Back to Journals » Cancer Management and Research » Volume 15
The Synergy of Gene Targeting Drug Icaritin Soft Capsule with Immunomodulator and TACE Brings New Hope for Drug Combination in Patients with Advanced Liver Cancer: A Case Report and Literature Review
Authors Tang X , Zhang Y , Dong X, Jiang G, Hong D, Liu X
Received 6 April 2023
Accepted for publication 2 July 2023
Published 18 July 2023 Volume 2023:15 Pages 707—717
DOI https://doi.org/10.2147/CMAR.S414487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Xiaoxia Tang,1 Yizhuo Zhang,2 Xinyu Dong,2 Guixing Jiang,2 Defei Hong,2,* Xiaolong Liu2,*
1Operating Room, Sir Run Run Shaw Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2General Surgery, Sir Run Run Shaw Hospital of Zhejiang University School of Medicine, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaolong Liu; Defei Hong, Email [email protected]; [email protected]
Abstract: At present, the average five-year survival rate of liver cancer in China is only 12.1%. The reason for this association lies in the diagnosis at its middle or/and advanced stage of liver cancer for lacking special clinical symptoms in almost 70% of patients without the chance of effective surgical resection. Epidemiological studies have shown that there are only 30% of patients with an initial diagnosis of liver cancer have the opportunity to undergo radical surgery. Therefore, systematic and comprehensive treatment would play an important role in liver cancer treatment at its middle or/and advanced stage, and the related therapeutic schedule still needs further improvement and optimization. We applied a gene-targeted drug of Icaritin soft capsule in the treatment of a liver cancer patient at its advanced stage. And the level of AFP was found to decrease to 6.4ng/mL from 10.86ng/mL; meanwhile, MRI showed that the primary tumor significantly reduced in size, with shrinking of the hepatogastric space, hepatic aortic side, and renal artery side lymph nodes. After treatment with TACE and Icaritin, the patient had no discomfort and no longer experienced abdominal pain and bloating and gained three kilograms of weight. The therapeutic effect of Icaritin-targeted drugs was completely demonstrated during the later treatment follow-up. That is to say, the multiple anti-tumor characteristics of Icaritin with good safety were fully displayed in this case, and it can be used in combination with other drugs to treat hepatocellular carcinoma in the clinical setting. The results show that Icaritin can put some effects on the combined treatment of patients with liver cancer.
Keywords: HCC, patient, systematic and comprehensive treatment, Icaritin, gene-targeted
Introduction
Hepatocellular carcinoma (HCC), the predominant histological type of liver cancer, is responsible for the vast majority of liver cancer diagnoses and deaths. Currently, chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) remain the most important global risk factors for HCC.1 Data collected in 2020 show that primary liver cancer is the sixth most common cancer in the world and the third most common cause of death.2 The highest incidence is in Asia and Africa, with China leading the number of liver cancer cases due to its higher morbidity (18.3 cases per 100,000 people) and the largest population (1.4 billion people).3
The “Guidelines for the Treatment of Primary Liver Cancer (2022 Edition)” and CSCO “Guidelines for the Treatment of Primary Liver Cancer 2022” are being updated and released with the efforts of the scholars and experts in China, which proposed CNLC staging in line with China’s national conditions, emphasizing MDT treatment, full management, treatment by line, emphasizing and optimizing the traditional Chinese medicine for treatment.
