Back to Journals » International Journal of General Medicine » Volume 16
The Symptoms of Benign Prostatic Hyperplasia Patients with Stromal-Dominated Hyperplasia Nodules May Be Associated with Prostate Fibrosis
Authors Cao Y, Zhang H , Tu GL, Tian Y , Tang XH, Tang L, Luo MX, Wang YD, Wang Z, Xia SJ, Luo GH
Received 30 November 2022
Accepted for publication 3 March 2023
Published 1 April 2023 Volume 2023:16 Pages 1181—1191
DOI https://doi.org/10.2147/IJGM.S395705
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Ying Cao,1,* Heng Zhang,2,* Gui-Lan Tu,3 Ye Tian,2 Xiao-Hu Tang,2 Lei Tang,1 Mu-Xia Luo,1 Yan-Dong Wang,2 Zhen Wang,2 Shu-Jie Xia,4 Guang-Heng Luo2
1Guizhou University Medical College, Guiyang, 550025, People’s Republic of China; 2Department of Urology, Guizhou Provincial People’s Hospital, Guiyang, 550002, People’s Republic of China; 3Department of Pathology, Guizhou Provincial People’s Hospital, Guiyang, 550002, People’s Republic of China; 4Department of Urology, Shanghai First People’s Hospital, Shanghai Jiaotong University, Shanghai, 200080, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guang-Heng Luo, Email [email protected]
Objective: The aim of the present study was to observe the effect of the stroma proportion in hyperplasia nodules on the clinical symptoms of benign prostatic hyperplasia (BPH) patients and to identify the different genes and pathways in prostatic hyperplasia nodules between patients with epithelial-dominated hyperplasia (EDH) and stromal-dominated hyperplasia (SDH) nodules.
Methods: Sixty-seven BPH patient samples underwent transurethral resection of the prostate (TURP) were collected and retrospectively analyzed. The differences in clinical parameters between the EDH and SDH groups were investigated. Collagen fiber percentage was assessed, and the correlation with clinical parameters was evaluated. mRNA sequencing in hyperplasia nodules of 8 BPH patients was performed, and differentially expressed genes (DEGs) between the EDH and SDH groups were screened. These DEGs were analyzed using GO, KEGG and PPI analysis.
Results: The results showed the IPSS was significantly higher in the SDH group than in the EDH group (p < 0.01). The collagen fiber percentage of BPH nodules was higher in the SDH group than in the EDH group (p < 0.05), and the collagen fiber percentage was positively correlated with the IPSS (r = 0.4058, p = 0.0007). A total of 172 DEGs were obtained, including 63 up-regulated genes and 109 down-regulated genes. GO and KEGG pathway enrichment analyses showed DEGs were mainly enriched in extracellular matrix structural constituents. The top 10 hub genes were associated to the components of extracellular matrix and fibrosis.
Conclusion: These results suggested that the symptoms of BPH patients with SDH nodules may be associated with prostate fibrosis and fibrosis may be a significant contributing factor in BPH/LUTS patients with SDH nodules.
