Back to Journals » Drug Design, Development and Therapy » Volume 16
The Therapeutic Effect and the Possible Mechanism of C-Phycocyanin in Lipopolysaccharide and Seawater-Induced Acute Lung Injury
Authors Zhang L, Kong D, Huang J, Wang Q, Shao L
Received 17 November 2021
Accepted for publication 26 March 2022
Published 6 April 2022 Volume 2022:16 Pages 1025—1040
DOI https://doi.org/10.2147/DDDT.S347772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Leifang Zhang,1 Deyi Kong,1 Junxia Huang,1 Qiongfen Wang,2 Lilin Shao1
1Zhejiang Provincial Engineering Technology Research Center of Marine Biomedical Products, School of Food and Pharmacy, Zhejiang Ocean University, Zhoushan, 316022, People’s Republic of China; 2Zhoushan Institute of Calibration and Testing for Quality and Technical Supervision, Zhoushan, 316012, Zhejiang, People’s Republic of China
Correspondence: Leifang Zhang, Zhejiang Provincial Engineering Technology Research Center of Marine Biomedical Products, School of Food and Pharmacy, Zhejiang Ocean University, 1 Haida South Road, Changzhi Island, Zhoushan, 316022, Zhejiang, People’s Republic of China, Tel +86 18 8570 63534, Fax +86 58 0 254 781, Email [email protected]
Background: Seawater drowning-induced acute lung injury (ALI) is a severe clinical condition characterized by increased alveolar-capillary permeability, excessive inflammatory response, and refractory hypoxemia. C-phycocyanin (C-PC), a biliprotein found in blue-green algae such as spirulina platensis, is widely used in the food and dietary nutritional supplement fields due to its beneficial pharmacological effects. Previous studies have revealed that C-PC has anti-inflammatory, antioxidant, and anti-apoptotic activities.
Purpose: Therefore, this study investigated the protective effect and underlying mechanisms of C-PC on lipopolysaccharide (LPS) and seawater (SW) induced ALI (SW and LPS-induced ALI).
Methods: An SW and LPS mouse model of ALI mice was established through intratracheal administration of 5mg/kg LPS and 25% SW. Different doses of C-PC (100, 200 and 400 mg/kg) were administered by intraperitoneal injection for seven days. In addition, gap junction communication in RAW264.7 and MLE-12 cells was determined following stimulation with 25% SW and 10 μg/ml LPS after treatment with C-PC (120 μg/ml). Moreover, the arterial partial pressure of oxygen, lung wet/dry weight ratios, total protein content and MPO levels in the bronchoalveolar lavage fluid (BALF), and the histopathologic and ultrastructure staining of the lung tissues were determined. The oxidative stress index, levels of the pro-inflammatory mediators, epithelial cell viability and apoptosis, and the regulatory effect of C-PC on the NF-κB/NLRP3 axis were investigated.
Results: The results showed that C-PC significantly alleviated pathological damages, suppressed oxidative stress, inflammation and apoptosis, and enhanced the viability of epithelial cells in the lung tissues. Furthermore, C-PC was shown to inhibit activation of the NF-κB/NLRP3 pathway and the formation of the NLRP3 inflammasome complex.
Conclusions: In conclusion, C-PC shows promising therapeutic value in SW and LPS-induced ALI/ARDS, providing new insight into ALI/ARDS treatment.
Keywords: C-phycocyanin, acute lung injury, inflammation, NF-κB/NLRP3, apoptosis
Introduction
Drowning is the second-leading cause of accidental death in the world. The World Health Organization (WHO) estimates that drowning leads to the loss of 372,000 people each year.1–4 Approximately 1/3 of near-drowning patients suffer from severe acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), which is a life-threatening disease with a mortality rate of between 25% and 40%.5–7 The number of people drowning in seawater has been increasing each year due to increased human marine activities. Studies have shown that drowning in seawater causes more significant lung damage than freshwater.8 Seawater has a high osmolality and complex composition. Therefore, aspiration of seawater leads to ALI/ARDS characterized by inflammation, edema of the pulmonary interstitium, and endothelial barrier damage induced by apoptosis of epithelial cells.5,9–13 Numerous inflammatory cytokines are released in the late stages of ALI/ARDS, leading to an imbalance of the inflammatory and anti-inflammatory mediators.10−13 Although the pathophysiological and molecular mechanisms are well known, there is no specific therapy for ALI/ARDS. Therefore, it is important to continue researching specific effective therapy for seawater drowning-induced ARDS.14
Nucleotide-binding domain, leucine-rich repeat (NLR) family, and pyrin domain-containing 3 (NLRP3) inflammasome are multiprotein complexes assembled in the cytoplasm following exposure to pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). The NLRP3 inflammasome is composed of the sensory protein NLRP3, the adaptor apoptosis-associated speck-like protein containing caspase-recruitment domain (ASC), and the effector protein caspase-1.15,16 Toll-like receptor 4 (TLR4) induces nuclear factor kappa B (NF-κB) pathway activation leading to increased expression of NLRP3, IL-1β, and IL-18. The NLRP3 directly interacts with the adaptor ASC to active caspase-1.17,18 The activated caspase-1 then splits gasdermin D (GSDMD), pro-IL-1β and pro-IL-18, to the biologically active secreted forms, IL-1β and IL-18, followed by cell pyroptosis.19,20 Pyroptosis is a type of programmed cell death mediated by gasdermin D (GSDMD). Following pyroptosis, numerous inflammatory mediators are released, inducing a strong inflammatory response.21 Recent studies have suggested that activation of NLRP3 is involved in the pathogenesis of ALI/ARDS.22–24 Grailer et al. revealed the critical role of NLRP3 inflammasome in the pathogenesis of LPS-induced lung injury. They demonstrated that full development of ALI requires the engagement of the NLRP3 inflammasome and caspase-1.23