Modern pharmacological studies prove Icaritin’s effects of promoting osteoblast growth, sedation, antidepressant, enhancing immunity, protecting the cardiovascular system, antioxidants and anti-aging, etc. The population enrichment met the following assays: ①AFP 400ng/mL ②TNF-+ 2.5ng/mL ③IFN-+ 7.0ng/mL. The median overall survival in the Icaritin group was 13.54 months, with a Hazard ratio of 0.43 and a 57% lower risk of death combined with the control group. The median time to disease progression was also nearly twice as long in the trial group as compared to controls, at 3.65 months, with a median progression-free survival of 2.79 months, as assessed by Independent Review Committee. The incidence of adverse reactions was extremely low, with an overall adverse reaction rate of less than 10% for grade 3 or higher.4 Based on the results of this III study, Icaritin soft capsules are recommended by the 2022 edition of the CSCO guidelines for the treatment of advanced hepatocellular carcinoma (Class 1B evidence) as a Class I recommendation (Class 1B evidence) from the CSCO guidelines in patients with poor liver function.5
Case Presentation
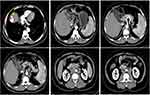
In early April 2022, a 54-year-old male presented to the hospital with an ultrasound showing a “mass in the liver near the top of the diaphragm, suspicious for malignancy”. His past history included hypertension, a small amount of alcohol consumption, and obesity with severe fatty liver. There were no typical positive signs on admission, including Bp 134/91mmHg, R 19bpm, HR 93bpm, T 36.3°C, and pain 0 points. Physical examination: abdomen flat, no hyperactive bowel sounds, negative shifting dullness indicates no seroperitoneumn, no pressure pain, rebound pain in the whole abdomen, no abnormal masses, no edema in both lower limbs. The important blood test indexes indicated that alpha-fetoprotein is 13.94ng/mL, slightly elevated, CEA 2.56ng/mL, CA19-9 23.84IU/mL, and CA125 11U/mL. Since there is no test for tumor necrosis factor and interferon, the patient was considered as hepatocellular carcinoma, not within the enrichment criteria, and all three types of hepatitis tests are negative without a history of hepatitis. The abdomen CT (2022.04.07) showed an occupancy in the VIII segment of the liver with a lesion of about 4 cm in size, with marginal enhancement and internal hemorrhage, which was considered to be HCC in the context of medical history (Figure 1). And there were large lymph node metastases between the liver and stomach located lateral to the common hepatic artery, on the SMA side, next to the renal artery, and on the abdominal aorta, which was not consistent with the diagnosis of primary hepatocellular carcinoma metastasis and needed a thorough examination to find its etiology (Figure 1). CT-guided liver puncture biopsy showed hepatocellular carcinoma with extensive necrosis; ultrasound endoscopic lymph node puncture biopsy of the hepatogastric space showed a large number of lymphatic anisocytic cells without malignancy, so the possibility of lymphoma was excluded; laparoscopic lymph node biopsy showed metastatic undifferentiated carcinoma; gastrointestinal PET-CT showed only liver lesions; small bowel capsule endoscopy showed normal (Figure 2); thyroid Ultrasound and laryngoscopy did not display any abnormality. Therefore, there were no findings of the primary foci other than the liver. Based on the pathological results and genetic traceability testing, primary HCC combined with undifferentiated hepatocellular carcinoma with extensive metastasis was proved.
 |
Figure 2 2022.04.20 Small bowel capsule endoscopy: duodenitis, jejunum, ileum with scattered spots and small patches of erythema, inflammation. |
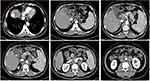
In accordance with the judgment mentioned above, a multidisciplinary MDT consultation was conducted, including general surgery, medical oncology, radiotherapy, pathology, gastroenterology, interventional medicine, radiology, etc. After careful discussion, lenvatinib 12mg qd was given as the targeted therapy combined with camrelizumab 200mg q3w as immunotherapy was recommended to treat hepatocellular carcinoma; capecitabine 1000mg po bid combined with oxaliplatin 130mg/m2/kg chemotherapy to treat the gastrointestinal malignancy and pancreatic tumor; TACE for HCC and hemostasis at the top of the liver. At present 2022CSCO Guidelines for Primary Liver Cancer Diagnosis and Treatment have approved carrilizumab for second-line treatment of hepatocellular carcinoma, and combined with oxaliplatin-based systemic chemotherapy has been approved as class 2B evidence for first-line treatment of hepatocellular carcinoma. The five-drug combination is more efficient but with greater side effects, including (1) Grade II myelosuppression with reduced platelets, which was normalized by multiple platelet-raising treatments with Giovanovene and Terbium after hematology consultation combined with transverse curvature; (2) mild RCCEP without special treatment; (3) mildly elevated liver enzymes, which was treated with adenosylmethionine tablets 500mg po tid combined with Tianqing Ganomax 100mg ig qd for liver protection; (4) hypertension, which was treated by the cardiology department. (4) hypertension and hypotension were lowered with Yashida after a cardiology consultation. After three months of treatment, the patient’s Alpha-fetoprotein decreased from the initial 13.78 ng/mL and was maintained at about 9 ng/mL (Figure 3). A repeat CT showed good iodine oil deposition on the top of the liver with no active tissue, as well as shrinking and new necrosis of lymph nodes in the hepatogastric space, necrosis of multiple lymph nodes in the mesentery over the renal artery, and only slightly enlarged lymph nodes in the gallbladder, so the patient’s tumor was judged to be in remission (Figure 4).
