Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
The Synaptic and Circuit Functions of Vitamin D in Neurodevelopment Disorders
Authors Ye X, Zhou Q, Ren P, Xiang W , Xiao L
Received 8 February 2023
Accepted for publication 7 June 2023
Published 3 July 2023 Volume 2023:19 Pages 1515—1530
DOI https://doi.org/10.2147/NDT.S407731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Xiaoshan Ye,1,* Qionglin Zhou,2,* Pengcheng Ren,1,3,4 Wei Xiang,1,3 Le Xiao1
1Hainan Women and Children’s Medical Center, School of Pediatrics, Hainan Medical University, Haikou, People’s Republic of China; 2International School of Public Health and One Health, Hainan Medical University, Haikou, People’s Republic of China; 3National Health Commission (NHC) Key Laboratory of Control of Tropical Diseases, Hainan Medical University, Haikou, People’s Republic of China; 4School of Basic Medicine and Life Science, Hainan Medical University, Haikou, People’s Republic of China
*These authors contributed equally to this workThese authors contributed equally to this work
Correspondence: Le Xiao; Wei Xiang, Hainan Women and Children’s Medical Center, Hainan Medical University, Haikou, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Vitamin D deficiency/insufficiency is a public health issue around the world. According to epidemiological studies, low vitamin D levels have been associated with an increased risk of some neurodevelopmental disorders, including autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder (ADHD). Animal models reveal that vitamin D has a variety of impacts on the synapses and circuits in the brain. A lack of vitamin D affects the expression of synaptic proteins, as well as the synthesis and metabolism of various neurotransmitters. Depending on where vitamin D receptors (VDRs) are expressed, vitamin D may also regulate certain neuronal circuits through the endocannabinoid signaling, mTOR pathway and oxytocin signaling. While inconsistently, some data suggest that vitamin D supplementation may be able to reduce the core symptoms of ASD and ADHD. This review emphasizes vitamin D’s role in the synaptic and circuit mechanisms of neurodevelopmental disorders including ASD and ADHD. Future application of vitamin D in these disorders will depend on both basic research and clinical studies, in order to make the transition from the bench to the bedside.
Keywords: vitamin D, neurodevelopmental disorders, autism spectrum disorder, ASD, attention-deficit hyperactivity disorder, ADHD, synapses, circuits
Introduction
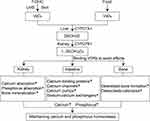
One of the liposoluble vitamins, vitamin D, exists in two distinct forms: vitamin D2 and vitamin D3. While vitamin D2 is obtained from diet, vitamin D3 is primarily produced from 7-dehydrocholesterol (7-DHC) in the skin by ultraviolet B (UVB) radiation.1 Vitamin D2 or vitamin D3 is first hydroxylated to 25-hydroxyvitamin D2 [25(OH)D2] or 25-hydroxyvitamin D3 [25(OH)D3] by sterol 27-hydroxylase (CYP27A1) in the liver. 25-hydroxyvitamin D, or 25(OH)D, is the collective name for the hydroxylated vitamin D. It is converted to 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] in the kidney by the second hydroxylation of 1, α-hydroxylase (CYP27B1).2,3 By binding to vitamin D receptors (VDRs), 1,25(OH)2D3 maintains the calcium and phosphorus homeostasis, as well as controls the bone metabolism, which has been discussed in great detail elsewhere.3–6 1,25(OH)2D3 is metabolized by 24-hydroxylase (CYP24A1) into 24,25-dihydroxyvitamin D3 [24,25(OH)2D3] in the kidney, where it is excreted from the body.3 Figure 1 summarizes the metabolic process and roles of vitamin D in maintaining the balance of calcium and phosphorus. Remarkably, recent studies have demonstrated that 1,25(OH)2D3 can influence a wide range of biological functions, including cell proliferation and differentiation, immune responses, and brain development.7,8 As a result, 1,25(OH)2D3 has been proposed as a neurosteroid hormone.9–12 In addition, epidemiological data and animal experiments have revealed a link between the lack of vitamin D and the occurrence of certain neurodevelopmental disorders.6,13 However, little is known about the molecular mechanisms that underlie vitamin D’s influence on these diseases. The purpose of this review is to provide a comprehensive overview about the synaptic and circuit functions of vitamin D in the neurodevelopmental disorders like autism spectrum disorder (ASD) and attention-deficit hyperactivity disorder (ADHD).
The Effects of Vitamin D on Synaptic Functions
The presence of vitamin D and its nuclear receptors (VDRs), as well as its metabolism enzymes (CYP27A1, CYP27B1 and CYP24A1) in the brain has been systematically reviewed elsewhere.14–16 All of the VDRs, CYP27B1 and CYP24A1 have been identified in neurons and glia cells throughout life, raising the notion that vitamin D might be involved in the fundamental functions of mammalian brains.15,17 These functions, such as learning, memory, cognition, and behavioral processes, all rely on the connection of neurons.18 The information transferred in the neural network is largely performed through synaptic transmission, which includes both electrical and chemical synapses.19 According to the literature, vitamin D participates in multiple processes that regulate synaptic transmission, particularly the chemical synapses.6,13,20 First, the absence of vitamin D increased cholesterol levels in the presynaptic membrane and vesicles, which altered the synaptic membrane’s fusion properties and, as a result, the efficiency of transmitter release.21 On the other hand, vitamin D supplementation could partially restore the capability of vesicle fusion.22 In addition, microarray sequencing revealed that vitamin D affected the transcription of proteins involved in the neurotransmitter release, including proteins in synaptic vesicles such as solute carrier family 17 member 6 (SLC17A6),23,24 proteins involved in exocytosis such as synaptojanin1 (synj1), complexin2, synaptotagmin1 (syt1), synaptotagmin2 (syt2), synaptotagmin10 (syt10), and synaptic vesicle glycoprotein 2c (SV2C),23,24 as well as proteins in the active zones such as double C2 gamma (DOC2G), synapsin2, and synapsin3.23–25 While the majority of the mRNA alterations in synaptic proteins revealed by sequencing were not validated, increased expression of syt2, synj1, and complexin2 were verified by polymerase chain reaction (PCR) or immunohistochemical (IHC) assays.23,26 Additionally, vitamin D could modulate synchronized transmitter release by either directly increasing the activity of L-type voltage-dependent calcium channels (LVDCCs)27 or by promoting the expression of calcium sensors such as syt1and syt2 in the brain.28 Therefore, vitamin D could potentially exert both immediate and long-term effects on synapses.
In addition to the expression of presynaptic release machinery, vitamin D could modulate the expression of transporters, receptors, as well as enzymes for the synthesis and metabolism of neurotransmitters like glutamate,22,29,30 GABA,29,31–33 glycine,32 dopamine,33–37 serotonin,33,37,38 and catecholamines.39
- Transporters: Vitamin D deficiency reduced the expression of excitatory amino acid transporters (EAATs) and GABA transporters 3 (GAT3), which in turn caused the dysfunction of glutamate and GABA reuptake systems.29 In addition, supplementing with vitamin D led to an increased expression of dopamine transporter gene-solute carrier family 6 member 3 (SLC6A3).36
- Receptors: Vitamin D deficiency reduced the mRNA expression of GABA receptors.31 On the other hand, vitamin D supplementation increased the expression of dopamine receptor D2 (DRD2).33,35,36
- The synthesis and metabolism enzymes of neurotransmitters: Vitamin D deficiency decreased the expression of glutamate synthetase 1 and GABA transmitter synthetase, which consisted of glutamate decarboxylase 65 and 67 (GAD65 and GAD67).30,32,33 Furthermore, vitamin D deficiency reduced the expression of catechol-O-methyltransferase (COMT), leading to decreased dopamine metabolism.34 Vitamin D supplementation upregulated the expression of dopamine transmitter synthase-tyrosine hydroxylase (TH).33,36 In addition, the first and rate-limiting enzyme in the biosynthesis of serotonin, tryptophan hydroxylase 2 (TPH2), could also be enhanced by vitamin D.33,37
Taken together, these findings suggested that vitamin D affected the process of synaptic transmission in various ways, likely by combining genomic and nongenomic mechanisms. Notably, vitamin D responsive elements (VDREs) could be identified in the promoter regions of certain genes, including syt1, syt2 and TPH2.23,37,40,41 Many vitamin D responsive genes, however, lack the VDRE sequence.40,41 Therefore, more VDRE sequences not now documented may exist, or some sequences may not directly respond to the VDR signaling.42 In addition, vitamin D could induce growth factors like nerve growth factor (NGF), glial cell derived neurotrophic factor (GDNF) and growth associated protein 43 (GAP43), which could enhance the growth and development of synapses and neurons.25,31,43 The effects of vitamin D on synaptic functions are summarized in Table 1. These findings suggested potential mechanisms by which inadequate vitamin D negatively impacted brain functions.