Furthermore, the activation of NF-κB is distinctly involved in LPS induced ALI.25,26 The NF-κB signaling pathway is one of the most widely recognized intracellular signaling pathways in inflammatory responses. Nuclear factor-kappa B regulates various genes involved in immune and acute-phase inflammatory reactions in almost all cells.27 Several stimuli, such as ROS and pro-inflammatory mediators, can activate the NF-κB pathway leading to the release of NF-κB through the degradation and phosphorylation of inhibitory proteins by the IκB kinase complex (Iκκ).25 The released NF-κB then leads to the transcriptional activation of several pro-inflammatory mediators, including tumor necrosis factor (TNF)-α, IL-1β, and IL-6.22,28 NF-κB is considered a promising pharmacological target for inflammatory diseases including ALI, asthma, and sepsis.26,29 According to Wang et al., the NLRP3 small-molecule inhibitor MCC950 alleviates lung injury induced by NLRP3 and NF-κB activation.30
C-phycocyanin (C-PC) is a porphyrin protein extracted from cyanobacteria. In addition, it is a natural food pigment with high nutritional value.31–33 Several studies have shown that C-PC possesses various biological activities, including antioxidant,34 anti-inflammatory,35,36 anti-cancer,37,38 immunomodulatory hepatoprotective and antiplatelet activities.39–41 Several mechanisms for the anti-inflammatory activity of C-PC have been reported, including selective inhibition of COX-2,42 inhibition of NF-κB activation, and consequent inhibition of the release of inflammatory mediators,43 eliminating reactive oxygen species (ROS), and reducing oxidative stress.44 Alzokaky et al. revealed that pre-treatment with C-PC significantly reduced HMGB1 expression via suppression of NLRP3/NF-κB, oxidative markers, IL-1β, TNF-α, and ulcer index value.45 Thus, there is a need to determine the role of C-PC in the seawater and LPS-induced ALI model. This study investigates the underlying mechanisms of seawater and LPS-induced ALI. Further, the study aims to determine whether C-PC could ameliorate lung inflammation in seawater and LPS-induced ALI.
Materials and Methods
Chemical and Reagents
C-phycocyanin (30–50% protein content) and lipopolysaccharide (LPS, compound from Pseudomonas aeruginosa) were purchased from Sigma (San Diego, MO, USA). Cell culture reagents, including Dulbecco’s Modified Eagle Medium, fetal bovine serum (FBS), Penicillin-Streptomycin, and Trypsin-EDTA (0.25%), were purchased from Gibco Life Technologies (Waltham, MA, USA). Antibodies against F4/80, NLRP3, PYCARD (ASC), Caspase-1, cleaved-Caspase-1, iNOS, NF-κB p65, p-NF-κB p65, IκBα, and p-IκBα were obtained from Affinity (Affinity Biosciences LTD, USA). In contrast, α-tubulin, horseradish peroxidase (HRP)-labeled secondary antibodies, and CoraLite488 and 555-conjugated secondary antibodies were purchased from Proteintech Group (Chicago, USA).
The experimental seawater was prepared according to the formula provided by the Third Institute of Oceanography, Ministry of Natural Resources, Fujian, China. The main components and contents of the seawater were close to those of the southeast coastal seawater of China, pH 8.2, specific gravity 1.05, and osmotic pressure 1300 mmol/L. The SW components were as follows: NaCl 26.518 g/L, MgCl2 2.447 g/L, MgSO4 3.305 g/L, CaCl2 1.141 g/L, NaBr 0.083 g/L, NaHCO3 0.202 g/L, KCl 0.725 g/L.
Animal Studies
The C57BL/6 mice were used to develop the seawater aspiration lung injury model. A total of 36 male, 6–8 weeks old C57BL/6 mice, weighing between 18–22 g, were purchased from Zi yuan Laboratory Animal Technology Co., Ltd (Hangzhou, China). All animal experiments were approved by the ethics committee of Zhejiang Ocean University. All animal experiments complied with the National Institute of Health’s guidelines for the care and use of laboratory animals (Publication No.85–23, revised 1985). The animals were maintained under constant conditions (temperature 24 ± 2°C, humidity 75%, and 12 h light-12 h dark cycle) in an air-conditioned room. The animals were adaptively bred for seven days before the experiment.
After one week of acclimation, the mice were randomly assigned to six groups: control group, LPS + 25% SW group as the model group (intratracheally administration of 5mg/kg LPS and 25% SW), model + dexamethasone (Dex) group (intraperitoneal administration of 5mg/kg dexamethasone), model + C-PC low, medium, and high-dose groups (intraperitoneal administration of 100, 200 and 400 mg/kg C-PC).
25% seawater prepared from seawater and distilled water at a ratio of 1:3, and then mixed with solid LPS to 5mg/kg of final concentration. The mixture was pH adjusted and filter sterilized (0.22-μm-pore-size filters) after mixing and stored at 4°C until use (within 48 h). Mice were anaesthetized using 0.5% sodium pentobarbital by intraperitoneal injection. The mice were placed under surgical light in the supine and 30-degree head-up position, and tracheal intubation (outer diameter, 0.5 mm) was performed under a laryngoscopy (Small Animal Laryngoscope for mouse Shanghai Yuyan Scientific Instrument Co., Ltd.). The mixture liquid of 25% SW and LPS (5 mg/kg) was perfused slowly into both lungs through the trachea. Within 7 days, the low, medium and high-dose groups of C-PC were intraperitoneally injected with 100, 200 and 400 mg/kg C-PC per day at a fixed time, respectively, the Dex group was intraperitoneally injected with 5 mg/kg dexamethasone solution per day at a fixed time. The model group and the control group were treated with the same volume of 0.9% saline.