 |
Figure 3 Change in AFP levels from April to July of patients. |
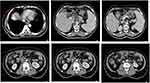
In August 2022, the patient experienced abdominal pain for the first time during the period of treatment, and enhanced CT showed additional foci of reinforcement on the top of the liver, suggesting an active tumor spreading, the lymph nodes in the hepatogastric space and the lymph nodes along the common hepatic artery continued to shrink, the lymph nodes along the superior mesenteric artery and abdominal aorta showed rapid expansion (Figure 5), while AFP elevated to 10.86 ng/mL (Figure 6), which also showed disease progression.
 |
Figure 6 Change in AFP levels from April to September of patients. |
Because of this, MDT consultation concluded that the original treatment was still effective. In order to treat lymph node metastases in the retroperitoneum and AFP elevation, Icaritin soft gels were recommended. Although continually responding from perihepatic lymph nodes, and lymph node metastases in the retroperitoneum, it was considered that although the original protocol was effective, there might be some degree of drug resistance or poor control of the disease. In addition, the target-immune combination treatment has been used for a while, considering the tolerance of patients, drugs with better safety are needed, and Icaritin combined with target-immune can play a synergistic role and delay drug resistance from the perspective of the mechanism of action. In addition, the preliminary study data of Icaritin has confirmed that it can play an anti-tumor effect and reduce the expression of AFP at the same time. Moreover, the majority of patients included in the Phase III clinical study of Icaritin were patients with metastasis of BCLC Stage C, accounting for 81.8%. Therefore, combined with these reasons, Icaritin may have a better effect on the presence of lymph node metastasis and increased AFP. Due to concerns about increased side effects after the addition of the drug, half a dose of Icaritin Soft Capsules was initially recommended, along with continued intensive liver protection treatment. The patient’s abdominal pain improved after the single drug addition of Icaritin Soft Capsules, and a review one month later showed a rapid decrease in Alpha-fetoprotein to 6.4 ng/mL (Figure 6). MRI showed that the primary foci were still active but significantly reduced in size, accompanied by the hepatogastric space, hepatic aortic side, and renal artery side lymph nodes, and nearly total cystic necrosis of the abdominal aortic side gonads (Figure 7). Therefore, it can be seen that the addition of Icaritin has achieved very good results.
A third MDT consultation was conducted based on the results of this treatment: TACE treatment was applied again for the active lesion liver; results of liver and kidney function, blood count, etc., suggested that Icaritin soft capsules did not increase the adverse effects of treatment, which recommended to increasing its dose to 6 BID and continuing to take platelet-raising drugs for maintenance. Due to the second TACE and drug dosing, we are concerned that the impairment of liver and kidney function and suppression of bone marrow will be exacerbated; therefore, after discussion with the patient, we decided to suspend the targeted therapy, and chemotherapy for one month and only perform TACE treatment on September 2, while taking a full dose of Icaritin Soft Capsules 6 capsules BID orally.
On September 16, the patient had no discomfort and no longer experience abdominal pain and bloating, gaining three kilograms of weight, evidencing improved quality of life. On September 24, CT showed a significant reduction in the lesion on the top of the liver, as well as a large cystic necrosis in the lymph nodes of the hepatogastric space and multiple lymph nodes in the posterior abdomen. This indicates that the patient had a sustained tumor remission after September 2 with one TACE and Icaritin soft gels at full dose (Figure 8).
Upon reexamination on October 25, abdominal CT showed continued remission of the primary foci and most metastatic lymph nodes, the patient continued Icaritin + chemotherapy, but the lymph nodes by the superior mesenteric artery increased to 6.3*4.3cm (Figure 9); two lymph nodes of 4cm in size nearby abdominal aorta showed a slight increase compared to past CT images of September, so after 4th MDT discussion, the second-line targeted drug was replaced with Regorafenib 120mg qd, d1-21, Q4W, and started packaged hepatic portal and retroperitoneal lymph node (GTV) SBRT on November 7. After imaging evaluation, LIRADS is 5 grades and perirenal lymph node enlargement is suitable for radiotherapy. The SBRT radiotherapy for the metastases in the hilar and retroperitoneal lymph nodes (GTV) started on November 7, 6MV-X SAD100 DT180cGy/1f/1d, to be reviewed after 8F and finished after 20F.