Keywords: benign prostate hyperplasia, lower urinary tract symptoms, prostate fibrosis, stroma, pathology
Introduction
Benign prostatic hyperplasia (BPH) is an age-related disease frequently associated with lower urinary tract symptoms (LUTS) that involves hyperplasia of epithelial and stromal tissues within the prostate glade. Surgical resection or medical approaches using anti-androgens or smooth muscle relaxers have proven effective for BPH patients to improve urinary flow and relieve LUTS.1 Despite the general effectiveness of current treatment method, a large proportion of patients are still fail to respond to medical therapy, unable to withstand surgery for physical reasons, or exhibit good initial responses to drug therapy before subsequent disease progression, which suggest other potentially pathobiology processes of prostate might also contribute to the development and progression of BPH/LUTS.2 Recently, studies showed that prostate fibrosis was associated with LUTD, and prostate fibrosis may be a previously unrecognized biological processes that contributes to BPH/LUTS but remains untargeted in the medical management of LUTS.3 Although the basic science research community is aware of the role of fibrosis and its potential as a therapeutic target, it is not yet targeted at the clinical level. Fibrosis is characterized with the fibroblasts proliferation and excessive secretion and accumulation of collagen fiber, which add sufficient rigidity to periurethral region and impact urinary function. The prostate tissue is composed of acini epithelium and stroma, including fibroblasts and smooth muscle. Histologically, BPH is characterized by overgrowth of the epithelium and stroma and is always considered to be a major stromal hyperplasia in BPH nodules. Therefore, in theory, the proportion of stroma in hyperplastic nodules may be associated with the development of prostate fibrosis and the symptoms of LUTS. To explore this problem, we investigated the proportion of stroma in hyperplastic nodules of 67 BPH patients to evaluate the association with the clinical parameters of patients. In addition, we also performed mRNA sequencing of prostatic nodules in 8 BPH patients to identify the differentially expressed genes (DEGs) and pathways between epithelial-dominated hyperplasia (EDH) and stromal-dominated hyperplasia (SDH) of nodules.
Materials and Methods
Patients and Groups
A total of 67 prostate samples from elderly male BPH patients who failed medical therapy and underwent transurethral resection of the prostate (TURP) at Guizhou Provincial People’s Hospital from August 2017 to December 2019 were collected and retrospectively analyzed. The age is from to 58 to 89 years, and the average age is 71.28 ± 7.43 years. Each BPH patient who had been pathologically proven by pathologist had complete clinical information, including age, prostate volume, preoperative international prostate symptom score (IPSS), quality of life score (QOL), maximum urinary flow rate (Qmax) and bladder outlet obstruction index (BOOI). These patients were divided into two groups, ie, the epithelial-dominated hyperplasia (EDH) group and the stromal-dominated hyperplasia (SDH) group, according to the stroma percentage in hyperplasia nodules. Briefly, the HE-stained sections were used for image analysis under the image analysis system, the stroma contours were delineated with the cursor, the images were collected and segmented, and the percentage of stroma area was calculated. The stroma percentage of the microscopic-field area <50% was defined as EDH, and the stroma percentage of the microscopic-field area ≥50% was defined as SDH. Furthermore, 8 prostate samples from elderly male BPH patients who failed medical therapy and underwent TURP at Guizhou Provincial People’s Hospital from Nov. 2021 were collected. The age is from to 67 to 77 years, and the average age is 71.25 ± 4.1 years. Each BPH patient who had been pathologically proven by pathologist and were also divided into the EDH (4 cases) group and the SDH (4 cases) group. The prostate samples of these 8 cases were collected and performed mRNA sequencing.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from each subject after a thorough explanation and all patients signed a written informed consent form prior to study commencement. The experiments were approved by the Human Medicine Experimental Ethical of Guizhou University (No. HMEE-GZU-2022-T005).
The Percentage of Collagen Fibers Was Evaluated by Masson’s Trichrome Staining
Masson’s trichrome staining was used to evaluate the percentage of collagen fibers. According to instructions (Solarbio, Beijing, CHN), after placing in Weigert’s working hematoxylin for 10 minutes (min), washing with distilled water, staining with Masson’s trichrome solution for 10 min, and rinsing in 95% alcohol, dehydrating, clearing, coverslipping, we observed the section under light microscopy. Collagen fiber was stained blue, and smooth muscle was stained red, respectively. The image analysis system (Image-Pro Plus 6.0) was used to evaluate the percentage of collagen fibers and smooth muscle. The collagen fiber (blue) and smooth muscle (red) contours were delineated with the cursor, images were collected and segmented, and the collagen fiber percentage and smooth muscle percentage of the microscopic-field area were calculated.