After 7 days of continuous administration, the whole blood of mice in each group was obtained by eyeball extraction and mice were euthanized by cervical dislocation, and the serum was separated by centrifugation. The thorax was opened rapidly, and lung tissue samples were collected. Lung tissue was removed from the thoracic cavity. In addition, right lungs were obtained to determine the wet-to-dry ratio, western blot HE staining, and immunofluorescence. Bronchoalveolar lavage fluid was obtained from the left lung for biochemical analysis.
Lung Wet-to-Dry Weight Ratio
The lung wet-to-dry ratio is used to characterize the degree of pulmonary edema. The weight of the harvested right lobes was recorded as the wet weight. The lobes were then dried in an oven at 50°C for 72 h and reweighed to determine the dry weight. The wet-to-dry ratio was then determined by dividing the wet weight by the dry weight.
Detection of the Total Cells and Proteins in the Bronchoalveolar Lavage Fluid (BALF)
Bronchoalveolar lavage was performed after the mice were sacrificed to detect lung infiltration by inflammatory cells. The chest cavity of the mice was opened, the right lung was ligatured, the lung was lavaged through the bronchus with 0.5 ml PBS, and then the liquid was pulled away slowly. After three times of bronchoalveolar lavage, the BALF was collected and centrifuged at 1000 r/min. The total number of cells in BALF was counted by a hemacytometer. The total protein concentration was determined using the Detergent Compatible Bradford Protein Assay Kit (Beyotime Biotechnology Co. Ltd., Shanghai, China).
Lung Histopathology
The superior lobe of the left-lung tissue was placed in 4% paraformaldehyde solution for fixation overnight. After fixation, different concentrations of ethanol were used for dehydration. The lobe was then paraffin-embedded and cut into 5μm sections that were stained with hematoxylin and eosin (HE). The slides were observed under a microscope to evaluate the degree of lung injury.
Cell Culture
The RAW264.7 and MLE-12 (Mouse lung epithelial) cell lines were purchased from Zhong Qiao Xin Zhou Biotechnology Co., Ltd (Shanghai, China). The RAW264.7 cells were cultured in DMEM (Dulbecco’s Modified Eagle Medium, High Glucose) with 10% FBS. In contrast, MLE-12 cells were cultured in DMEM/F-12 containing 10% FBS. Cells were maintained at 37°C, and 5% CO2 humidified incubator. The cell lines were then treated with C-PC (120μg/ml) after 24 h of treatment with LPS (10μg/ml) and 25% SW. The cells and the supernatant were then collected and stored at −80°C for further analysis.
CCK-8 Assay
The MLE-12 cells were plated onto 96 well plates at a density of 3000 to 5000 cells per well and treated with LPS + 25% SW and C-PC at a concentration of 40, 80, and 120 μg/ml for 24 h. Thereafter, 10 μl CCK-8 reagent was added to each well. After 4 h of incubation at 37°C, optical density was read at a wavelength of 450 nm using the multi-function microplate reader (Molecular Device, Sunnyvale, USA).
Immunofluorescence Analysis
Immunofluorescence analysis was carried out to study the formation of inflammasome complexes. The right-lung lobe was fixed in 4% paraformaldehyde solution and permeabilized with 0.5% Triton X. The tissues were then embedded in paraffin and cut into 5μm sections, which were then incubated in 0.5% BSA for 1 h to block non-specific protein sites. The lung tissues were probed with F4/80, NLRP3, PYCARD (ASC), Caspase-1, and Cleaved-Caspase-1 antibodies at 4°C overnight. The slides were then washed with PBS three times and incubated in Coralite488 or Coralite594 conjugated secondary antibodies (Proteintech, USA) for 2 h at room temperature. The slides were observed under a laser scanning confocal microscope (Olympus, Japan). Image J (Rockville, USA) software was used to analyze the co-localization of the merged images.
Determination of Inflammatory Cytokine Levels
Levels of IL-1β, TNF-α, IL-6, and MCP-1 in the mouse serum were determined using an ELISA kit purchased from Neobioscience Co., Ltd (Shanghai, China). Absorbance was read at 450 nm using a multi-function microplate reader (Molecular Devices, Sunnyvale, USA). The method was carried out per the manufacturer’s instructions.
Western Blot Analysis
The lung tissues were ground under liquid nitrogen, transferred to RIPA buffer (Beyotime Biotechnology Co. Ltd., Shanghai, China) for lysis, and centrifuged to collect the supernatant. The total protein concentration was determined using the Detergent Compatible Bradford Protein Assay Kit (Beyotime Biotechnology Co. Ltd., Shanghai, China). The protein concentration of the samples was adjusted for consistency. A loading buffer was then added and boiling was done to denature the protein. Samples containing 20 μg protein were separated using 10% or 12% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked with tris-buffered saline (TBS) containing 5% non-fat milk for 1 h. The PVDF membranes were then incubated with primary antibodies against NLRP3, ASC, Caspase-1, cleaved-Caspase-1, BAX, Bcl-2, Caspase-3, or α-tubulin (Affinity Biosciences LTD, USA) overnight at 4°C. After washing three times with a TBS Buffer containing 0.1% Twain 20, the membranes were incubated with a secondary antibody (HRP-conjugated) for 1 h at room temperature. The signals of ECL chemiluminescence intensity were captured using the FluorChem FC3 system (Protein Simple, USA). The optical density of the protein bands was analyzed using ImageJ software (NIH, Bethesda, USA).