On November 15th, the patient’s AFP had reached a new low level of 5.018 ng/mL (Figure 10). And on Dec 5th, the CT scan indicated a significant reduction in multiple lymph node metastases compared to the previous CT scan (Figure 11). The biggest lesion has decreased from around 6.2 cm to 2.3 cm, and the patient has once more reached PR.
 |
Figure 10 Changes in overall AFP levels of patients. |
Discussion
Based on a CT scan taken in early April, the patient was thought to have an unconventional advanced primary liver cancer diagnosed as primary HCC combined with undifferentiated hepatocellular carcinoma and extensive metastases. The first multidisciplinary consultation recommended a combination of target-free drugs to be used for liver cancer, chemotherapy to treat the gastrointestinal tract and pancreatic tumor, and TACE to treat the top liver tumor and bleeding. The AFP level decreased and most of the lymph nodes shrunk throughout the combination treatment of multiple drugs. The main adverse effects are second-degree myelosuppression with thrombocytopenia, mild RCCEP, and elevated liver enzymes. However, the side effects were controlled with effective symptomatic treatment.
It showed a limited reduction of AFP level after the first treatment, and it increased after the third month, which suggested that the disease has not been controlled optimally. Although most of the lymph nodes became necrotic, while, the lymph nodes next to the gallbladder were slightly enlarged, and the drug control was not comprehensive, eventually, it progressed in four months. The possible reasons include (1) hypoxia after TACE: it will cause elevated levels of HIF-1α, VEGF, and other growth factors that could promote neovascularization, induce residual tumor cells to highly express the immunosuppressive molecule PD-L1, and inhibit the anti-tumor immune response; TACE treatment will also lead to the release of pro-inflammatory cytokines (such as IL-6), which can affect the prognosis; (2) drug resistance: the heterogeneity of tumor cells may also reduce the effectiveness of TKI due to the target specificity; the efficacy of IO drugs is also deeply influenced by PD-L1 expression. The results of the present approved studies with the first-line therapeutic agents for hepatocellular carcinoma showed that progression-free survival (PFS) ranged from 3.7 to 7.4 months, mainly due to the emergence of acquired drug resistance. (3) Side effects: This patient, treated with a combination of drugs and approaches including targeted, immune, chemotherapy, and TACE, developed second-degree myelosuppression. (4) Restricted liver function: The patients included in the approved phase II/III clinical studies of first- and second-line systemic therapies for hepatocellular carcinoma were mainly with Child-Pugh grade A liver function, and there is a lack of efficacy data for patients with Child-Pugh grade B and C. Patients with poorer liver function are difficult to benefit from it.6 Therefore, there is an urgent need for a drug with a good safety profile that can delay TKI resistance and synergize with other drugs for hepatocellular carcinoma.
It has been reported that Icaritin can inhibit the transcription of the AFP gene to inhibit cancer cells. Icaritin enhances the stability of p53 by inhibiting the ubiquitination and degradation of p53 and inhibits the degradation of p53 mediated by Mdm2 stabilizing the p53 protein, thereby inhibiting AFP transcription, leading to the reduction of AFP protein expression, inhibiting the proliferation of hepatocellular carcinoma (HCC) cells, and promoting the apoptosis of HCC cells.7 In addition, Icaritin can induce ROS production of tumor cells, activate DNA damage and make tumor cells age early.8 Cell aging is also another major factor that inhibits tumor growth.