mRNA Sequencing of the Prostate and Bioinformatics Analysis
The prostate samples were collected in sterile tubes with TRIzol reagent. Then, the quality of the RNA samples was analyzed by an Agilent Bioanalyzer (Agilent), and the cDNA libraries were generated using TruSeq RNA Sample Preparation (Illumina). Each library was sequenced using single reads on a HiSeq2000 (Illumina). R software 3.4.0 (https://www.r-project.org/) was applied to perform to analyze the dataset and search DEGs between EHD and SHD groups. Genes with correcting P < 0.05 and log fold change (FC) >1 were deemed as DEGs. The Gene Ontology (GO), including biological process (BP), cellular component (CC), and molecular function (MF), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses of the DEGs were carried out using DAVID database4 (https://david.ncifcrf.gov/). A p value <0.05 was specified for statistical significance. Protein–protein interaction (PPI) network analysis of DEPs was constructed by the STRING database (http://string-db.org/), and a visualization analysis was performed. According to the centrality score, the key nodes in PPI network were determined, and then the top ten hub genes were deduced.
Statistical Analysis
Statistical analysis was performed with SPSS software version 20.0 (IBM, NY, USA). Data were expressed as the means ± standard deviations (SD). Means of two groups were compared by two-tailed Student’s t-test, and correlations were evaluated by Spearman correlation analysis. A statistically significant difference was accepted when p < 0.05.
Results
The Difference in BPH Patient Clinical Parameters Between the EDH and SDH Groups
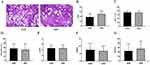
We calculated the percentage of stroma area in hyperplasia nodules, and defined 20 cases EDH patients (the stroma percentage of the microscopic-field area <50%), and 47 cases SDH patients (the stroma percentage of the microscopic-field area ≥50%). In this study, 67 BPH patients had 20 cases of EDH (29.9%) and 47 cases of SDH (70.1%) (Figure 1A). The IPSS was significantly higher in the SDH group than in the EDH group (p < 0.01) (Figure 1B). However, there were no significant differences in the age, prostate size, QOL, Qmax or BOOI between the EDP and SDP groups (Figure 1C–G). The clinical parameters of the BPH patients in the EDP and SDP groups are shown in Table 1.
 |
Table 1 Comparison of Clinical Parameters in 67 BPH Patients Between EDH and SDH Groups (mean±SD) |
Collagen Fibers Percentage in the EDH and SDH Groups and Correlation Analysis with BPH Patient Clinical Parameters
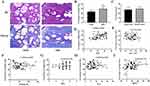
Collagen fiber and smooth muscle in prostatic hyperplasia nodules were stained blue and red by Masson’s trichrome staining (Figure 2A), and the percentages of collagen fibers and smooth muscle were evaluated. The data showed that collagen fiber percentage in hyperplasia nodules was higher in the SDH group than in the EDH group (p < 0.01) (Figure 2B), and the percentages of collagen fibers and smooth muscle were roughly the same (Figure 2C). Furthermore, we performed a correlation analysis between the percentage of collagen fibers and the clinical parameters of patients. Our results showed that the percentage of collagen fibers was positively correlated with the IPSS (r = 0.4058, p = 0.0007) (Figure 2D) but uncorrelated with the age, prostate size, QOL, Qmax and BOOI (Figure 2E–I).
The Transcriptomic Changes in the Prostate of BPH Patients Between the EDH and SDH Groups by RNA-Seq Analysis
To investigate the different genes between the EDH and SDH groups, we performed mRNA sequencing on hyperplasia nodules of 8 BPH patients (4 cases of EDH and 4 cases of SDH).