 |
Table 1 The Effects of Vitamin D on Synaptic Functions |
The Effects of Vitamin D on the Cognitive Function and Behaviors
There is growing evidence that vitamin D influences on cognition and behaviors in a variety of manners.4,44,45 The cortex and hippocampus, two crucially important brain areas for cognition, learning and memory, both have VDRs.16,19 According to epidemiological studies, low vitamin D levels have been linked to cognitive impairment.20,46–49 For instance, inadequate vitamin D levels (25(OH)D<30ng/mL) were associated with poorer cognitive performance in individuals older than 60.46–48 Furthermore, daily 800IU vitamin D oral administration for a period of 12 months could improve the cognitive function and reduce amyloid beta (Aβ)-related biomarkers in patients with Alzheimer’s disease.49 Nevertheless, some randomized clinical trials (RCTs) found no correlation between vitamin D supplementation and cognitive improvement.50–52 The inconsistent results might be due to diverse research designs, varied intervention doses and different analysis of confounding factors. Large multicenter RCTs will be necessary in the future to provide more reliable clinical evidence.
Studies using animal models may also provide crucial biological explanations for how vitamin D influences cognition and behaviors. Unfortunately, systemic ablation of VDR or CYP27B1 caused severe rickets and osteomalacia in mice.53–55 Therefore, the mice’s motor dysfunction made it impossible to draw proper conclusions from standard cognitive and social behavioral assessments. Thus, studies using developmental vitamin D (DVD) deficient animal models, adult vitamin D (AVD) deficient animal models and vitamin D supplementation animal models will be used in the following part to discuss how vitamin D affects cognition and behaviors.
In the DVD deficient model, female rodents (rats or mice) were fed a vitamin D deficient diet for 3–4 weeks before and during mating, as well as throughout pregnancy.56–58 At the same time, they were kept without UV light to prevent vitamin D synthesis from the skin.59–61 Therefore, the offsprings were deficient in vitamin D since the fertilized egg-stage until birth, and in some cases, until the time of weaning.59–61 In AVD deficient animal models, rodents around 4 months old were fed with a vitamin D-deficient diet for 10 weeks.32,62 At the same time, these mice were housed in an environment without UV light.32,62 In this way, the animals were deficient in vitamin D due to restricted dietary intake and limited vitamin D3 production from the skin. In vitamin D supplementation animal models, mice were fed with the vitamin D3-enriched diet for several months, and the calcium and phosphorus levels in sera were carefully monitored to be stable.23,63,64 Excellent reviews have elaborated these vitamin D-related animal models.6,20,65 Hereby, we focused on the effects of vitamin D on cognition and behaviors from studies using these animal models and summarized the key points in Table 2.
 |
Table 2 The Effects of Vitamin D on Cognition and Behaviors |
The Potential Effects of Vitamin D in the Etiology Behind ASD and ADHD
The optimal range of serum 25(OH)D concentrations is between 30 and 90ng/mL. Vitamin D deficiency is defined as a serum 25(OH)D level below 10ng/mL, and vitamin D insufficiency as a level between 10 and 30ng/mL.76 Currently, vitamin D deficiency or insufficiency is a major global health issue.77–88 Obese people, people of color, and those individuals who live in high altitudes are more likely to have vitamin D insufficiency or deficiency.78–80,84,89 Additionally, children and pregnant women are particularly vulnerable to have vitamin D deficiency or insufficiency.77,90 A multi-center cross-sectional study conducted in England indicated that up to 14% children under the age of seven were vitamin D deficient.91 Notably, a number of studies have suggested a strong correlation between low vitamin D levels during pregnancy and a higher likelihood of being diagnosed with neurodevelopmental disorders.85–87 Here, we will provide an overview of recent findings on the functions of vitamin D in the physiological mechanisms in neurodevelopmental disorders, taking ASD and ADHD as two examples.
The Role of Vitamin D in ASD
ASD is a neurodevelopmental disorder characterized by social impairment, restricted interests and repetitive behaviors.92 ASD affects about 1% of people worldwide.93,94 According to a recent meta-analysis, children and adolescents with ASD had considerably lower vitamin D concentrations than the controls.95 Additionally, children with insufficient vitamin D levels (<30ng/mL) displayed more severe core symptoms.96,97 Besides, some studies suggested that vitamin D supplementation could alleviate the core symptoms of ASD.96,98 In a clinical trial conducted in 2016, vitamin D supplementation, a dosage of 150000IU/per month i.d. plus a dosage of 400IU/per day orally, was given to ASD children (mean age of 5.1 years old) for three months, and their symptoms were significantly alleviated.98 However, Kerley et al reported that ASD children (N = 40, mean age of 7.1 years old) treated with 2000 IU of vitamin D per day for 20 weeks did not show any significant improvement when compared to the placebo group in a double-blind RCT.99 The discrepancy of the therapeutic effects reported by these studies might be due to difference not only in sample sizes but also the ages of treatment, since the therapeutic effect of vitamin D could be related to the plasticity of the nervous system.95,100 Therefore, vitamin D might be more effective for younger patients. Another reason for the diversity of outcomes could be that ASD is a heterogeneous population, and vitamin D might only have an impact on one fraction of the patients. The precise ASD subgroup sensitive to vitamin D remains to be identified. The majority of clinical studies in the literature are based on observations and are unable to address the causality link between the lack of vitamin D and ASD. The exact role that vitamin D plays in the pathogenesis of ASD is still unclear.101 Here, we summarized the potential synaptic and circuit mechanisms through which inadequate vitamin D contributed to the etiology of ASD.
Vitamin D Regulates the Synaptic Functions
One hypothesis about ASD pathophysiology is the disruption of synaptic functions.102,103 According to autopsy findings, ASD patients’ brains had an abnormally high density of dendritic spines and irregularly shaped spines.104–106 Mutations in ASD-risk genes like shank3, neuroligin3, neurexin1 and sapap3 have been associated with aberrant dendritic spine formation in animal models.107,108 As was previously mentioned in this review, vitamin D modulated a variety of synaptic proteins, such as SLC17A6, synj1 and syt1.23–25,30 Among them, SLC17A6 is a ASD-risk gene.109 Studies showed that vitamin D regulated the expression of growth factors NGF and GDNF in vitro, which were essential for the formation and development of synapses.8,9,43,110 In addition, high vitamin D dosages could promote the expression of synaptic proteins such as synj1 and syt1.23 Remarkably, vitamin D was shown to rescue the ASD-like behaviors in animal models.111,112 For example, mice that had phosphatase and tensin homolog (PTEN) selectively deleted from the granule cells of hippocampus displayed an osteoporosis phenotype as well as impairments similar to autism.113,114 These mice became more sociable after receiving a vitamin D-enriched treatment (vitamin D3 20000IU/kg per day, orally) for 5 weeks.68 Moreover, vitamin D administration was found to decrease the levels of phospho-AKT (pAKT) and phospho-S6 (pS6), both of which were the downstream molecules of mammalian target of rapamycin (mTOR).68 The mTOR signaling is an important pathway for synaptic growth and pruning.106,115,116 These results raise the question of whether vitamin D deficiency-related synaptic dysfunctions can contribute to the development of ASD. More studies are required to answer this question in the future.
