ser. 1971;469:1–57.
39. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
40. Mainz J, Krog BR, Bjørnshave B, Bartels P. Nationwide continuous quality improvement using clinical indicators: the Danish national indicator project. Int J Qual Health Care. 2004;16 Suppl 1:i45–50. doi:10.1093/intqhc/mzh031
41. Olsen TS, Dehlendorff C, Andersen KK. Sex-related time-dependent variations in post-stroke survival–evidence of a female stroke survival advantage. Neuroepidemiology. 2007;29(3–4):218–225. doi:10.1159/000112464
42. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
43. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi:10.1016/j.jbi.2019.103208
44. Wilson E. Probable inference, the law of succession, and statistical inference. J Am Stat Assoc. 1927;22:209–212. doi:10.1080/01621459.1927.10502953
45. Royston P. PTREND: stata module for trend analysis for proportions; 2014. Available from: https://ideas.repec.org/c/boc/bocode/s426101.html.
46. Corraini P, Henderson VW, Ording AG, Pedersen L, Horváth-Puhó E, Sørensen HT. Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke. 2017;48(1):180–186. doi:10.1161/STROKEAHA.116.015242
47. Pottegård A, García Rodríguez LA, Poulsen FR, Hallas J, Gaist D. Antithrombotic drugs and subarachnoid haemorrhage risk. A nationwide case-control study in Denmark. Thromb Haemost. 2015;114(5):1064–1075. doi:10.1160/TH15-04-0316
48. Østergaard L, Andersson NW, Kristensen SL, et al. Risk of stroke subsequent to infective endocarditis: a nationwide study. Am Heart J. 2019;212:144–151. doi:10.1016/j.ahj.2019.03.010
49. Pedersen MGB, Olsen MS, Schmidt M, et al. Ischemic stroke in adults with congenital heart disease: a population-based cohort study. J Am Heart Assoc. 2019;8(15):e011870. doi:10.1161/JAHA.118.011870
50. Gaist D, García Rodríguez LA, Hald SM, et al. Antidepressant drug use and subdural hematoma risk. J Thromb Haemost. 2020;18(2):318–327. doi:10.1111/jth.14658
51. Cordonnier C, Rutgers MP, Dumont F, et al. Intra-cerebral haemorrhages: are there any differences in baseline characteristics and intra-hospital mortality between hospital and population-based registries? J Neurol. 2009;256(2):198–202. doi:10.1007/s00415-009-0030-3
52. Lavados PM, Sacks C, Prina L, et al. Incidence of lobar and non-lobar spontaneous intracerebral haemorrhage in a predominantly Hispanic-Mestizo population–the PISCIS stroke project: a community-based prospective study in Iquique, Chile. Neuroepidemiology. 2010;34(4):214–221. doi:10.1159/000289353
53. Pasi M, Charidimou A, Boulouis G, et al. Mixed-location cerebral hemorrhage/microbleeds: underlying microangiopathy and recurrence risk. Neurology. 2018;90(2):e119–e126. doi:10.1212/WNL.0000000000004797
54. Petri CN. Decrease in the frequency of autopsies in Denmark after the introduction of a new autopsy act. Qual Assur Health Care. 1993;5(4):315–318. doi:10.1093/intqhc/5.4.315
55. Poulsen FR, Halle B, Pottegård A, García Rodríguez LA, Hallas J, Gaist D. Subdural hematoma cases identified through a Danish patient register: diagnosis validity, clinical characteristics, and preadmission antithrombotic drug use. Pharmacoepidemiol Drug Saf. 2016;25(11):1253–1262. doi:10.1002/pds.4058
56. Feigin V, Norrving B, Sudlow CLM, Sacco RL. Updated criteria for population-based stroke and transient ischemic attack incidence studies for the 21st century. Stroke. 2018;49(9):2248–2255. doi:10.1161/STROKEAHA.118.022161
57. Ref.program. Referenceprogram for behandling af patienter med apopleksi og TCI; 2013. Available from: http://www.dsfa.dk/wpcontent/uploads/REFERENCEPROGRAMFINAL20131.pdf.
58. Henriksen DP, Rasmussen L, Hansen MR, Hallas J, Pottegård A. Comparison of the five danish regions regarding demographic characteristics, healthcare utilization, and medication use–a descriptive cross-sectional study. PLoS One. 2015;10(10):e0140197. doi:10.1371/journal.pone.0140197
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