Figure 3 (the volcano map) shows that 172 DEGs (63 upregulated genes and 109 downregulated genes) were obtained in the SDH group compared to the EDH group (Table 2). Figure 4 shows the statistical results of GO enrichment analysis. In BP category, the enriched DEGs were related to immune response, immune system process, G-protein-coupled receptor signaling pathway, antigen processing and presentation, and other functions. In CC category, DEGs were concentrated in the processes of collagen trimer, extracellular region, extracellular region part, extracellular matrix, proteinaceous extracellular matrix, Golgi apparatus, and other CCs. In MF category, DEGs were mainly enriched in extracellular matrix structural constituent, chemokine activity, chemokine receptor binding, G protein-coupled receptor binding, and other MFs. KEGG enrichment analyses revealed that the DEGs were enriched in pathways associated with protein digestion and absorption, ECM-receptor interaction, renin-angiotensin system, human papillomavirus infection, neuroactive ligand‒receptor interaction, focal adhesion, NF-κB signaling pathway, and other signaling pathways (Figure 5). The proteins expressed of 172 DEGs were analyzed in the PPI network, Figure 6A shows the PPI network formed by them. Then, we ranked the top 10 hub genes including COL1A1, COL1A2, COL3A1, POSTN, SPARC, COL5A1, COL6A2, TGFβ1, COL5A2, and VIM by combination with PPI network results and CytoHubba, which are shown in Figure 6B and Table 3.
 |
Table 2 The Up-Regulated and Down-Regulated DEGs in SDH Groups Compared to EDH Groups Were Screened by Integrated Microarray |
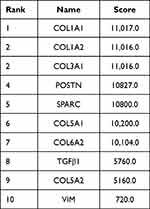 |
Table 3 Top 10 Hub Genes in PPI Network Ranked |
 |
Figure 4 GO enrichment analysis of DEGs on the −log10 (P-value). BP for red bar, CC for green bar and MF for blue bar. |
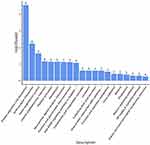 |
Figure 5 KEGG signal pathway enrichment analysis of DEGs on the −log10(P-value). |
Discussion
Currently, a growing number of researchers emphasize that BPH histological diagnosis is not only “BPH” but also the proportion and composition of hyperplastic nodules, which might influence LUTS and clinicians’ management of patients.4,5 Studies have demonstrated that obstructive symptoms of BPH patients are not necessarily related to prostate size but are correlated with the proportion and composition of its volume occupied by stromal tissue.6,7 Some patients showed a small prostate size, predominance of fibromuscular stroma nodules and obvious urination disorders. However, another patient showed a large prostate size, primarily epithelial nodules and relatively mild urination symptoms.8,9 The prostate is composed of acinar epithelial tissues and supporting stromal tissues. Histopathologically, hyperplastic nodules of BPH are composed of different proportions of stroma and epithelium tissue and primarily of stroma.5 In the present study, 67 BPH patients were retrospectively analyzed and divided into EDH and SDH groups according to the stroma percentage in hyperplasia nodules. There were 20 cases of EDH (29.9%) and 47 cases of SDH (70.1%), which also supported BPH as primarily stromal hyperplasia. At present, it is thought that the stroma in BPH nodules may play a key role in LUTS. Studies by Shapiro et al showed that the average ratio of stroma to epithelium is 2.7 in asymptomatic hyperplasia, whereas the ratio is 4.6 in patients of obstruction, suggesting that the stroma may make a significant contribution to BPH/LUTS.8 In this study, we investigated the patients’ clinical parameters between the EDH and SDH groups and found significant differences in the IPSS, which is always used to assess male LUTS. Our data showed that the patients in the SDH group had a higher IPSS than those in the EDH group, which suggested that the patients with predominant stromal hyperplasia had more severe clinical obstructive symptoms and that the stroma in BPH nodules may play a significant role in contributing to LUTS, consistent with Shapiro et al’s studies.8 However, our data did not show a significant difference in age, prostate size, QOL, Qmax or BOOI between these two groups. The QOL category indicates the general level of unhappiness and bother. Although useful, it is now thought that using QOL scores to gauge BPH/LUTS extent might be a subjective and inaccurate approach. The conscious symptoms of urinary tract obstruction (IPSS) and objective indications (Qmax) are often inconsistent. IPSS is influenced by the patient’s subjective factors, while Qmax is influenced by the urine volume and the temporary physical factors. BOOI, as calculated by the results of the urodynamic examination, may help to determine the presence or absence of BOO. Our data showed that Qmax was slightly low and BOOI was slightly high in patients in the SDH group compared to patients in the EDH group, indicating the presence of bladder outlet obstruction in these two types. The symptoms of LUTS and BOO were more severe in the SDH group. However, there were no statistical differences in QOL, Qmax, BOOI or prostate size between the two types, and we speculate that these differences are related to the less number of cases in our study.
