Back to Journals » Journal of Asthma and Allergy » Volume 15
The Specific microRNA Profile and Functional Networks for Children with Allergic Asthma
Authors Zhang X , Zhang X, Feng S, Wang X, Guo B, Liu J, Xu D, Liu F
Received 14 June 2022
Accepted for publication 14 August 2022
Published 29 August 2022 Volume 2022:15 Pages 1179—1194
DOI https://doi.org/10.2147/JAA.S378547
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Xiyan Zhang,1 Xude Zhang,1 Shaojie Feng,1 Xijuan Wang,1 Beibei Guo,1 Jingjing Liu,1 Donghua Xu,2,3 Fengxia Liu1
1Department of Allergy, Weifang People’s Hospital, Weifang, People’s Republic of China; 2Clinical Medicine College, Weifang Medical University, Weifang, People’s Republic of China; 3Department of Rheumatology, The First Affiliated Hospital of Weifang Medical University, Weifang, People’s Republic of China
Correspondence: Fengxia Liu; Donghua Xu, Email [email protected]; [email protected]
Background: Allergic asthma is the most common type of asthma and often occurs in early life with increasing comorbidities, including atopic dermatitis and allergic rhinitis. MicroRNAs (miRNAs) are involved in the pathogenesis of numerous immune and inflammatory disorders, particularly allergic inflammation. The specific miRNA profiles of children with allergic asthma have not been fully delineated and still require in-depth study.
Objective: This study aimed to identify the expression profile of miRNAs and constructed a network of the interactions between differentially expressed miRNAs and target mRNAs to provide novel insights into understanding the pathogenesis of allergic asthma.
Materials and Methods: In this study, we performed high-throughput sequencing of peripheral blood mononuclear cells (PBMCs) from children in the acute phase of asthma. Bioinformatics approaches, including miRanda, Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) databases, were employed to predict novel therapeutic and diagnostic targets for allergic asthma. Real-time quantitative PCR was conducted to detect the expression of aberrantly expressed miRNAs.
Results: One hundred and sixty-one differentially expressed miRNAs were identified in children with allergic asthma, including 140 conserved miRNAs and 21 novel miRNAs. A total of 8929 targeted mRNAs (44,186 transcripts) associated with differentially expressed miRNAs were predicted and significantly enriched in the cGMP-PKG signalling pathway, cholinergic synapse, and salivary secretion. We also found that miRNA-370-3p targeted PKG and MLCP molecules in the cGMP-PKG signalling pathway and was involved in the pathogenesis of allergic asthma.
Conclusion: We identified the miRNA profile of PBMCs in children with allergic asthma and also found that miRNA-370-3p targeted PKG and MLCP molecules in the cGMP-PKG signalling pathway, which provides a novel insight into understanding the pathogenesis of allergic asthma and investigating new targets for the treatment of allergic asthma in children.
Keywords: allergic asthma, MicroRNAs, inflammation, house dust mite, children
Introduction
Asthma is one of the most common chronic childhood diseases and is characterized by chronic inflammation and airway hyperreactivity. Up to 4.4% of preschoolers and 6.4% of elementary school children are reported to have asthma. Elucidating allergic factors for children with asthma is essential.1 Exposure to allergens, such as house dust mites (HDM), cockroaches, pets, and pollen, triggers or even exacerbates bronchial hyperreactivity. In addition, allergic asthma is usually persistent and typically continues into adulthood as a chronic condition, cooccurring with several diseases, such as allergic rhinitis and atopic dermatitis.2 In recent decades, the mechanisms of allergic asthma have been extensively studied. Notably, increasing evidence indicates that noncoding RNAs (ncRNAs), including long noncoding RNAs (lncRNAs), microRNAs (miRNAs), and circular RNAs (circRNAs), among others, play important roles in allergic asthma.3
MicroRNAs (miRNAs), which are small noncoding RNA molecules of 18–24 nucleotides in length, play a fundamental role in regulating gene expression by degrading mRNAs or inhibiting translation.4 Due to their rich biofunctions and stability in serum, miRNAs show excellent potential to become biomarkers for the diagnosis of allergic asthma. Differentially expressed miRNAs are gradually being identified in the sputum, serum, and airway epithelium of patients with asthma and are closely related to asthma severity, asthma type, and asthma outcomes.5 Despite advances in allergic asthma research, the accurate diagnosis and individualized treatment of allergic asthma still require in-depth study.
This study aimed to identify the expression profile of miRNAs in children diagnosed with allergic asthma with a clear etiology (house dust mites) using small RNA sequencing. A specific profile of miRNAs in PBMCs associated with allergic asthma in children was confirmed. Pathway enrichment analysis of targeted mRNAs of differentially expressed miRNAs revealed signalling pathways closely related to allergic asthma pathogenesis, such as the cGMP-PKG signalling pathway, cholinergic synapse, and salivary secretion.