Quantitative Real-Time PCR (qRT-PCR)
Total RNA from RAW264.7 cells was extracted and purified using the GeneJET RNA Purification Kit (Thermo Scientific, USA) to quantify IL-1β, TNF-α, IL-6, MCP-1, and MIF mRNA levels. All steps were carried out per the manufacturer’s instructions. The integrity and quality of the extracted RNA were determined using Qubit™ RNA IQ Assay Kits (Thermo Scientific, USA). Reverse transcription was then performed using HiScript® III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing). Real-time PCR reactions were performed using the BeyoFast™ SYBR Green qPCR Mix (2X) (Beyotime Biotechnology Co. Ltd., Shanghai, China) on a real-time PCR system (Bio-Rad, USA). mRNA expression was detected using the 2-ΔΔCq method and normalized to 18s RNA. The primers used in the present study are listed in Table 1.
 |
Table 1 Primer Sequences and Conditions Used in the Present Study |
Flow Cytometry
The cells were washed in PBS twice and subsequently incubated with Annexin V and PI staining (BD Biosciences, CA) for 15 min in the dark at 4°C as per the manufacturer’s instructions. The cells were then counted, and flow cytometry was performed on an FACscan flow cytometer (Becton Dickinson, US). The analysis was done using CytExpert software V2.3.0.84.
Statistical Analysis
Data were expressed as mean ± SED. Differences between groups were determined using Student’s t-test or one-way ANOVA, and multiple comparisons among groups using the Tukey method were made. All analyses were performed using GraphPad Prism software version 8.0 (La Jolla, USA). A P-value <0.05 was considered statistically significant.
Results
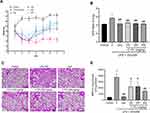
C-PC Alleviates Lung Injury Induced by SW and LPS
The weight changes in mice were recorded over seven days to determine the effect of C-PC on appetite (Figure 1A). The results revealed improved weight gain following treatment with C-PC. In addition, the wet-to-dry ratio suggested the presence of pulmonary edema. Furthermore, the HE staining showed pathological changes in the lungs. The model group showed a significantly increased proportion of empty lacunae in HE staining compared with the control group. HE staining showed that the alveolar wall thickened, edema and hemorrhage, less alveolar space and obvious inflammatory cells infiltration into interstitial and alveolar spaces in the LPS+SW group compared with the Control group (Figure 1B). However, treatment with C-PC was shown to significantly alleviate lung edema, reduce alveolar wall thickening, and limit the damage to the alveolar structure (Figure 1B and C). Seawater and LPS lead to an increase in the MPO levels. However, this observation was significantly reversed following treatment with C-PC (Figure 1D). Overall, the results showed that C-PC could alleviate the severity of lung injury induced by SW and LPS in mice.
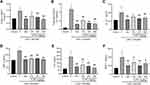
Effect of C-PC on SW and LPS-Induced Pulmonary Inflammation
The effect of C-PC on SW and LPS-induced pulmonary inflammation was examined. The total protein concentration and the total cell count in BALF were determined to reflect inflammatory cells’ infiltration in the alveoli. Treatment with C-PC (40, 80, and 120 μg/ml) significantly reversed the infiltration by inflammatory cells and decreased the total protein concentration in BALF (Figure 2A and B). Moreover, treatment with C-PC and Dex decreased the serum levels of TNF-α, IL-1β, IL-6, and MCP-1 compared with the SW and LPS groups (Figure 2C–F). Overall, the results showed that C-PC ameliorated lung infiltration by inflammatory cells induced by SW and LPS-induced mice.
Antioxidant Effect of C-PC in Lung Tissue
In order to explore the effect of C-PC on antioxidation in lung tissue of SW & LPS-induced mice, we detected the level of MDA, total antioxidant capacity (T-AOC) and antioxidant enzymes in lung tissue. SW and LPS stimulation increased the MDA level in lung tissue compared with controls, but the changes were prevented by C-PC or Dex treatment (Figure 3B). And the levels of T-AOC, SOD, CAT and GPx were all decreased in SW & LPS group, and these trends were reversed by C-PC or Dex (Figure 3A, C and D). These results indicated that C-PC treatment could reduce the level of oxidative stress induced by SW & LPS in lung tissue.
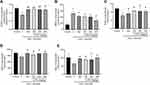
Effect of C-PC on Gene Expression of Inflammatory Mediators in Macrophages
Gene expression of TNF-α, IL-1β, IL-6, MCP-1, and MIF in macrophages was determined using qRT-PCR. In the RAW264.7 cell lines, SW and LPS were shown to increase the expression of inflammatory mediators. However, treatment with C-PC decreased the mRNA levels of TNF-α, IL-1β, IL-6, MCP-1, and MIF (Figure 4A–E). These results suggest that C-PC could inhibit the expression of inflammatory mediators induced by SW and LPS in macrophages.
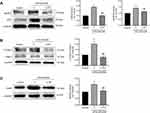
Effect of C-PC on the Expression of NLRP3 Inflammasome-Related Proteins
Western blot was used to analyze the effect of C-PC on the expression of NLRP3 inflammasome-related proteins induced by SW and LPS in RAW264.7 cells. The expression of NLRP3, ASC, cleaved-Caspase-1, and iNOS were increased in the SW and LPS groups. However, treatment with C-PC decreased the levels of NLRP3, ASC, cleaved-Caspase-1, and iNOS in the RAW264.7 cells (Figure 5A–C). These results demonstrate that C-PC can inhibit activation of the NLRP3 inflammasome in macrophages.