Prostate stroma mainly comprises smooth muscle cells (SMCs) and fibroblasts.10 Previous research has suggested that in the pathomorphogenesis of hyperplasia, the role of the stroma, mainly of the activated smooth muscle cells, is more important. Under the effect of noradrenergic sympathetic nerves SMC can constrict the urethra, which is considered to be the main cause of LUTS, and this also is the rationale for using α-1 blockers to relieve obstructive symptoms of patients.11 In recent years, people have come to recognize that prostate fibrosis other than androgen-mediated proliferation and smooth muscle dysfunction might thus contribute to the progression of BPH/LUTD in some patients. Prostate fibrosis is characterized by an increase in fibroblasts, excessive collagen deposition and alterations in ECM remodeling.3 Prostate collagen deposition is significantly associated with LUTS, and the occurrence of LUTS may be partially secondary to lower urinary tract fibrosis due to ECM deposition and increased tissue stiffness.12,13 In addition, one proposed reason for BPH treatment failure with a-blockers or 5a-RIs is collagen deposition within regions of the prostate as a result of fibrosis.3,12,13 In this study, we assessed the proportion of collagen fibers and smooth muscle in BPH nodules using Masson’s trichrome staining and found that the proportions of collagen fibers and smooth muscle were approximately equal. Robert et al14 and Shapiro et al15 also showed that stroma comprises an equal proportion of fibroblasts and smooth muscle elements using immunohistochemistry and computerized image analysis. Furthermore, our results showed that the proportion of collagen fibers was increased in BPH nodules of the SDH group compared to the EDH group, and the proportion of collagen fibers was positively correlated with the IPSS. Previous studies by Ma et al12 and Francesco et al16 also demonstrated that collagen content was positively correlated with the IPSS. Therefore, we suggest that fibroblasts, which secrete collagen fibers and have half the proportion of stromal components in BPH nodules, also play an important role in contributing to LUTS. All these results implied that collagen fiber excessive deposition into the ECM and hence fibrosis may contribute to clinical symptom in some BPH patients. Anti-fibrotic therapeutics might provide a new treatment strategy for men with LUTD who do not respond to conventional medical treatment approaches.
To identify the altered gene levels in hyperplastic prostate tissue of BPH patients between the SDH and EDH groups, mRNA sequencing in prostate hyperplasia of 8 patients was performed. A total of 172 DEGs were found, including 63 upregulated and 109 downregulated genes in the SDH group compared to the EDH group. Among these DEGs, we observed a list of fibrosis-related and profibrotic genes with significantly increased mRNA expression and potential implications in maintaining the chronic fibrotic process in the SDH group compared to the EDH group. These genes encode collagens (COL10A1, COL1A1, COL3A1, COL1A2, COL5A2, COL6A2 and COL5A1), cytokines and chemokines (CCL13, CCL21, CXCL14), and growth factors (TGFβ1). Collagen is one of the most important ECM components responsible for the structural integrity of the extracellular matrix, tissues, and organs.17,18 However, collagen fiber excessive accumulation has been shown to be detrimental in lots of other disease states,19–21 and prostate fibrosis is usually accompanied by an increased collagen content in prostate tissue. The upregulation of collagen genes in the SDH group indicated that there was more collagen deposition into the ECM, which contributed to the symptomology of these patients. Inflammation is one major factor associated with the development of BPH. Acute and chronic inflammation are implicated in BPH pathogenesis by the increased presence of inflammatory infiltrates and elevated cytokines and chemokines,22–24 and chronic inflammation has