The network analysis of the interactions between differentially expressed miRNAs and targeted mRNAs/pathways illustrated the pathogenesis of allergic asthma. Our study provides novel insights into understanding the pathogenesis of allergic asthma and illuminates some potential mechanisms of allergic asthma pathogenesis by analyzing differentially expressed miRNAs, which may be used to design novel therapeutic strategies and diagnostic biomarkers of allergic asthma.
Materials and Methods
Experimental Design
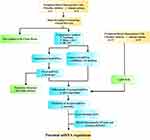
RNAs were extracted from PBMCs for high-throughput sequencing and a subsequent systematic bioinformatics analysis to explore differences in the expression profiles between healthy children and children suffering from allergic asthma. The study flowchart is provided in Figure 1.
Study Population and Ethics Statement
Thirty-six participants aged 3–12 years old were involved in this experiment at Weifang People’s Hospital between October 2020 and August 2021. Patients with systemic inflammatory diseases or cancers were excluded. In addition, lung and respiratory-related diseases were not present in healthy subjects.
The inclusion criteria were as follows: (I) clinical history of wheezing symptoms and expiratory wheezing (diagnosed by a physician); (II) age of 3–12 years; and (III) skin testing and specific IgE tests for a single allergen (house dust mite) using the ImmunoCAP system (Thermo Fisher-Phadia, Uppsala, Sweden). The following exclusion criteria were used: (I) mycoplasma or virus-induced asthma; (II) specific IgE tests for multiple allergens or non-house dust mite allergens. According to GINA guidelines, we established the diagnosis of allergic asthma based on the medical history, clinical evaluation, and skin testing or specific IgE in serum.
Our experiments are comprised of two parts. The children were initially diagnosed in the acute phase of asthma (moderate or severe house dust mite allergy) (n=3), and healthy children (n=3) were included in the small RNA sequencing group. The validation groups consisted of 30 subjects, including 15 children initially diagnosed in the acute phase of asthma (moderate or severe house dust mite allergy) and 15 healthy children (Table 1). The ethics committee of Weifang People’s Hospital (KYLL2021120) approved the study, and written informed consent was obtained from all participants and/or their parents or legal guardians in accordance with the revised version of the Helsinki Declaration regarding research involving human subjects.
 |
Table 1 Summary Characteristics of Allergic Asthma Patients and Controls |
PBMC Collection
We collected peripheral blood mononuclear cells (PBMCs) from fasting subjects in the early morning. Briefly, 6 mL of peripheral blood was gently collected using a vacuum blood collection tube (anticoagulated with 1.5–2 mg/mL EDTA). First, equal amounts of peripheral blood and phosphate-buffered saline (PBS) were mixed. Then, the mixture was slowly added to the top of a centrifuge tube containing the same amount of lymphocyte isolate (TBD Bio. Co., Tianjin, China). PBMCs were collected from the white mist layer after centrifugation (room temperature, 400 g, 30 minutes) and washed thoroughly and uniformly twice with PBS. Finally, we resuspended the PBMCs using TRIzol (Invitrogen, USA) and stored them in liquid nitrogen until the RNA was extracted.
RNA Extraction and Library Construction
According to the manufacturer’s protocol, a mirVana™ miRNA Isolation Kit (Ambion) and Nanodrop 2000 (Thermo Fisher Scientific Inc., USA) were used to extract and quantify the total RNA from PBMCs, respectively. RNA integrity was assessed by an Agilent 2100 Bioanalyzer (Agilent Technology, USA). Afterwards, small RNA libraries (1 μg) were constructed using TruSeq Small RNA Sample Prep Kits (Cat. No. RS-200-0012, Illumina, USA.). Briefly, total RNA was ligated to adapters at each end. Then, the adapter-ligated RNA was reverse transcribed to cDNA and performed PCR amplification. The PCR products ranging from 140–160 bp were isolated and purified as small RNA libraries. Then, the quality of the libraries was assessed with an Agilent Bioanalyzer 2100 system using a DNA high-sensitivity microarray. Finally, the library was sequenced using the Illumina Novaseq 6000 platform, generating 150 bp single-end reads for analysis. OE Biotech Co., Ltd. (Shanghai, China) conducted small RNA sequencing and the analysis.
Small RNA Sequencing and Comparative Analysis
The basic reads from the Illumina analysis were converted into raw reads in FASTQ format by base calling, and clean reads from raw reads were used for all downstream analyses. Briefly, clean reads with high quality were obtained from raw reads by removing the low-quality and specific reads, such as reads with 5′ primer contaminants and poly (A), reads without 3’ adapters and insert tags, and reads shorter than 15 nt and longer than 41 nt.