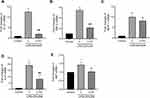
Effect of C-PC on the NLRP3 Inflammasome and NF-κB Signaling Pathway in Lung Tissues
The NF-κB signaling pathway plays an essential role in NLRP3 inflammasome activation and acute inflammation. Western blot analysis was used to determine the protein expression of NLRP3, ASC, cleaved-Caspase-1, iNOS, p-P65, P65, p-IκBα, and IκBα in lung tissues of mice. The results showed that treatment with different concentrations of C-PC decreased the expression of NLRP3 inflammasome-related proteins and the levels of p-P65 and p-IκBα in SW and LPS-induced lung injury (Figure 6A–D). These results demonstrate that treatment with C-PC could suppress the activation of the NLRP3 inflammasome and NF-κB signaling pathway in lung tissues.
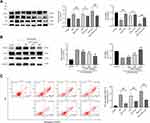
Effect of C-PC on the Formation of NLRP3 Inflammasome Complex in Pulmonary Macrophages
F4/80 is considered a marker of resident macrophages, while NLRP3 is the main component of the NLRP3 inflammasome. Immunofluorescent analysis revealed that the expression of NLRP3 was co-localized with F4/80 in the lung tissue sections obtained from the SW and LPS mice groups. However, in the (Figure 7A), Cleaved-Caspase-1 is the active form of Caspase-1. Release of large amounts of Cleaved-Caspase-1 can exacerbate inflammation and induce cell pyroptosis. Intratracheal administration of SW and LPS induced the expression of Cleaved-Caspase-1 in lung-resident macrophages. However, Dex and different concentrations of C-PC decreased the expression of Cleaved-Caspase-1 (Figure 7B). Co-localization of NLRP3 and ASC suggests the formation of the NLRP3 inflammasome. Stimulation with SW and LPS led to a marked increase in NLRP3 co-localization with ASC. In contrast, different concentrations of C-PC significantly inhibited the increased co-localization of NLRP3 with ASC (Figure 7C). These results demonstrate that C-PC could inhibit the formation of the NLRP3 inflammasome complex in pulmonary macrophages.
Effect of C-PC on Apoptosis in Lung Tissues and MLE-12 Cells
Protein expression of BAX, Bcl-2, and Caspase-3 in lung tissues and MLE-12 cells was analyzed to explore the effect of C-PC on apoptosis following stimulation with SW and/or LPS. As shown in Figure 8, treatment with C-PC inhibited the expression of Caspase-3 and BAX, and promoted the expression of Bcl-2 in MLE-12 cells following stimulation with SW and/or LPS (Figure 8A). In addition, different concentrations of C-PC showed similar effects in the lung tissues following stimulation with SW and LPS (Figure 8B). The flow cytometry analysis revealed increased MLE-12 cell apoptosis in the SW and LPS groups. However, treatment with C-PC significantly decreased cell apoptosis (Figure 8C). These findings indicate that C-PC could inhibit apoptosis in lung tissue and MLE-12 cells induced by SW and LPS.
Discussion
Acute lung injury and acute respiratory distress syndrome remain a significant health challenge, with no current Food and Drug Administration (FDA) approved drug for its management. Seawater drowning results in pathological damage to the respiratory system. Approximately 1/3 of seawater drowning patients exhibit characteristics of ALI or ARDS.6 Seawater is hypertonic with a high microbial composition.5 The hyperosmotic seawater induces a deficiency of pulmonary surfactant, destruction of the blood-gas barrier, formation of pulmonary edema, inflammation, oxidative stress, autophagy, and apoptosis.5,46 In the present study, saline water and LPS were used to establish SW and LPS-induced ALI models. The study aimed to elucidate the molecular mechanisms of C-PC in alleviating inflammation and apoptosis in seawater near-drowning induced ALI. The results showed that treatment with C-PC could ameliorate seawater exposure-induced lung pathological damage, lung edema, and macrophage infiltration. In addition, treatment with dexamethasone showed a similar protective effect. The protective mechanism may be associated with inhibiting the excessive inflammatory response by suppressing the NF-κB/NLRP3 axis and inhibiting lung epithelial cell apoptosis and the oxidative stress reaction (Figure 9).
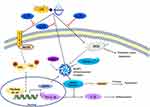 |
Figure 9 Schematic model for the effect of C-PC in LPS and SW-induced ALI. |
In the mouse model, SW and LPS were shown to induce ALI evidenced by significant weight loss, pathological damage to the lungs (including alveolar edema, hemorrhage, inflammatory cell infiltration, diffuse alveolus collapse and increased alveolar wall thickness), high W/D ratio, increased levels of MPO and increased total protein concentration and total cells in BALF. Various studies have shown that entry of SW into the lungs produces a large amount of ROS, which destroys the redox balance and induces oxidative stress response causing cell death.5,47 In this study, treatment with C-PC was shown to mitigate lung pathology. In the ALI models, C-PC was shown to alleviate SW and LPS-induced lung tissue edema, inflammation, and oxidative stress (Figures 1 and 2). Treatment with C-PC decreased the total protein concentration and total cells in BALF, reduced W/D ratio and MPO activity, increased SOD, CAT, Gpx, and T-AOC activity, and reduced levels of MDA in lung tissues. Oxidative stress is closely related to inflammation. Alveolar macrophages, activated neutrophils, and pro-inflammatory mediators, such as IL-1β, IL-6, IL-8, TNF-α, and MCP-1, are considered biomarkers of inflammation in ALI.48 These markers have been shown to trigger aggravation of lung tissue injury.49 The present study showed that treatment with C-PC significantly decreased the levels of inflammatory mediators in ALI. It has been reported that the course of acute lung injury caused by seawater inhalation is more acute and faster than that caused by LPS.12 In previous reports, C-PC has been reported to have a therapeutic effect on LPS-induced ALI by inhibiting lung cell apoptosis and NF-kB-mediated inflammatory responses.50 We also verified this in seawater and LPS models, and further found that it is related to the NLRP3 inflammasome.