Vitamin D Could Modulate the Excitation and Inhibition Balance
Another theory for the etiology of ASD from a neuroscience perspective is an excitation to inhibition (E/I) imbalance.117 ASD animal models demonstrated abnormalities in glutamatergic and GABAergic activities, which lead to an E/I imbalance in the brain.111,118–120 Recent work illustrated how vitamin D could potentially modulate the ratio of excitation to inhibition by regulating the synthesis of neurotransmitters.22,29,45 Vitamin D-deficient animals had lower levels of dopamine and glutamate, while having higher amounts of glycine and GABA.29,32,34 Mechanistically, a lack of vitamin D prevented glutamate and GABA transporters from being expressed, which would have led to a possible E/I imbalance in the brain.29 Further experimental research is necessary to determine whether inadequate vitamin D directly contributes to the pathogenesis of ASD through affecting the E/I balance.
The Roles of Vitamin D in the Neural Circuits Involved in the Core Symptoms of ASD
Diagnosing mental disorders such as ASD is mainly based on symptoms.92 However, these classifications, which are based on clinical manifestations, may not fully capture the fundamental mechanisms underlying mental diseases. Thus, the “Research Domain Criteria (RDoC)” was introduced as a new classification system for the research on mental disorders.121 The RDoC conceptualized mental illness as brain disorders that could be addressed by altered function of neural circuits.121,122 The focus of this concept was on researching the functional abnormalities of the brain circuits underlying the symptoms rather than the disease itself.121,122 The main characteristics of ASD are social deficit, restricted interests and repetitive behaviors.92 In the past decade, the research on the relevant neural circuits has made significant progress.123 In the section below, we will discuss the potential roles of vitamin D in ASD, focusing on the underlying neural circuits.
- Recent studies have suggested that abnormalities in the social-reward circuitry may contribute to the social deficit in ASD patients.124,125 And this system is mostly involved in basolateral amygdala (BLA), nucleus accumbens (NAC), dorsal anterior cingulate cortex (ACC), hypothalamus and midbrain.126–129 VDRs were found in the aforementioned brain regions.16,130,131 According to neuroimaging studies, ASD patients exhibited a feature of reduced activity in the BLA-NAC reward circuit.132 Interestingly, it was found that increasing 2-arachidonoylglycerol (2-AG), an endocannabinoid signal, might reduce presynaptic glutamate release in the BLA-NAC pathway, thereby alleviating the social avoidance in Shank3−/− model mice.133 Vitamin D deprivation lowered cannabinoid receptor expression in the spinal cord as well as 2-AG in the intestines of mice.134 These findings raise the possibility that vitamin D deficiency may modulate the level of 2-AG in the BLA-NAC circuitry, contributing to the onset of ASD. However, more experiments are needed for the direct evidence supporting this hypothesis.
- In addition, an important feature of ASD is the impairment in social functioning, particularly a lack of empathy.135 The ACC-BLA circuit is one of the brain networks implicated in emotional empathy.136,137 In contrast to healthy controls, children with ASD showed decreased connectivity between the amygdala and ACC, according to the functional magnetic resonance imaging (fMRI).138 This reduced connectivity was correlated with the degree of social deficits.139 The BLA and ACC brain regions were found to express VDRs, suggesting a biological basis of vitamin D to act in ACC-BLA circuit.16,131 Additionally, vitamin D deficiency was linked to a thinner cingulate cortex.140,141 These indirect evidences imply that vitamin D deficiency may impair the morphology of the cingulate gyrus, which in turn may affect the function of this brain region.
- The instability of the cortico-striatal circuit has been proposed as the primary cause of repetitive behaviors manifested by ASD patients.142–144 Children with idiopathic ASD showed cortico-striatal hyperconnectivity in fMRI, and this functional connectivity feature was related to the overactivation of mTOR signaling pathway.145 Vitamin D supplementation could reduce pAKT and pS6 levels in the mTOR signaling in mice.68 These results indicate that vitamin D may alter the clinical manifestations of ASD by modulating relevant neural circuits. However, there are still a lot of unanswered questions regarding how inadequate vitamin D contributes to the onset and development of ASD. For example, there is still a lack of experimental evidence to support vitamin D’ direct action on the ACC-BLA circuit. Application of latest technique progress in neuroscience, such as the use of optogenetics and pharmacogenetics might help in exploring these questions.138
Vitamin D and Oxytocin/Vasopressin Signaling
The paraventricular (PVN) and supraventricular nuclei of the hypothalamus produce the hormones oxytocin and vasopressin, which have been linked to social behaviors.146,147 According to a 12-week RCT, oxytocin treatment improved the social performance in patients with ASD (mean age 10.3 years, n = 35).148 Another study reported that children with ASD (aged 9.6–12.9 years, n = 30) who received a 4-week intranasal vasopressin treatment showed a decrease in anxiety symptoms and repetitive behaviors.149 But according to a different placebo-controlled clinical trial, ASD children (aged 3–17 years, n = 277) who received intranasal oxytocin once a day for 24 weeks did not show any significant improvement in social or cognitive assessments when compared to the control group.150 The discrepancy in the therapeutic effects reported by these studies might be due to variations in medication delivery methods, treatment ages, and training program compliance.
Despite the fact that the clinical outcomes were controversial, oxytocin has been demonstrated to reduce the abnormal social behaviors in the animal models of ASD.151–153 In Shank3−/− model mice, oxytocin supplementation could activate endogenous oxytocin neurons in PVN, and thus alleviate their social deficit.151 It is interesting to note that in the hypothalamus, VDRs partially co-localize with vasopressin and oxytocin receptors.16 In addition, the presence of VDREs in the genes encoding oxytocin precursor proteins, oxytocin receptors and vasopressin receptors suggests that vitamin D can regulate their transcripts.154 Furthermore, VDRs are expressed in pro-opiomelanocortin (POMC) neurons in the arcuate nucleus (ARC) of the hypothalamus, and POMC could be directly stimulated by vitamin D.155 Interestingly, POMC neurons project to the oxytocin-secreting PVN neurons.156 These results imply that vitamin D may play a role in the social process by stimulating POMC neurons, which in turn activates oxytocin secretion.
All of the aforementioned evidence together provided the experimental foundation for hypothetic link between vitamin D deficiency/insufficiency and ASD susceptibility. It has been proposed that vitamin D exerted multi-dimensional effects on the synapses and circuits. However, the exact synaptic and circuit mechanisms through which vitamin D contributes to the development of ASD remain to be further investigated. In addition, whether vitamin D can be administered as a supplement to treat ASD needs to be determined.