After the second round of MDT discussions, the original regimen was kept for lymph nodes shrinking but considering the primary lesions with metastasis, especially the rapidly progressing lymph nodes on the edge of the abdominal aorta. Due to the partial progression of the patient’s disease, considering that it may be caused by taking the original drug resistance, people considered changing drugs or drug addiction. Since the patient’s regimen covered all types of drugs and the current treatment was still effective, there were almost no drugs to change or replace, so the addition of drugs was preferred. There are some side effects, including liver and kidney impairment, bone marrow suppression, etc. The patient may not be able to tolerate further enhancement of targeted, immune or chemotherapeutic agents, so based on our center’s past experiences, we recommend the use of Icaritin soft gels, which have fewer side effects and are approved for this indication. Its unique dual-channel mechanism of it mainly acts on inflammatory signaling pathways to improve the tumor microenvironment and exert anti-tumor effects through anti-inflammation and regulation of immune function. Its pathways include direct binding of MyD88/IKKα, inhibition of TLR-MyD88-IKK-NF-κB inflammatory pathway, reduction of TNF-α, IL-6, and other inflammatory factors production;9 in addition, down-regulation of IL6-JAK-STAT3 pathway, which in turn inhibits the effect of PD-L1 expression and MDSC, activation of IFN-γ-positive CD8retro+ T cells, exerting anti-tumor effect.10 Down-regulation of alpha-fetoprotein (AFP) directly inhibits tumor cell growth.7 Studies prove that Icaritin does not act directly on PD-1 but rather on the regulation of the immune microenvironment.
A patient who had been in remission during the first treatment achieved cancer remission in the second phase by adding Icaritin soft gels exclusively. The reason for this lies in the efficacy of Icaritin soft gels to delay TKI resistance and synergize with PD-1 antibodies. Research shows that Icaritin can effectively reverse the multiple drug resistance of tumor cells by downregulating the expression of the MDR1 gene and P-gp protein.11 Dal Z. showed that activating the IL-6/JAKs/STAT3 pathway plays an important role in inducing resistance to TKIs when sorafenib/regorafenib treatment induces IL-6Rα production, which promotes IL-6/STAT3 signaling pathway activation and HCC cell leads to drug resistance,12 while the inhibition of IL-6 expression will significantly increase the ability of sorafenib to suppress HCC cell proliferation in basic studies and also significantly increases sorafenib-induced apoptosis in HCC cells.13 At the same time, Icaritin reduced the mRNA level of Jak2, thereby weakening the phosphorylation of Stat3 and inhibiting the growth of cancer cells.14 Icaritin can delay TKI resistance by inhibiting the IL-6/STAT3 signaling pathway, which restrains proliferation and pro-survival gene activation, or by blocking the formation of the IKK complex to repress the NF-κB signaling path, thus reducing the P65 nuclear translocation on IL-6. Immune checkpoint inhibitors mainly work on the upstream signaling pathway by blocking the binding of PD-L1 to PD-1 of T cells, restoring T cell activity, and activating T cells to kill tumors. An article published in Cell Commun Signal in 2020 showed that the tumor immune microenvironment plays an important role in tumor growth, local infiltration, and distant metastasis.15 Immunotherapy should also not be limited to a single target but also focus on the regulation of the microenvironment. According to basic research, Icaritin significantly increases the number and activity status of CD3+ and CD8+ T cells and exerts antitumor effects. Mechanistically, it can improve the tumor immune microenvironment and synergistically enhance the anti-PD-1 efficacy in combination with immune checkpoint inhibitors.
In addition to the inflammatory signal pathway and immune regulation function mentioned above, Icaritin has much evidence that it regulates the growth, proliferation, invasion, and metastasis of cancer cells at the gene level, for example, PLK1 gene, AFP gene, MDR1 gene, and HIF-1 α. These genes can inhibit the growth of tumor cells at the cellular level, reduce the multiple drug resistance of tumor cells, and inhibit the migration and invasion of tumor cells.
Accordingly, we conducted a third MDT consultation based on the results of this treatment: (1) liver MR indicated that the tumor lesion at the top of the diaphragm still existed, and a second TACE was recommended. (2) Most of the metastatic lymph nodes were cystic necrosis with declined tumor indicators, and abdominal pain had completely resolved; examination of liver and kidney function, blood count, etc. indicated that Icaritin softgels did not increase the adverse effects of treatment, and it was recommended to increase the dosage to (3) Continue to take platelet-raising drugs for maintenance. Because of the need for secondary TACE and drug dosing for the factors of liver and kidney function impairment and bone marrow suppression of the patient, we decided to suspend target-free and chemotherapy drug treatment for one month on a trial basis after discussion with the patient and only performed TACE treatment on September 2, while taking Icaritin soft capsule 6 capsules BID orally.