Alveolar macrophages are closely related to ALI inflammation. The NLRP3 inflammasome is a large intracellular signaling complex in macrophages, which plays a critical role in the innate immune response. Previous studies have shown that activation of the NLRP3 inflammasome plays an important role in the pathogenesis of ALI.23 Alzokaky et al. showed that C-PC exhibited anti-inflammatory effects in ethanol-induced gastric ulcers by targeting the HMGB1/NLRP3/NF-κB pathway.45 The present study investigated the underlying mechanism of C-PC in mitigating the inflammatory response in ALI. Subsequently, we investigate the role of C-PC in the NF-κB/NLRP3 axis in RAW264.7 cells and SW- and LPS-induced ALI in the mouse model. Western blot analysis showed that SW and LPS increased the expression of ASC, cleaved-Caspase-1, and NLRP3 in RAW264.7 cells. The qRT-PCR analysis showed that the mRNA levels of inflammatory mediators were up-regulated following the administration of SW and LPS. However, treatment with C-PC was shown to decrease the mRNA levels of inflammatory mediators. Furthermore, western blot and immunofluorescence analysis revealed that C-PC inhibited the expression of the NLRP3 inflammasome-related proteins and prevented the formation of the NLRP3 inflammasome complexes. The NLRP3 inflammasomes were co-localized with F4/80 and cleaved-caspase-1 in the alveolar macrophages. Different concentrations of C-PC showed an inhibitory effect on the activation of the NLRP3 inflammasomes and activation of Caspase-1. In addition, C-PC was shown to inhibit the formation and assembly of the NLRP3 inflammasomes, as evidenced by reduced co-localization of ASC and NLRP3. The NF-κB signaling pathway is closely related to the NLRP3 inflammasome activation.51 Results of the western blot analysis showed that C-PC suppressed the phosphorylation of P65 and IκBα. These results show that C-PC mainly regulates SW and LPS-induced ALI inflammation through the NLRP3/NF-κB axis.
Apoptosis of the lung epithelial cells is closely related to the pathogenesis of ALI.52 Several studies have shown that inflammatory cells and cytokines can induce cell death.53,54 Therefore, we studied the effect of C-PC on apoptosis induced by SW in MLE-12 cells. The western blot and flow cytometry analysis showed that exposure to SW and LPS lead to apoptosis in MLE-12 cells. In contrast, treatment with C-PC was shown to inhibit apoptosis. However, the specific underlying mechanism needs to be further explored.
There are still some issues that need to be considered in the study. First, when reviewing the literature, we found that some studies suggest that the lung injury induced by seawater is more serious than that induced by freshwater, but the clinical treatment of these two types of lung injury has not been distinguished, which deserves further study.55,56 Secondly, the reason why we use the model of seawater and LPS is to better simulate the clinical patient situation, but our model needs more improvement to be as close to clinical confirmation as possible. Finally, our experiments need to consider more clinical indicators, such as blood oxygen concentration, lung capacity, and so on, in order to better explore the clinical applicability of phycocyanin.
Some studies have found that there is a correlation between hypoxia-inducible factor (HIF)-α and seawater-induced lung injury.12 We will further explore the relationship between C-PC and HIF-α in affecting the inflammation of seawater-induced lung injury in our next research. We will further explore the designing efficacious formulations and dosage forms of C-PC to improve its bioavailability and lung targeting.