The Role of Vitamin D in ADHD
ADHD is a neurodevelopmental disorder characterized by hyperactivity, inattention and impulsivity performance.92,157,158 It affects 8~12% of children worldwide.159 Although ADHD is highly inheritable, many biological and environmental factors, such as food additives, lead pollution, prenatal and postnatal toxicant exposures, and low birth weight, have been identified as risk factors.160–162
In recent years, numerous clinical studies suggest that vitamin D may be an environmental risk factor for ADHD.163–166 According to a meta-analysis, children with ADHD had serum 25(OH)D concentrations lower than healthy controls.166 When compared to children with sufficient vitamin D, children with vitamin D insufficiency had a 2.57-fold higher risk of developing ADHD than children.166 Prospective studies have revealed a negative correlation between the severity of ADHD symptoms and maternal 25(OH)D levels.164 The incidence of ADHD-like symptoms in children decreased by 11% for every 10ng/mL increase in maternal 25(OH)D levels.163 Interestingly, there is growing evidence suggesting that vitamin D supplementation could help reduce the symptoms of ADHD.167 In addition, treating ADHD patients with a methylphenidate and vitamin D combination was more effective than using methylphenidate alone.168–170 Another study, however, reported ADHD children (aged 5–12 years, n = 54) who received 1440 IU of vitamin D daily for eight weeks did not show any improvement from baseline.171 This outcome diversity between these studies might be due to different sample sizes and large individual variations. Therefore, current evidence is not sufficient to conclude that vitamin D supplementation could reduce ADHD-related aberrant behaviors. Understanding the neuronal mechanisms will help address the question, and the following mechanisms have been proposed according to the literature:
Alterations of Dopaminergic and Serotoninergic Pathways in Vitamin D Deficient or Supplemented Animals
ADHD susceptibility may be increased by altered gene expression in dopaminergic pathways, including those encoding the dopamine transporter (SLC6A3), DRD2, dopamine D4 receptor (DRD4), dopamine D5 receptor (DRD5) and COMT.35,172 Genes related to dopamine metabolic pathways, such as DRD2, COMT, TH, and SLC6A3, significantly decreased in vitamin D-deficient mice.34,36,154 In addition, ADHD has also been linked to the dysregulation of serotoninergic system, including the serotonin transporter (SERT), 5-hydroxytryptamine (5-HT), and monoamine oxidase A (MAO-A).173,174 Interestingly, vitamin D was shown to regulate the genes involved in the serotoninergic pathways, including 5-HT, SERT and MAO-A.38,175
The Link Between Inadequate Vitamin D and the Dysfunctions in ADHD-Related Neural Circuits
- Impairment in cognition: Response inhibition, which relies on circuits from frontal cortex to striatum and from frontal cortex to subthalamic circuits, was significantly impaired in ADHD to various degrees.176–178 Animals without enough vitamin D exhibited a phenotype of increased impulsive behavior as a result of impaired response inhibition.73,179 These indirect evidences suggested that the lack of vitamin D might have an impact on the cortical-striatal and cortical-subthalamic circuits, leading to impulsive behaviors. This hypothesis needs to be verified through a maneuver on the specific circuit.
- Impairment in executive function: Many studies suggested that the primary cause of executive dysfunction in ADHD is the impairment in frontal cortex.180–182 Furthermore, the frontal cortices of ADHD patients showed volume reduction as well as disruption in the networks.183,184 The DVD model rodents, on the other hand, had cortex that was thinner,56 implying a possible link between vitamin D deficiency/insufficiency and the executive dysfunction of ADHD. Experiments using techniques that can trace the brain circuitry underpinning executive function will be valuable to fully address the mechanisms.
Table 3 provides an overview of vitamin D’s effects on the ASD and ADHD. These findings offered potential rationales for how vitamin D affects the neurodevelopmental disorders. However, it is still unclear whether vitamin D deficiency/insufficiency directly contributes to the etiology of ADHD. Future studies will be required to further clarify the causal linkages, including animal experiments, prospective cohort studies and intervention trials.
 |
Table 3 The Effects of Vitamin D in ASD and ADHD |
Conclusions and Future Directions
As a neurosteroid hormone, vitamin D exerts multi-dimensional influence on the nervous system. It regulates synaptic transmission and synapse growth, as well as influences cognition and behaviors (Figure 2). Numerous epidemiological, molecular, and animal studies have revealed a link between vitamin D deficiency/insufficiency and an increased risk of ASD and ADHD. On the other hand, some studies demonstrated that vitamin D supplementation could reduce the symptoms in children with ASD and ADHD. Animal studies indicated that vitamin D might influence social process-related neural circuits like BLA-NAC and ACC-BLA pathways. Moreover, vitamin D might reduce the repetitive and aberrant social behaviors in ASD via regulating the mTOR pathway and oxytocin pathway. In addition, the prefrontal cortex circuits, as well as the dopaminergic and serotonergic pathways, which are frequently linked to the etiology of ADHD, may be impacted by inadequate vitamin D. More direct evidence on how vitamin D might affect the onset and progress of these disorders mechanistically is still missing. Nevertheless, vitamin D has the potential to be a treatment for neurodevelopmental disorders such as ASD and ADHD. It has the benefits including high safety, little side effects, and low cost. However, the precise therapeutic dose and effects, treatment duration and age of intervention for vitamin D remain to be determined. More clinical evidence is required before vitamin D can be extensively applied as a treatment strategy for ASD and ADHD. Most importantly, understanding how vitamin D contributes to the neurodevelopmental disorders will provide a solid foundation for the transition from the bench to the bedside.
Acknowledgments
We thank Wenbin Pang, Hongai Li, Qingshang Bi, Meijuan Wang, Dan Ye, and Xinmei Lin for kind suggestions to our manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by National Natural Science Foundation of China (NO. 31960170) and Hainan Province Science and Technology Special Fund (NO. ZDYF2020216) to L.X.; Hainan Province Science and Technology Special Fund (NO. ZDYF2021SHFZ088) and Hainan Major Science and Technology Projects (NO. ZDKJ2019010) to W.X.; Hainan Graduate Students Innovation Projects (NO. HYYS2021B19) to X.S.Y.; The open grant of NHC Key Laboratory of Tropical Disease Control, Hainan Medical University (NO. 2021NHCTDCKFKT22014) to P.C.R. The study also received financial support from Hainan Province Clinical Medical Center Grant (NO. QWYH202175) and the Excellent Talent Team of Hainan Province (NO. QRCBT202121).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am. 2010;39(2):243–253. doi:10.1016/j.ecl.2010.02.002
2. DeLuca HF. Vitamin D: the vitamin and the hormone. Fed Proc. 1974;33(11):2211–2219.
3. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6):1689s–1696s. doi:10.1093/ajcn/80.6.1689S
4. Moretti R, Morelli ME, Caruso P. Vitamin D in neurological diseases: a rationale for a pathogenic impact. Int J Mol Sci. 2018;19(8):2245. doi:10.3390/ijms19082245
5. Berridge MJ. Vitamin D deficiency: infertility and neurodevelopmental diseases (attention deficit hyperactivity disorder, autism, and schizophrenia). Am J Physiol Cell Physiol. 2018;314(2):C135–c151. doi:10.1152/ajpcell.00188.2017
6. DeLuca GC, Kimball SM, Kolasinski J, Ramagopalan SV, Ebers GC. Review: the role of vitamin D in nervous system health and disease. Neuropathol Appl Neurobiol. 2013;39(5):458–484. doi:10.1111/nan.12020
7. Ali A, Cui X, Eyles D. Developmental vitamin D deficiency and autism: putative pathogenic mechanisms. J Steroid Biochem Mol Biol. 2018;175:108–118. doi:10.1016/j.jsbmb.2016.12.018
8. Rihal V, Khan H, Kaur A, Singh TG. Vitamin D as therapeutic modulator in cerebrovascular diseases: a mechanistic perspectives. Crit Rev Food Sci Nutr. 2022;1–23. doi:10.1080/10408398.2022.2050349
9. Gezen-Ak D, Dursun E, Yilmazer S. The effect of Vitamin D treatment on Nerve Growth Factor (NGF) release from hippocampal neurons. Noro psikiyatri arsivi. 2014;51(2):157–162. doi:10.4274/npa.y7076
10. Faye PA, Poumeaud F, Miressi F, et al. Focus on 1,25-Dihydroxyvitamin D3 in the peripheral nervous system. Front Neurosci. 2019;13:348. doi:10.3389/fnins.2019.00348
11. Cui X, Eyles DW. Vitamin D and the central nervous system: causative and preventative mechanisms in brain disorders. Nutrients. 2022;14(20):1. doi:10.3390/nu14204353
12. Kalueff AV, Tuohimaa P. Neurosteroid hormone vitamin D and its utility in clinical nutrition. Curr Opin Clin Nutr Metab Care. 2007;10(1):12–19. doi:10.1097/MCO.0b013e328010ca18
13. Mayne PE, Burne THJ. Vitamin D in synaptic plasticity, cognitive function, and neuropsychiatric illness. Trends Neurosci. 2019;42(4):293–306. doi:10.1016/j.tins.2019.01.003
14. Gáll Z, Székely O. Role of Vitamin D in cognitive dysfunction: new molecular concepts and discrepancies between animal and human findings. Nutrients. 2021;13(11):4. doi:10.3390/nu13113672
15. Landel V, Stephan D, Cui X, Eyles D, Feron F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J Steroid Biochem Mol Biol. 2018;177:129–134. doi:10.1016/j.jsbmb.2017.09.008
16. Liu H, He Y, Beck J, et al. Defining vitamin D receptor expression in the brain using a novel VDR(Cre) mouse. J Comp Neurol. 2021;529(9):2362–2375. doi:10.1002/cne.25100
17. Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi:10.1016/j.jchemneu.2004.08.006
18. Shanahan M. The brain’s connective core and its role in animal cognition. Philos Trans R Soc Lond B Biol Sci. 2012;367(1603):2704–2714. doi:10.1098/rstb.2012.0128
19. Kandel ER, Schwartz JH, Jessell TM, Siegelbaum S, Hudspeth AJ, Mack S. Principles of Neural Science. Vol. 4. McGraw-hill New York; 2000.