On September 16, there was no abdominal pain or distension, nor was there any weight loss, suggesting that the quality of life had improved. CT images showed a significant reduction in the active lesion at the top of the liver and necrosis of most lymph nodes. This indicates that the tumor continued to remit after September 2 with only one TACE and a full dose of Icaritin softgels. Physiologically, Icaritin soft gels can inhibit tumor growth, increase CD8+ T cell counts and activity, and regulate the immune microenvironment.16 Moreover, Icaritin can down-regulate the expression of EMMPRIN in cells at the level of mRNA and protein, thus inhibiting the migration and invasion of tumor cells. Icaritin regulates HIF-1 by inhibiting de novo synthesis and promoting degradation α Expression, then causing a series of changes in gene transcription and proteins related to the behavior of various tumor cells, including cell apoptosis, invasion, and metastasis.17
On the other hand, Icaritin also inhibits the activation of the IL-6/STAT3 pathway caused by hypoxia after TACE treatment, which might increase the expression of HIF-1α, and HIF-1α can directly act as a transcription factor, upregulate SNAI1, and induce epithelial cell mesenchymalization (EMT). And inhibition of this pathway can organize the growth and metastasis of residual HCC cells.18 Therefore, when combined with TACE, which can reduce the tumor vascular growth by hypoxia-treated TACE and effectively delay tumor recurrence.
Moreover, Icaritin can inhibit tumor growth at the cellular molecular level. The latest evidence shows that Icaritin inhibits the PLK1 gene and leads to DNA damage response (DDR). RNA sequencing showed that the Polo-like kinase 1 (PLK1) gene and DNA damage response (DDR) were the targets of Icaritin. In mechanism, Icaritin inhibits the PLK1 gene to promote checkpoint kinase 2 (Chk2) homodimerization and T387 phosphorylation, which further activates p53 and leads to the activation of the DDR pathway. In addition, inhibition of the PLK1 gene increased the location of the forkhead box O3a nucleus. The increased location of the forkhead box O3a nucleus activated the ataxia telangiectasia mutation (ATM, an early sensor of DNA damage), and then ATM phosphorylated Chk2 T68 and activated Chk2.19 In terms of inhibiting the PLK1 gene, Icaritin induced DDR in two ways and inhibited the growth of tumor cells. Icaritin up-regulates miR-124 to induce tumor cell apoptosis. Icaritin up-regulates miR-12420 by inhibiting the Sp1/DNMT1 signal pathway, and miRNA-124 combines with the 3’ - untranslated regions (3’ - UTRs) of the target mRNA to induce mRNA degradation or/and inhibit translation, thus regulating the target gene,21 and inducing cell apoptosis through the intrinsic mitochondrial pathway.
In late October, the patient’s CT images showed continued remission of the primary lesion and most of the metastatic lymph nodes, but the SMA-sided and abdominal aorta-sided lymph nodes were slightly larger than before September. The results suggested that it could maintain the patient’s tumor remission for almost two months despite taking only, according to TACE. The decrease in CT lesions and AFP in November suggested further tumor remission with the combination of radiotherapy.
In this case, Icaritin soft capsule was added as the first-line therapy, and quality of life was significantly improved after one month with internal tumor necrosis and decreased blood indexes. It displays the sustained remission and the possibility of the synergistic effect of combined target-free drugs based on their efficacy. It opened a new chapter for the systematic combination therapy of liver cancer patients at an advanced stage of the tumor.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, [D.H. and X.L.], upon reasonable request.
Consent for Publication
The patient provided written informed consent for the case details to be published.
Informed Consent Statement
All patients or participants provided written informed consent to participate in this research.
Acknowledgments
The output of this paper cannot be achieved without the contribution of all, and we would like to offer our sincere thanks to all the authors of this paper and the patients who participated in the experiments.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was sponsored by the Zhejiang Provincial Natural Science Foundation [grant number: LQ20H160034].
Disclosure
The authors declare that the study was implemented in the absence of any business or financial relationships that could be regarded as a potential conflict of interest.