Conclusion
In summary, this study revealed that C-PC has a protective effect on SW and LPS-induced ALI. The protective mechanism was associated with alleviating inflammation by inhibiting the NLRP3/NF-κB axis in macrophages and inhibiting apoptosis in lung epithelial cells. These results show that C-PC may have a potential therapeutic value in seawater near-drowning induced ALI/ARDS.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article was submitted, gave final approval for the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work received financial support from the National Natural Science Foundation of China (Grant No. 81903693), Fundamental Research Funds for Zhejiang Provincial Universities and Research Institutes (Grant No. 2019J00013), Science and Technology Plan Project of Zhoushan (Grant No. 2020C21018), and the National Undergraduate Training Program for Innovation and Entrepreneurship (Grant No. 201910340019).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sugawara M, Ishiyama K, Takahashi S, et al. Postmortem computed tomographic features in the diagnosis of drowning: a comparison of fresh water and salt water drowning cases. JPN J Radiol. 2019;37:220–229. doi:10.1007/s11604-018-0802-8
2. Tester DJ, Ackerman MJ. Drowning. N Engl J Med. 2012;367:
3. Omar HR, Mirsaeidi M, Bosco G, et al. Cardiovascular complications and mortality determinants in near drowning victims: a 5-year retrospective analysis. J Crit Care. 2017;37:237–239. doi:10.1016/j.jcrc.2016.09.010
4. The L. Drowning: a silent killer. Lancet. 2017;389:1859. doi:10.1016/S0140-6736(17)31269-2
5. Jin F, Li C. Seawater-drowning-induced acute lung injury: from molecular mechanisms to potential treatments. Exp Ther Med. 2017;13:2591–2598. doi:10.3892/etm.2017.4302
6. Gregorakos L, Markou N, Psalida V, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187:93–97. doi:10.1007/s00408-008-9132-4
7. Dushianthan A, Cusack R, Goss V, et al.. Clinical review: exogenous surfactant therapy for acute lung injury/acute respiratory distress syndrome - where do we go from here?. Criti Care. 2012;16. doi:10.1186/cc11512
8. Giammona ST, Modell JH. Drowning by total immersion: Effects on pulmonary surfactant of distilled water, Isotonic Saline, and sea water. Am J Dis Child. 1967;114:612–616. doi:10.1001/archpedi.1967.02090270068005
9. Han F, Luo Y, Li Y, et al. Seawater induces apoptosis in alveolar epithelial cells via the Fas/FasL-mediated pathway. Respir Physiol Neurobiol. 2012;182:71–80. doi:10.1016/j.resp.2012.05.012
10. Congcong L, Liyan B, Pengcheng L, et al. Losartan, a selective antagonist of AT1 receptor, attenuates seawater inhalation induced lung injury via modulating JAK2/STATs and apoptosis in rat. Pulm Pharmacol Ther. 2017;45:69–79. doi:10.1016/j.pupt.2017.05.002
11. Ma L, Zhao Y, Li B, et al. 3,5,4’-Tri-O-acetylresveratrol attenuates seawater aspiration-induced lung injury by inhibiting activation of nuclear factor-kappa B and hypoxia-inducible factor-1 alpha. Respir Physiol Neurobiol. 2013;185:608–614. doi:10.1016/j.resp.2012.11.016
12. Liu Z, Xi R, Zhang Z, et al. 4-hydroxyphenylacetic acid attenuated inflammation and edema via suppressing HIF-1 alpha in seawater aspiration-induced lung injury in rats. Int J Mol Sci. 2014;15:12861–12884. doi:10.3390/ijms150712861
13. Zhang M, Wang L, Dong M, et al. Endothelial Semaphorin 7A promotes inflammation in seawater aspiration-induced acute lung injury. Int J Mol Sci. 2014;15:19650–19661. doi:10.3390/ijms151119650
14. Yuan JJ, Zhang XT, Bao YT, et al. Heme oxygenase-1 participates in the resolution of seawater drowning-induced acute respiratory distress syndrome. Respir Physiol Neurobiol. 2018;247:12–19. doi:10.1016/j.resp.2017.08.016
15. Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–489. doi:10.1038/s41577-019-0165-0
16. Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76:100889. doi:10.1016/j.mam.2020.100889
17. He X, Qian Y, Li Z, et al. TLR4-upregulated IL-1β and IL-1RI promote alveolar macrophage pyroptosis and lung inflammation through an autocrine mechanism. Sci Rep. 2016;6:31663. doi:10.1038/srep31663
18. Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi:10.1111/imr.12286
19. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi:10.1038/nature15514
20. Chae JJ, Cho Y-H, Lee G-S, et al. Gain-of-function pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi:10.1016/j.immuni.2011.02.020
21. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi:10.1016/j.tibs.2016.10.004
22. Yang H, Lv H, Li H, et al. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-kappaB pathways. Cell Commun Signal. 2019;17:62. doi:10.1186/s12964-019-0366-y
23. Grailer JJ, Canning BA, Kalbitz M, et al. Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol. 2014;192:5974–5983. doi:10.4049/jimmunol.1400368
24. Zhang Y, Li X, Grailer JJ, et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J Pineal Res. 2016;60:405–414. doi:10.1111/jpi.12322
25. Wang B, Wang J, Lu D, et al. The defensive action of LYRM03 on LPS-induced acute lung injury by NF-kappaB/TLR4/NLRP3 signals. J Invest Surg. 2021;34:284–296. doi:10.1080/08941939.2019.1634165
26. Zhang B, Wang B, Cao S, et al. Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-kappaB signaling and NLRP3 activation. Int J Mol Med. 2017;39:1111–1118. doi:10.3892/ijmm.2017.2935
27. Zhang M, Dong M, Liu W, et al. 1 alpha,25-dihydroxyvitamin D3 ameliorates seawater aspiration-induced acute lung injury via NF-kappaB and RhoA/Rho kinase pathways. PLoS One. 2014;9:e104507. doi:10.1371/journal.pone.0104507
28. Li W, Zhang W, Liu J, et al. Down-regulation of miR-let-7e attenuates LPS-induced acute lung injury in mice via inhibiting pulmonary inflammation by targeting SCOS1/NF-kappaB pathway. Biosci Rep. 2021;41:54.
29. Rahman A, Fazal F. Blocking NF-kappaB: an inflammatory issue. Proc Am Thorac Soc. 2011;8:497–503. doi:10.1513/pats.201101-009MW
30. Wang X, Chen S, Zhao L, et al. Protective effect of combination of anakinra and MCC950 against acute lung injury is achieved through suppression of the NF-kappaB-mediated-MAPK and NLRP3-caspase pathways. Int Immunopharmacol. 2021;97:107506. doi:10.1016/j.intimp.2021.107506
31. Yoshida A, Takagaki Y, Nishimune T. Enzyme immunoassay for phycocyanin as the main component of spirulina color in foods. Biosci Biotechnol Biochem. 1996;60:57–60. doi:10.1271/bbb.60.57
32. Patil G, Chethana S, Sridevi AS, et al. Method to obtain C-phycocyanin of high purity. J Chromatogr A. 2006;1127:76–81. doi:10.1016/j.chroma.2006.05.073
33. Kuddus M, Singh P, Thomas G, et al. Recent developments in production and biotechnological applications of C-phycocyanin. Biomed Res Int. 2013;2013:742859. doi:10.1155/2013/742859
34. Romay C, Armesto J, Remirez D, et al. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47:36–41. doi:10.1007/s000110050256
35. Shih C-M, Cheng S-N, Wong C-S, et al. Antiinflammatory and antihyperalgesic activity of C-phycocyanin. Anesth Analg. 2009;108:1303–1310.