20. Groves NJ, McGrath JJ, Burne TH. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr. 2014;34:117–141. doi:10.1146/annurev-nutr-071813-105557
21. Kasatkina LA, Gumenyuk VP, Sturm EM, Heinemann A, Bernas T, Trikash IO. Modulation of neurosecretion and approaches for its multistep analysis. Bio et Bio acta General Sub. 2018;1862(12):2701–2713. doi:10.1016/j.bbagen.2018.08.004
22. Kasatkina LA, Tarasenko AS, Krupko OO, Kuchmerovska TM, Lisakovska OO, Trikash IO. Vitamin D deficiency induces the excitation/inhibition brain imbalance and the proinflammatory shift. Int J Biochem Cell Biol. 2020;119:105665. doi:10.1016/j.biocel.2019.105665
23. Latimer CS, Brewer LD, Searcy JL, et al. Vitamin D prevents cognitive decline and enhances hippocampal synaptic function in aging rats. Proc Natl Acad Sci U S A. 2014;111(41):E4359–4366. doi:10.1073/pnas.1404477111
24. Landel V, Millet P, Baranger K, Loriod B, Féron F. Vitamin D interacts with Esr1 and Igf1 to regulate molecular pathways relevant to Alzheimer’s disease. Mol Neurodegener. 2016;11:22. doi:10.1186/s13024-016-0087-2
25. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769–780. doi:10.1002/pmic.200600392
26. Ferrer-Mayorga G, Niell N, Cantero R, et al. Vitamin D and Wnt3A have additive and partially overlapping modulatory effects on gene expression and phenotype in human colon fibroblasts. Sci Rep. 2019;9(1):8085. doi:10.1038/s41598-019-44574-9
27. Gooch H, Cui X, Anggono V, et al. 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Transl Psychiatry. 2019;9(1):281. doi:10.1038/s41398-019-0626-z
28. Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4(1):a011353. doi:10.1101/cshperspect.a011353
29. Krisanova N, Pozdnyakova N, Pastukhov A, et al. Vitamin D3 deficiency in puberty rats causes presynaptic malfunctioning through alterations in exocytotic release and uptake of glutamate/GABA and expression of EAAC-1/GAT-3 transporters. Food Chemical Toxicol. 2019;123:142–150. doi:10.1016/j.fct.2018.10.054
30. Eyles D, Almeras L, Benech P, et al. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol. 2007;103(3–5):538–545. doi:10.1016/j.jsbmb.2006.12.096
31. Féron F, Burne TH, Brown J, et al. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res Bull. 2005;65(2):141–148. doi:10.1016/j.brainresbull.2004.12.007
32. Groves NJ, Kesby JP, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Adult vitamin D deficiency leads to behavioural and brain neurochemical alterations in C57BL/6J and BALB/c mice. Behav Brain Res. 2013;241:120–131. doi:10.1016/j.bbr.2012.12.001
33. Jiang P, Zhang LH, Cai HL, et al. Neurochemical effects of chronic administration of calcitriol in rats. Nutrients. 2014;6(12):6048–6059.
34. Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett. 2009;461(2):155–158. doi:10.1016/j.neulet.2009.05.070
35. Pertile RA, Cui X, Eyles DW. Vitamin D signaling and the differentiation of developing dopamine systems. Neuroscience. 2016;333:193–203. doi:10.1016/j.neuroscience.2016.07.020
36. Trinko JR, Land BB, Solecki WB, et al. Vitamin D3: a role in dopamine circuit regulation, diet-induced obesity, and drug consumption. eNeuro. 2016;3(2):ENEURO.0122–15.2016. doi:10.1523/ENEURO.0122-15.2016
37. Kaneko I, Sabir MS, Dussik CM, et al. 1,25-Dihydroxyvitamin D regulates expression of the tryptophan hydroxylase 2 and leptin genes: implication for behavioral influences of vitamin D. FASEB J. 2015;29(9):4023–4035. doi:10.1096/fj.14-269811
38. Sabir MS, Haussler MR, Mallick S, et al. Optimal vitamin D spurs serotonin: 1,25-dihydroxyvitamin D represses serotonin reuptake transport (SERT) and degradation (MAO-A) gene expression in cultured rat serotonergic neuronal cell lines. Genes Nutr. 2018;13:19. doi:10.1186/s12263-018-0605-7
39. Baksi SN, Hughes MJ. Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res. 1982;242(2):387–390. doi:10.1016/0006-8993(82)90331-6
40. Ramagopalan SV, Heger A, Berlanga AJ, et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20(10):1352–1360. doi:10.1101/gr.107920.110
41. Wang TT, Tavera-Mendoza LE, Laperriere D, et al. Large-scale in silico and microarray-based identification of direct 1,25-dihydroxyvitamin D3 target genes. Molecular Endocrinology. 2005;19(11):2685–2695. doi:10.1210/me.2005-0106
42. Carlberg C. Genome-wide (over)view on the actions of vitamin D. Front Physiol. 2014;5:167. doi:10.3389/fphys.2014.00167
43. Brown J, Bianco JI, McGrath JJ, Eyles DW. 1,25-dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons. Neurosci Lett. 2003;343(2):139–143. doi:10.1016/S0304-3940(03)00303-3
44. Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34(1):47–64. doi:10.1016/j.yfrne.2012.07.001
45. Cui X, McGrath JJ, Burne THJ, Eyles DW. Vitamin D and schizophrenia: 20 years on. Mol Psychiatry. 2021;26(7):2708–2720. doi:10.1038/s41380-021-01025-0
46. Kuźma E, Soni M, Littlejohns TJ, et al. Vitamin D and memory decline: two population-based prospective studies. JAD. 2016;50(4):1099–1108. doi:10.3233/JAD-150811
47. Wilson VK, Houston DK, Kilpatrick L, et al. Relationship between 25-hydroxyvitamin D and cognitive function in older adults: the health, aging and body composition study. J Am Geriatr Soc. 2014;62(4):636–641. doi:10.1111/jgs.12765
48. Slinin Y, Paudel M, Taylor BC, et al. Association between serum 25(OH) vitamin D and the risk of cognitive decline in older women. J Gerontol a Biol Sci Med Sci. 2012;67(10):1092–1098. doi:10.1093/gerona/gls075
49. Jia J, Hu J, Huo X, Miao R, Zhang Y, Ma F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: a randomised, double-blind, placebo-controlled trial. J Neurol Neurosurg Psychiatry. 2019;90(12):1347–1352. doi:10.1136/jnnp-2018-320199
50. Kang JH, Vyas CM, Okereke OI, et al. Effect of vitamin D on cognitive decline: results from two ancillary studies of the VITAL randomized trial. Sci Rep. 2021;11(1):23253. doi:10.1038/s41598-021-02485-8
51. Rossom RC, Espeland MA, Manson JE, et al. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J Am Geriatr Soc. 2012;60(12):2197–2205. doi:10.1111/jgs.12032
52. Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of Vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi:10.1001/jama.2020.16909
53. Kato S, Takeyama K, Kitanaka S, Murayama A, Sekine K, Yoshizawa T. In vivo function of VDR in gene expression-VDR knock-out mice. J Steroid Biochem Mol Biol. 1999;69(1–6):247–251. doi:10.1016/S0960-0760(99)00042-4
54. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–776. doi:10.1210/er.2008-0004
55. Panda DK, Miao D, Tremblay ML, et al. Targeted ablation of the 25-hydroxyvitamin D 1alpha -hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98(13):7498–7503. doi:10.1073/pnas.131029498
56. Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118(3):641–653. doi:10.1016/S0306-4522(03)00040-X