been associated with the subsequent development of tissue fibrosis. CCL-type chemokines are secreted glycoproteins. CCL upregulated levels might signify the presence of massive T-lymphocyte populations in prostate tissue, in turn, promote prostate fibrosis.25 CXC-type chemokine is of secreted protein family that mediate myofibroblast phenoconversion and ECM deposition and may thereby promote fibrotic changes in prostate tissue architecture associated with the development and progression of male lower urinary tract dysfunction. Men with moderate/severe LUTS expressed outstanding higher levels of CXCR4, the receptor for CXCL12 in periurethral tissues from the prostates than men without LUTS.26 Transforming growth factor β (TGFβ) is a multifunctional cytokine with extensive regulatory activities, and it is known to play an important role in fibrosis disease.27,28 TGFβ has five isoforms, and TGFβ1 is the most active and the most widely distributed. TGFβ1 might promote fibrosis by a variety of ways, including driving phenoconversion of fibroblast to myofibroblast, ECM deposition, and epithelial-mesenchymal transition (EMT).29,30 In addition, our data also showed that vimentin (VIM), a marker of stromal cells, was also increased, indicating the expansion of the stromal compartment. GO enrichment analysis of DEGs, we found that these genes were mainly enriched in ECM structural constituent, extracellular region, extracellular region part, collagen trimer, immune response, immune system process, chemokine activity and receptor binding. KEGG enrichment analyses revealed that the DEGs were enriched in pathways associated with protein digestion and absorption, ECM-receptor interaction, and the NF-κB signaling pathway. Next, cytoHubba of Cytoscape was used to build the PPI network and determine the top 10 hub genes: COL1A1, COL1A2, COL3A1, POSTN, SPARC, COL5A1, COL6A2, TGFβ1, COL5A2, and VIM. The top 10 hub genes were mostly related to the ECM, and 6 of them were associated to collagen. Overall, the mRNA sequencing and bioinformatics analysis results may provide a comprehensive indication that the upregulated fibrosis-related and profibrotic gene expression was responsible for the symptoms of BPH patients with SDH.
Conclusions
In conclusion, despite our sample size was small, our data also suggested that the symptoms of BPH patients with SDH nodules may be associated with prostate fibrosis. Although stromal fibrosis is not an unrecognized contributor. It is not currently a therapeutic target, but people realized the role of prostate fibrosis in BPH/LUTS development and progression. However, our study has the limitation that we only compared two types of BPH and thus cannot say what genes are increased in BPH vs non-BPH tissues. We will explore the problem in our future studies.
Funding
The present study is supported by National Natural Science Foundation of China (81860141 and 82160149), Guizhou Provincial Science and Technology Foundation ([2021]378), Guizhou University introduces talents Foundation ([2021]8).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Marberger M. Medical management of lower urinary tract symptoms in men with benign prostatic enlargement. Adv Ther. 2013;30:309–319. doi:10.1007/s12325-013-0022-7
2. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387–2398. doi:10.1056/NEJMoa030656
3. Rodriguez-Nieves JA, Macoska JA. Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol. 2013;10:546–550. doi:10.1038/nrurol.2013.149
4. Sciarra A, Voria G, Mariotti G, et al. Histopathological aspects associated with the diagnosis of benign prostatic hyperplasia: clinical implications. Urol Int. 2002;69:253–262. doi:10.1159/000066128
5. Roehrborn CG. Pathology of benign prostatic hyperplasia. Int J Impot Res. 2008;20(Suppl 3):S11–8. doi:10.1038/ijir.2008.55
6. Deering RE, Bigler SA, King J, et al. Morphometric quantitation of stroma in human benign prostatic hyperplasia. Urology. 1994;44:64–70. doi:10.1016/s0090-4295(94)80011-1