36. Cherng S-C, Cheng S-N, Tarn A, et al. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007;81:1431–1435. doi:10.1016/j.lfs.2007.09.009
37. Jiang L, Wang Y, Liu G, et al. C-phycocyanin exerts anti-cancer effects via the MAPK signaling pathway in MDA-MB-231 cells. Cancer Cell Int. 2018;18:12. doi:10.1186/s12935-018-0511-5
38. Li B, Gao M-H, Chu X-M, et al. The synergistic antitumor effects of all-trans retinoic acid and C-phycocyanin on the lung cancer A549 cells in vitro and in vivo. Eur J Pharmacol. 2015;749:107–114. doi:10.1016/j.ejphar.2015.01.009
39. Grover P, Bhatnagar A, Kumari N, et al. C-phycocyanin-a novel protein from spirulina platensis- in vivo toxicity, antioxidant and immunomodulatory studies. Saudi J Biol Sci. 2021;28:1853–1859. doi:10.1016/j.sjbs.2020.12.037
40. Vadiraja BB, Gaikwad NW, Madyastha KM. Hepatoprotective effect of C-phycocyanin: protection for carbon tetrachloride andR-(+)-pulegone-mediated hepatotoxicity in rats. Biochem Biophys Res Commun. 1998;249:428–431. doi:10.1006/bbrc.1998.9149
41. Chiu H-F, Yang S-P, Kuo Y-L, et al. Mechanisms involved in the antiplatelet effect of C-phycocyanin. Br J Nutr. 2006;95:435–440. doi:10.1079/BJN20051643
42. Reddy CM, Bhat VB, Kiranmai G, et al. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from spirulina platensis. Biochem Biophys Res Commun. 2000;277:599–603. doi:10.1006/bbrc.2000.3725
43. Sun Y, Zhang J, Yan Y, et al. The protective effect of C-phycocyanin on paraquat-induced acute lung injury in rats. Environ Toxicol Pharmacol. 2011;32:168–174. doi:10.1016/j.etap.2011.04.008
44. Bhat VB, Madyastha KM. C-phycocyanin: a potent peroxyl radical scavenger in vivo and in vitro. Biochem Biophys Res Commun. 2000;275:20–25. doi:10.1006/bbrc.2000.3270
45. Alzokaky AA, Abdelkader EM, El-Dessouki AM, et al. C-phycocyanin protects against ethanol-induced gastric ulcers in rats: role of HMGB1/NLRP3/NF-kappaB pathway. Basic Clin Pharmacol Toxicol. 2020;127:265–277. doi:10.1111/bcpt.13415
46. Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi:10.1146/annurev-pathol-011110-130158
47. Li PC, Wang BR, Li CC, et al. Seawater inhalation induces acute lung injury via ROS generation and the endoplasmic reticulum stress pathway. Int J Mol Med. 2018;41:2505–2516. doi:10.3892/ijmm.2018.3486
48. Butt Y, Kurdowska A, Allen TC. Acute lung injury: a clinical and molecular review. Arch Pathol Lab Med. 2016;140:345–350. doi:10.5858/arpa.2015-0519-RA
49. Mokra D, Mikolka P, Kosutova P, et al. Corticosteroids in acute lung injury: the dilemma continues. Int J Mol Sci. 2019;20:4765. doi:10.3390/ijms20194765
50. Leung PO, Lee HH, Kung YC, et al. Therapeutic effect of C-phycocyanin extracted from blue green algae in a rat model of acute lung injury induced by lipopolysaccharide. Evid Based Complement Alternat Med. 2013;2013:916590. doi:10.1155/2013/916590
51. Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-κb activating pattern recognition and cytokine receptors license nlrp3 inflammasome activation by regulating nlrp3 expression. J Immunol. 2009;183:787–791. doi:10.4049/jimmunol.0901363
52. Fujita M, Kuwano K, Kunitake R, et al. Endothelial cell apoptosis in lipopolysaccharide–induced lung injury in mice. Int Arch Allergy Immunol. 1998;117:202–208. doi:10.1159/000024011
53. Martin TR, Nakamura M, Matute-Bello G. The role of apoptosis in acute lung injury. Crit Care Med. 2003;31:S184–S188.
54. Chopra M, Reuben JS, Sharma AC. Acute lung injury: apoptosis and signaling mechanisms. Exp Biol Med. 2009;234:361–371. doi:10.3181/0811-MR-318
55. Ma L, Chen X, Wang R, et al. 3,5,4’-Tri-O-acetylresveratrol decreases seawater inhalation-induced acute lung injury by interfering with the NF-kappaB and i-NOS pathways. Int J Mol Med. 2016;37(1):165–172. doi:10.3892/ijmm.2015.2403
56. Szpilman D, Bierens JJ, Handley AJ, et al. Drowning. N Engl J Med. 2012;366(22):2102–2110. doi:10.1056/NEJMra1013317
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.