57. Al-Harbi AN, Khan KM, Rahman A. Developmental Vitamin D deficiency affects spatial learning in Wistar rats. J Nutr. 2017;147(9):1795–1805. doi:10.3945/jn.117.249953
58. Ali A, Vasileva S, Langguth M, et al. Developmental Vitamin D deficiency produces behavioral phenotypes of relevance to Autism in an animal model. Nutrients. 2019;11(5):1187. doi:10.3390/nu11051187
59. Nemere I, Farach-Carson MC, Rohe B, et al. Ribozyme knockdown functionally links a 1,25(OH)2D3 membrane binding protein (1,25D3-MARRS) and phosphate uptake in intestinal cells. Proc Natl Acad Sci U S A. 2004;101(19):7392–7397. doi:10.1073/pnas.0402207101
60. Hu W, Zhang L, Li MX, et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy. 2019;15(4):707–725. doi:10.1080/15548627.2018.1557835
61. Chen J, Olivares-Navarrete R, Wang Y, Herman TR, Boyan BD, Schwartz Z. Protein-disulfide isomerase-associated 3 (Pdia3) mediates the membrane response to 1,25-dihydroxyvitamin D3 in osteoblasts. J Biol Chem. 2010;285(47):37041–37050. doi:10.1074/jbc.M110.157115
62. Byrne JH, Voogt M, Turner KM, Eyles DW, McGrath JJ, Burne TH. The impact of adult vitamin D deficiency on behaviour and brain function in male Sprague-Dawley rats. PLoS One. 2013;8(8):e71593. doi:10.1371/journal.pone.0071593
63. Durk MR, Han K, Chow EC, et al. 1α,25-Dihydroxyvitamin D3 reduces cerebral amyloid-β accumulation and improves cognition in mouse models of Alzheimer’s disease. J Neuros. 2014;34(21):7091–7101. doi:10.1523/JNEUROSCI.2711-13.2014
64. Morello M, Landel V, Lacassagne E, et al. Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2018;55(8):6463–6479. doi:10.1007/s12035-017-0839-1
65. Eyles DW. Vitamin D: brain and behavior. JBMR Plus. 2021;5(1):e10419. doi:10.1002/jbm4.10419
66. Taghizadeh M, Djazayery A, Salami M, Eshraghian MR, Zavareh SA. Vitamin-D-free regimen intensifies the spatial learning deficit in Alzheimer’s disease. Int J Neurosci. 2011;121(1):16–24. doi:10.3109/00207454.2010.523132
67. Groves NJ, Burne TH. Sex-specific attentional deficits in adult vitamin D deficient BALB/c mice. Physiol Behav. 2016;157:94–101. doi:10.1016/j.physbeh.2016.01.033
68. Womble PD, Hodges SL, Nolan SO, et al. A vitamin D enriched diet attenuates sex-specific behavioral deficits, increases the lifespan, but does not rescue bone abnormalities in a mouse model of cortical dysplasia. Epilepsy Andbeha. 2021;124:108297. doi:10.1016/j.yebeh.2021.108297
69. Satterstrom FK, Kosmicki JA, Wang J, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of Autism. Cell. 2020;180(3):568–584.e523. doi:10.1016/j.cell.2019.12.036
70. Burne TH, Becker A, Brown J, Eyles DW, Mackay-Sim A, McGrath JJ. Transient prenatal Vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav Brain Res. 2004;154(2):549–555. doi:10.1016/j.bbr.2004.03.023
71. Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: an animal model of schizophrenia. Biol Psychiatry. 2006;60(6):591–596. doi:10.1016/j.biopsych.2006.02.033
72. Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187(2):343–350. doi:10.1016/j.bbr.2007.09.032
73. Turner KM, Young JW, McGrath JJ, Eyles DW, THJBbr B. Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behavioural Brain Research. 2013;242:47–53. doi:10.1016/j.bbr.2012.12.029
74. Pan P, Jin DH, Chatterjee-Chakraborty M, et al. The effects of vitamin D3 during pregnancy and lactation on offspring physiology and behavior in Sprague-Dawley rats. Dev Psychobiol. 2014;56(1):12–22. doi:10.1002/dev.21086
75. Yates NJ, Tesic D, Feindel KW, et al. Vitamin D is crucial for maternal care and offspring social behaviour in rats. J Endocrinol. 2018;237(2):73–85. doi:10.1530/JOE-18-0008
76. Godel JC; Canadian Paediatric Society. Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health. 2007;12(7):583–598. doi:10.1093/pch/12.7.583
77. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–165. doi:10.1007/s11154-017-9424-1
78. Vearing RM, Hart KH, Charlton K, et al.. Vitamin D status of the British African-Caribbean residents: analysis of the UK biobank cohort. Nutrients. 2021;13(11). doi:10.3390/nu13114104
79. Zhao Y, Zhao W, Hao Q, et al. Vitamin D status and obesity markers in older adults: results from West China health and aging trends study. BMC Geriatr. 2021;21(1):528. doi:10.1186/s12877-021-02449-7
80. Vearing RM, Hart KH, Darling AL, et al. Global perspective of the Vitamin D status of African-Caribbean populations: a systematic review and meta-analysis. Eur J Clin Nutr. 2022;76(4):516–526. doi:10.1038/s41430-021-00980-9
81. Pang X, Yang Z, Wang J, et al. Relationship between Serum 25OH-Vitamin D2 Level and Vitamin D status of children aged 3–5 years in China. Nutrients. 2021;13(11):1. doi:10.3390/nu13114135
82. Weiler HA, Vanstone CA, Razaghi M, et al. Disparities in Vitamin D status of newborn infants from a diverse sociodemographic population in Montreal, Canada. J Nutr. 2022;152(1):255–268. doi:10.1093/jn/nxab344
83. Li LL, Li XN, Jia FY, et al. 李娈娈, 李晓南, 贾飞勇, 等. 中国部分地区7岁以下儿童维生素D营养状况分析 [J] . 中华儿科杂志 [Analysis of vitamin D status among children under 7 years of age in some regions of China]. Zhonghua er ke za zhi. 2022;60(5):413–420. Chinese. doi:10.3760/cma.j.cn112140-20220126-00087
84. Christoph P, Challande P, Raio L, Surbek D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med Wkly. 2020;150:w20238. doi:10.4414/smw.2020.20238
85. Gould JF, Anderson AJ, Yelland LN, et al. Association of cord blood vitamin D with early childhood growth and neurodevelopment. J Paediatr Child Health. 2017;53(1):75–83. doi:10.1111/jpc.13308
86. Morales E, Guxens M, Llop S, et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics. 2012;130(4):e913–920. doi:10.1542/peds.2011-3289
87. Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics. 2012;129(3):485–493. doi:10.1542/peds.2011-2644
88. Tylavsky FA, Kocak M, Murphy LE, et al. Gestational Vitamin 25(OH)D status as a risk factor for receptive language development: a 24-month, longitudinal, observational study. Nutrients. 2015;7(12):9918–9930. doi:10.3390/nu7125499
89. Cashman KD, Dowling KG, Škrabáková Z, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–1044. doi:10.3945/ajcn.115.120873
90. Kiely ME, Wagner CL, Roth DE. Vitamin D in pregnancy: where we are and where we should go. J Steroid Biochem Mol Biol. 2020;201:105669. doi:10.1016/j.jsbmb.2020.105669
91. Basatemur E, Horsfall L, Marston L, Rait G, Sutcliffe A. Trends in the diagnosis of Vitamin D deficiency. Pediatrics. 2017;139(3). doi:10.1542/peds.2016-2748
92. Battle DE. Diagnostic and statistical manual of mental disorders (DSM). CoDAS. 2013;25(2):191–192. doi:10.1590/s2317-17822013000200017
93. Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160–179. doi:10.1002/aur.239
94. Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35(4):100–136.