References
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. doi:10.1002/hep.31288
2. Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. doi:10.1016/j.jhep.2022.08.021
3. Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res. 2018;30(6):571–579. doi:10.21147/j.issn.1000-9604.2018.06.01
4. Yan S, Shukui Q, Li W, et al. A randomized, double-blinded, phase III study of icaritin versus huachashu as the first-line therapy in biomarker-enriched HBV-related advanced hepatocellular carcinoma with poor conditions: interim analysis result. J Clin Oncol. 2021;39(15_suppl):4077. doi:10.1200/JCO.2021.39.15_suppl.4077
5. Ryder SD. Guidelines for the diagnosis and treatment of hepatocellular carcinoma. Gut. 2019;52:1–8.
6. Anwanwan D, Singh SK, Singh S, et al. Challenges in liver cancer and possible treatment approaches. Rev Cancer. 2020;1873(1):188314. doi:10.1016/j.bbcan.2019.188314
7. Li H, Liu Y, Jiang W, et al. Icaritin promotes apoptosis and inhibits proliferation by down-regulating AFP gene expression in hepatocellular carcinoma. BMC Cancer. 2021;21(1):318. doi:10.1186/s12885-021-08043-9
8. Wang S, Wang Q, Wang H, et al. Induction of ROS and DNA damage-dependent senescence by icaritin contributes to its antitumor activity in hepatocellular carcinoma cells. Pharm Biol. 2019;57(1):424–431. doi:10.1080/13880209.2019.1628073
9. Zhu S, Wang Z, Li Z, et al. Icaritin suppresses multiple myeloma, by inhibiting IL-6/JAK2/STAT3. Oncotarget. 2015;6(12):10460–10472. doi:10.18632/oncotarget.3399
10. Tao H, Liu M, Wang Y, et al. Icaritin induces anti-tumor immune responses in hepatocellular carcinoma by inhibiting splenic myeloid-derived suppressor cell generation. Front Immunol. 2021;12:609295. doi:10.3389/fimmu.2021.609295
11. Sun L, Chen W, Qu L, Wu J, Si J. Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma cells via downregulation of MDR1 and P-glycoprotein expression. Mol Med Rep. 2013;8(6):1883–1887. doi:10.3892/mmr.2013.1742
12. Dai Z, Wang X, Peng R, et al. Induction of IL-6Rα by ATF3 enhances IL-6 mediated sorafenib and regorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2022;524:161–171. doi:10.1016/j.canlet.2021.10.024
13. Li Y, Chen G, Han Z, et al. IL-6/STAT3 signaling contributes to sorafenib resistance in hepatocellular carcinoma through targeting cancer stem cells. Onco Targets Ther. 2020;13:9721–9730. doi:10.2147/OTT.S262089
14. Zhao H, Guo Y, Li S, et al. A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget. 2015;6(31):31927–31943. doi:10.18632/oncotarget.5578
15. Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:59. doi:10.1186/s12964-020-0530-4
16. Li Q, Sun J, Cao Y, et al. Icaritin inhibited cigarette smoke extract-induced CD8+ T cell chemotaxis enhancement by targeting the CXCL10/CXCR3 axis and TGF-β/Smad2 signaling. Phytomedicine. 2022;96:153907. doi:10.1016/j.phymed.2021.153907
17. Bo X, Chuanwu J, Hongxing H, et al. Icaritin inhibits the invasion and epithelial-to-mesenchymal transition of glioblastoma cells by targeting EMMPRIN via PTEN/AKt/HIF-1α signalling. Clin Exp Pharmacol Physiol. 2015. doi:10.1111/1440-1681.12488
18. Kudo M. A new treatment option for intermediate-stage hepatocellular carcinoma with high tumor burden: initial lenvatinib therapy with subsequent selective TACE. Liver Cancer. 2019;8(5):299–311. doi:10.1159/000502905
19. Zhang C, Xu H, Sui X, et al. Icaritin inhibits PLK1 to activate DNA damage response in NK/T cell lymphoma and increases sensitivity to GELOX regime. Mol Ther Oncolytics. 2022;25:288–304. doi:10.1016/j.omto.2022.04.012
20. Jin L, Miao J, Liu Y, et al. Icaritin induces mitochondrial apoptosis by up-regulating miR-124 in human oral squamous cell carcinoma cells. Biomed Pharmacother. 2017;85:287–295. doi:10.1016/j.biopha.2016.11.023
21. HeeSun Y, JaeYong K, JuHye L, et al. Celastrol isolated from Tripterygium regelii induces apoptosis through both caspase-dependent and -independent pathways in human breast cancer cells. Food Chem Toxicol. 2011;49(2):0278–6915. doi:10.1016/j.fct.2010.11.044
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.