7. Foster CS. Pathology of benign prostatic hyperplasia. Prostate Suppl. 2000;9:4–14.
8. Shapiro E, Becich MJ, Hartanto V, et al. The relative proportion of stromal and epithelial hyperplasia is related to the development of symptomatic benign prostate hyperplasia. J Urol. 1992;147:1293–1297. doi:10.1016/s0022-5347(17)37546-8
9. Schuster GA, Schuster TG. The relative amount of epithelium, muscle, connective tissue and lumen in prostatic hyperplasia as a function of the mass of tissue resected. J Urol. 1999;161:1168–1173. doi:10.1016/S0022-5347(01)61620-3
10. Chagas MA, Babinski MA, Costa WS, et al. Stromal and acinar components of the transition zone in normal and hyperplastic human prostate. BJU Int. 2002;89:699–702. doi:10.1046/j.1464-410x.2002.02724.x
11. Lepor H, Shapiro E. This month in investigative urology: alpha adrenergic innervation of the prostate: insights into pharmacotherapy of BPH. J Urol. 1990;143:590–591. doi:10.1016/s0022-5347(17)40035-8
12. Jinjin M, Gharaee-Kermani M, Kunju L, et al. Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol. 2012;188:1375–1381. doi:10.1016/j.juro.2012.06.007
13. Bauman TM, Nicholson TM, Abler LL, et al. Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One. 2014;9:e109102. doi:10.1371/journal.pone.0109102
14. Robert M, Costa P, Bressolle F, et al. Percentage area density of epithelial and mesenchymal components in benign prostatic hyperplasia: comparison of results between single biopsy, multiple biopsies and multiple tissue specimens. Br J Urol. 1995;75:317–324. doi:10.1111/j.1464-410x.1995.tb07342.x
15. Shapiro E, Hartanto V, Lepor H. Quantifying the smooth muscle content of the prostate using double-immunoenzymatic staining and color assisted image analysis. J Urol. 1992;147:1167–1170. doi:10.1016/s0022-5347(17)37508-0
16. Cantiello F, Cicione A, Salonia A, et al. Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: a prospective cohort study. Urology. 2013;81:1018–1023. doi:10.1016/j.urology.2013.01.053
17. Gelse K, Pöschl E, Aigner T. Collagens: structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi:10.1016/j.addr.2003.08.002
18. Péterszegi G, Andrès E, Molinari J, et al. Effect of cellular aging on collagen biosynthesis: i. Methodological considerations and pharmacological applications. Arch Gerontol Geriatr. 2008;47:356–367. doi:10.1016/j.archger.2007.08.019
19. Diaz Encarnacion MM, Griffin MD, Slezak JM, et al. Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am J Transplant. 2004;4:248–256. doi:10.1046/j.1600-6143.2003.00311.x
20. Wynn TA. Thomas A Wynn.Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi:10.1084/jem.20110551
21. Grewal P, Martin P. Care of the cirrhotic patient. Clin Liver Dis. 2009;13:331–340. doi:10.1016/j.idc.2012.08.009
22. Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202–1216. doi:10.1016/j.eururo.2006.12.011
23. Fibbi B, Penna G, Morelli A, et al. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33:475–488. doi:10.1111/j.1365-2605.2009.00972.x
24. Penna G, Fibbi B, Amuchastegui S, et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009;182:4056–4064. doi:10.4049/jimmunol.0801875
25. McDowell KL, Begley LA, Mor-Vaknin N, et al. Leukocytic promotion of prostate cellular proliferation. Prostate. 2010;70:377–389. doi:10.1002/pros.21071
26. Gharaee-Kermani M, Kasina S, Moore BB, et al. CXC-type chemokines promote myofibroblast phenoconversion and prostatic fibrosis. PLoS One. 2012;7:e49278. doi:10.1371/journal.pone.0049278
27. Mingfang A, Franco OE, Park D, et al. Cross-talk between paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007;67:4244–4253. doi:10.1158/0008-5472.CAN-06-3946
28. Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J Exp Med. 2020;217:e20190103. doi:10.1084/jem.20190103
29. Bierhoff E, Vogel J, Benz M, et al. Stromal Nodules in Benign Prostatic Hyperplasia. Eur Urol. 1996;29:345–354. doi:10.1159/000473774
30. Verrecchia F, Mauviel A. Transforming growth factor-beta and fibrosis. World J Gastroenterol. 2007;13:3056–3062. doi:10.3748/wjg.v13.i22.3056
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.