95. Kittana M, Ahmadani A, Stojanovska L, Attlee A. The role of Vitamin D supplementation in children with autism spectrum disorder: a narrative review. Nutrients. 2021;14(1):26. doi:10.3390/nu14010026
96. Saad K, Abdel-Rahman AA, Elserogy YM, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19(8):346–351. doi:10.1179/1476830515Y.0000000019
97. Qi X, Yang T, Chen J, et al. Vitamin D status is primarily associated with core symptoms in children with autism spectrum disorder: a multicenter study in China. Psychiatry Res. 2022;317:114807. doi:10.1016/j.psychres.2022.114807
98. Feng J, Shan L, Du L, et al. Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder. Nutr Neurosci. 2017;20(5):284–290. doi:10.1080/1028415X.2015.1123847
99. Kerley CP, Power C, Gallagher L, Coghlan D. Lack of effect of vitamin D(3) supplementation in autism: a 20-week, placebo-controlled RCT. Arch Dis Child. 2017;102(11):1030–1036. doi:10.1136/archdischild-2017-312783
100. Wang J, Huang H, Liu C, et al. Research progress on the role of Vitamin D in Autism spectrum disorder. Front Behav Neurosci. 2022;16:859151. doi:10.3389/fnbeh.2022.859151
101. Waddington CH. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183(4676):1654–1655. doi:10.1038/1831654a0
102. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392(10146):508–520. doi:10.1016/S0140-6736(18)31129-2
103. Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16(9):551–563. doi:10.1038/nrn3992
104. Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi:10.1016/j.brainres.2009.09.120
105. Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. doi:10.1038/nn.2741
106. Tang G, Gudsnuk K, Kuo SH, et al. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83(5):1131–1143. doi:10.1016/j.neuron.2014.07.040
107. Santini E, Klann E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Sci Signal. 2014;7(349):re10. doi:10.1126/scisignal.2005832
108. Sala C, Vicidomini C, Bigi I, Mossa A, Verpelli C. Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders. J Neurochem. 2015;135(5):849–858. doi:10.1111/jnc.13232
109. Moreira ES, Silva IM, Lourenço N, et al.. Detection of small copy number variations (CNVs) in autism spectrum disorder (ASD) by custom array comparative genomic hybridization (aCGH). Res Autism Spectr Disord. 2016;23:145–151.
110. Cass WA, Peters LE, Fletcher AM, Yurek DM. Evoked dopamine overflow is augmented in the striatum of calcitriol treated rats. Neurochem Int. 2012;60(2):186–191. doi:10.1016/j.neuint.2011.11.010
111. Bauman MD, Schumann CM. Advances in nonhuman primate models of autism: integrating neuroscience and behavior. Exp Neurol. 2018;299(Pt A):252–265. doi:10.1016/j.expneurol.2017.07.021
112. Ribeiro MC, Moore SM, Kishi N, Macklis JD, MacDonald JL. Vitamin D supplementation rescues aberrant NF-κB pathway activation and partially ameliorates rett syndrome phenotypes in Mecp2 mutant mice. eNeuro. 2020;7(3):5. doi:10.1523/ENEURO.0167-20.2020
113. Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D’Arcangelo G. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech. 2009;2(7–8):389–398. doi:10.1242/dmm.002386
114. Lugo JN, Smith GD, Arbuckle EP, et al. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front Mol Neurosci. 2014;7:27. doi:10.3389/fnmol.2014.00027
115. Tariq K, Cullen E, Getz SA, et al. Disruption of mTORC1 rescues neuronal overgrowth and synapse function dysregulated by Pten loss. Cell Rep. 2022;41(5):111574. doi:10.1016/j.celrep.2022.111574
116. Lipton JO, Sahin M. The neurology of mTOR. Neuron. 2014;84(2):275–291. doi:10.1016/j.neuron.2014.09.034
117. Lopatina OL, Komleva YK, Gorina YV, et al. Oxytocin and excitation/inhibition balance in social recognition. Neuropeptides. 2018;72:1–11. doi:10.1016/j.npep.2018.09.003
118. Lee E, Lee J, Kim E. Excitation/Inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry. 2017;81(10):838–847. doi:10.1016/j.biopsych.2016.05.011
119. Zhao H, Jiang YH, Zhang YQ. Modeling autism in non-human primates: opportunities and challenges. Autism Res. 2018;11(5):686–694. doi:10.1002/aur.1945
120. Hori K, Yamashiro K, Nagai T, et al. AUTS2 regulation of synapses for proper synaptic inputs and social communication. iScience. 2020;23(6):101183. doi:10.1016/j.isci.2020.101183
121. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi:10.1176/appi.ajp.2010.09091379
122. Lilienfeld SO. The Research Domain Criteria (RDoC): an analysis of methodological and conceptual challenges. Behav Res Ther. 2014;62:129–139. doi:10.1016/j.brat.2014.07.019
123. Takumi T, Tamada K, Hatanaka F, Nakai N, Bolton PF. Behavioral neuroscience of autism. Neurosci Biobehav Rev. 2020;110:60–76. doi:10.1016/j.neubiorev.2019.04.012
124. Cox A, Kohls G, Naples AJ, et al. Diminished social reward anticipation in the broad autism phenotype as revealed by event-related brain potentials. Soc Cogn Affect Neurosci. 2015;10(10):1357–1364. doi:10.1093/scan/nsv024
125. Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord. 2012;42(2):147–160. doi:10.1007/s10803-011-1221-1
126. Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3(7):563–573. doi:10.1038/nrn875
127. Hu RK, Zuo Y, Ly T, et al. An amygdala-to-hypothalamus circuit for social reward. Nat Neurosci. 2021;24(6):831–842. doi:10.1038/s41593-021-00828-2
128. Bagot RC, Parise EM, Peña CJ, et al. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi:10.1038/ncomms8062
129. Chen YC, Huang YH, Yen NS. Role of anterior midcingulate cortex in self-reward representation and reward allocation judgments within social context. Hum Brain Mapp. 2022;43(7):2377–2390. doi:10.1002/hbm.25793
130. Veenstra TD, Prüfer K, Koenigsberger C, Brimijoin SW, Grande JP, Kumar R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res. 1998;804(2):193–205. doi:10.1016/S0006-8993(98)00565-4
131. Walbert T, Jirikowski GF, Prüfer K. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the limbic system of the rat. Hormone Metab Res. 2001;33(9):525–531. doi:10.1055/s-2001-17210
132. Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7(2):160–172. doi:10.1093/scan/nsq095
133. Folkes OM, Báldi R, Kondev V, et al. An endocannabinoid-regulated basolateral amygdala-nucleus accumbens circuit modulates sociability. J Clin Invest. 2020;130(4):1728–1742. doi:10.1172/JCI131752
134. Guida F, Boccella S, Belardo C, et al. Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immun. 2020;85:128–141. doi:10.1016/j.bbi.2019.04.006
135. Smith A. The empathy imbalance hypothesis of autism: a theoretical approach to cognitive and emotional empathy in autistic development. Psychol Res. 2009;59(3):489–510.
136. Smith ML, Asada N, Malenka RC. Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science. 2021;371(6525):153–159. doi:10.1126/science.abe3040
137. Kim SW, Kim M, Baek J, et al. Hemispherically lateralized rhythmic oscillations in the cingulate-amygdala circuit drive affective empathy in mice. Neuron. 2023;111(3):418–429.e414. doi:10.1016/j.neuron.2022.11.001
138. Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi:10.1146/annurev-neuro-071013-014048
139. Bartolotti J, Sweeney JA, Mosconi MW. Functional brain abnormalities associated with comorbid anxiety in autism spectrum disorder. Dev Psychopathol. 2020;32(4):1273–1286. doi:10.1017/S0954579420000772
140. Sultan S. Neuroimaging changes associated with vitamin D Deficiency - a narrative review. Nutr Neurosci. 2022;25(8):1650–1658. doi:10.1080/1028415X.2021.1888206
141. Foucault G, Duval GT, Simon R, Beauchet O, Dinomais M, Annweiler C. Serum Vitamin D and cingulate cortex thickness in older adults: quantitative MRI of the brain. Curr Alzheimer Res. 2019;16(11):1063–1071. doi:10.2174/1567205016666191113124356
142. Fuccillo MV. Striatal circuits as a common node for autism pathophysiology. Front Neurosci. 2016;10:27. doi:10.3389/fnins.2016.00027
143. Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17(1):45–59. doi:10.1038/nrn.2015.8
144. Wu WL, Cheng SJ, Lin SH, Chuang YC, Huang EY, Chen CC. The effect of ASIC3 knockout on corticostriatal circuit and mouse self-grooming behavior. Front Cell Neurosci. 2019;13:86. doi:10.3389/fncel.2019.00086
145. Pagani M, Barsotti N, Bertero A, et al. mTOR-related synaptic pathology causes autism spectrum disorder-associated functional hyperconnectivity. Nat Commun. 2021;12(1):6084. doi:10.1038/s41467-021-26131-z
146. Cataldo I, Azhari A, Esposito G. A review of oxytocin and arginine-vasopressin receptors and their modulation of autism spectrum disorder. Front Mol Neurosci. 2018;11:27. doi:10.3389/fnmol.2018.00027
147. Caria A, Ciringione L, Falco S. Morphofunctional alterations of the hypothalamus and social behavior in autism spectrum disorders. Brain Sci. 2020;10(7):435. doi:10.3390/brainsci10070435
148. Kong XJ, Liu J, Liu K, et al. Probiotic and oxytocin combination therapy in patients with autism spectrum disorder: a randomized, double-blinded, placebo-controlled pilot trial. Nutrients. 2021;13(5):1552. doi:10.3390/nu13051552
149. Parker KJ, Oztan O, Libove RA, et al.. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci Transl Med. 2019;11(491). doi:10.1126/scitranslmed.aau7356
150. Sikich L, Kolevzon A, King BH, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med. 2021;385(16):1462–1473. doi:10.1056/NEJMoa2103583
151. Peñagarikano O, Lázaro MT, Lu XH, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7(271):271ra278. doi:10.1126/scitranslmed.3010257
152. Choe KY, Bethlehem RAI, Safrin M, et al. Oxytocin normalizes altered circuit connectivity for social rescue of the Cntnap2 knockout mouse. Neuron. 2022;110(5):795–808.e796. doi:10.1016/j.neuron.2021.11.031
153. Harony-Nicolas H, Kay M, du Hoffmann J, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. eLife. 2017;2017:6.
154. Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28(6):2398–2413. doi:10.1096/fj.13-246546
155. Sisley SR, Arble DM, Chambers AP, et al. Hypothalamic Vitamin D improves glucose homeostasis and reduces weight. Diabetes. 2016;65(9):2732–2741. doi:10.2337/db16-0309
156. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8(5):571–578. doi:10.1038/nn1455
157. Matthews M, Nigg JT, Fair DA. Attention deficit hyperactivity disorder. Curr Top Behav Neurosci. 2014;16:235–266.
158. Sharma A, Couture J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann Pharmacother. 2014;48(2):209–225. doi:10.1177/1060028013510699
159. Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–1250. doi:10.1016/S0140-6736(15)00238-X
160. Faraone SV, Biederman J, Milberger S. An exploratory study of ADHD among second-degree relatives of ADHD children. Biol Psychiatry. 1994;35(6):398–402.
161. Banerjee TD, Middleton F, Faraone SV. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediat. 2007;96(9):1269–1274. doi:10.1111/j.1651-2227.2007.00430.x
162. Nigg JT. Temperament and developmental psychopathology. J Child Psychol Psychiatry. 2006;47(3–4):395–422. doi:10.1111/j.1469-7610.2006.01612.x
163. Morales E, Julvez J, Torrent M, et al. Vitamin D in pregnancy and attention deficit hyperactivity disorder-like symptoms in childhood. Epidemiology. 2015;26(4):458–465. doi:10.1097/EDE.0000000000000292
164. Sucksdorff M, Brown AS, Chudal R, et al. Maternal Vitamin D levels and the risk of offspring attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2021;60(1):142–151.e142. doi:10.1016/j.jaac.2019.11.021
165. Ideraabdullah FY, Belenchia AM, Rosenfeld CS, et al. Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD). J Endocrinol. 2019;241:R65–R80. doi:10.1530/JOE-18-0541
166. Khoshbakht Y, Bidaki R, Salehi-Abargouei A. Vitamin D status and attention deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. Adv Nutrit. 2018;9(1):9–20. doi:10.1093/advances/nmx002
167. Gan J, Galer P, Ma D, Chen C, Xiong T. The effect of Vitamin D supplementation on attention-deficit/hyperactivity disorder: a systematic review and meta-analysis of randomized controlled trials. J Child Adolesc Psychopharmacol. 2019;29(9):670–687. doi:10.1089/cap.2019.0059
168. Elshorbagy HH, Barseem NF, Abdelghani WE, et al. Impact of Vitamin D supplementation on attention-deficit hyperactivity disorder in children. Ann Pharmacother. 2018;52(7):623–631. doi:10.1177/1060028018759471
169. Dehbokri N, Noorazar G, Ghaffari A, Mehdizadeh G, Sarbakhsh P, Ghaffary S. Effect of vitamin D treatment in children with attention-deficit hyperactivity disorder. WJP. 2019;15(1):78–84. doi:10.1007/s12519-018-0209-8
170. Naeini AA, Fasihi F, Najafi M, Ghazvini MR, Hasanzadeh AJEJo IM. The effects of vitamin D supplementation on ADHD (Attention Deficit Hyperactivity Disorder) in 6–13 year-old students: a randomized, double-blind, placebo-controlled study. Eur J Integr Med. 2019;25:28–33.
171. Mohammadpour N, Jazayeri S, Tehrani-Doost M, et al. Effect of vitamin D supplementation as adjunctive therapy to methylphenidate on ADHD symptoms: a randomized, double blind, placebo-controlled trial. Nutr Neurosci. 2018;21(3):202–209. doi:10.1080/1028415X.2016.1262097
172. Hawi Z, Cummins TD, Tong J, et al. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 2015;20(3):289–297. doi:10.1038/mp.2014.183
173. Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet. 2009;126(1):51–90. doi:10.1007/s00439-009-0694-x
174. Chatterjee M, Saha S, Sinha S, Mukhopadhyay K. A three-pronged analysis confirms the association of the serotoninergic system with attention deficit hyperactivity disorder. WJP. 2022;18(12):825–834. doi:10.1007/s12519-022-00614-5
175. Tekes K, Gyenge M, Folyovich A, Csaba G. Influence of neonatal vitamin A or vitamin D treatment on the concentration of biogenic amines and their metabolites in the adult rat brain. Hormone Metab Res. 2009;41(4):277–280. doi:10.1055/s-0028-1103287
176. Doyle AE. Executive functions in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2006;67(Suppl 8):21–26.
177. Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27(5):393–402. doi:10.1023/A:1021980002473
178. Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi:10.1016/j.tics.2004.02.010
179. Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne THJPO. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS One. 2012;7(4):e35896. doi:10.1371/journal.pone.0035896
180. Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. J Child Psychol Psychiatry. 1996;37(1):51–87. doi:10.1111/j.1469-7610.1996.tb01380.x
181. Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi:10.1037/0033-2909.121.1.65
182. Buss AT, Spencer JP. Changes in frontal and posterior cortical activity underlie the early emergence of executive function. Dev Sci. 2018;21(4):e12602. doi:10.1111/desc.12602
183. Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288(14):1740–1748. doi:10.1001/jama.288.14.1740
184. Firouzabadi FD, Ramezanpour S, Firouzabadi MD, Yousem IJ, Puts NAJ, Yousem DM. Neuroimaging in attention-deficit/hyperactivity disorder: recent advances. AJR Am J Roentgenol. 2022;218(2):321–332. doi:10.2214/AJR.21.26316
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.