For the primary analysis, a distribution of the lengths of the clean sequences in the reference genome was generated. Then, the clean reads were aligned with the small RNAs from the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/) and Rfam v.10.1 (http://www.sanger.ac.uk/software/Rfam)6 to annotate noncoding RNA sequences using the Basic Local Alignment Search Tool (BLAST).7 Subsequently, the known miRNAs and unannotated small RNAs were analysed by aligning them with the miRBase v.21.0 database (http://www.mirbase.org/)8 and mirdeep2,9 respectively. Meanwhile, RNAfold software10 was used to predict the secondary structures of the novel miRNAs based on the hairpin structure of a pre-miRNA and the miRbase database.
Identification and Bioinformatics Analysis of Differentially Expressed miRNAs
Differentially expressed miRNAs were calculated with the DESeq2 R package and identified with a threshold of p value < 0.05 and |log2FC|>1.
Next, miRanda software predicted targeted mRNAs associated with differentially expressed miRNAs using the following parameters: S ≥ 150, ΔG ≤ −30 kcal/mol, and strict 5’ seed pairing. We performed GO enrichment and KEGG pathway enrichment analyses of targeted mRNAs with R based on the hypergeometric distribution. The significant GO terms and pathways were identified by performing multiple Benjamini-Hochberg procedure, and a p value <0.05 was considered statistically significant. Finally, Cytoscape_v3.5.1 software11 was used to visualize the miRNA–mRNA network in the KEGG pathway.
Validation of miRNA Expression by qRT–PCR
The expression of miRNAs was estimated by performing a quantitative real-time polymerase chain reaction (qRT–PCR). Briefly, the TransScript miRNA First-Strand cDNA Synthesis SuperMIX kit was utilized to transcribe the RNA samples (0.5 μg) into cDNAs. The qRT-PCRs were performed with a LightCycler® 480 II fluorescent qPCR instrument (Roche, Switzerland) using the PerfectStartTM Green qPCR SuperMix kit with cDNAs as the template in a 10 μL reaction system (2×PerfectStartTM Green qPCR SuperMix (5 μL), 10 μM Universal primer (0.2 μL), 10 μM microRNA-specific primer (0.2 μL) shown in Table S1, cDNAs (1 μL), and nuclease-free H2O (3.6 μL)). The qRT–PCR program was 94 ℃ for 30 s, followed by 45 cycles of denaturation at 94 ℃ for 5 s and annealing and extension for 30 s at 60 ℃.
Statistical Analysis
The relative expression of miRNAs was calculated by applying the comparative cycle threshold method (2−∆∆Ct), where 5S rRNA was an internal control. Student’s t-test or the nonparametric Mann–Whitney test was used to calculate the difference in the expression of a particular miRNA between children with allergic asthma and normal children, with p<0.05 representing a statistically significant difference. SPSS 22 software (IBM Corp., NY, USA) and Prism 8 software (GraphPad Software, CA, USA) were employed to analyze the results and visualize the results, respectively. Data from at least three replicates were analyzed.
Results
Overview of the Small RNA Sequencing Datasets
Small RNA sequencing, which provides extremely large amounts of detailed genomic data, is a powerful tool for discovering the types and amounts of small RNAs expressed in children with allergic asthma (house dust mites) and healthy children. Notably, 26,317,201, 24,303,427, 27,232,340, 34,175,661, 22,946,397, and 25,271,873 raw reads were obtained from the six RNA libraries, generating 22,742,046, 20,965,855, 24,167,237, 30,657,749, 20,422,852, and 22,712,854 clean reads. In the distribution of small RNA sequences, the most abundant size category was 22 nt, followed by 21 and 23 nt, consistent with the length of miRNAs (21–23 nt), as shown in Figure 2. Based on these results, small RNA sequencing provides a detailed description of the length and abundance of small RNAs, suggesting that miRNAs are an important and major component of small RNAs present in children with allergic asthma. Subsequently, the unique reads of the six small RNA libraries were described as the following numbers for further analysis: 916,387, 899,099, 859,490, 910,604, 967,065, and 890,647 (Table S2).
We mapped the clean reads to the Rfam database for annotation processing to assess the efficiency of small RNA sequencing in detecting miRNAs, and this analysis eventually detected several types of small RNAs, including rRNA, scRNA, Cis-reg, snRNA, tRNA, repeats, unannotated, and others. By annotating the small RNAs according to the category, unannotated reads were the most represented type of small RNA library, followed by known miRNAs, indicating the dominance of miRNAs in the pathogenesis of allergic asthma (Figure 3).
Identification of Conserved and Novel miRNAs in Children with Allergic Asthma
We identified conserved miRNAs and novel miRNAs using the miRBase v21.0 database (http://www.mirbase.org/) and mirdeep2, respectively, to elucidate the role of miRNAs in regulating allergic asthma in children and obtained at least 1076 conserved miRNAs and 593 novel miRNAs in each of the small RNA libraries (Table S3). Similarly, the length of conserved miRNAs was mainly distributed from 21 to 24 nt (Figure 4A). In addition, no significant difference in the bias of the bases of conserved miRNAs was observed between children with allergic asthma and healthy children (Figure 4B).
Identification of Differentially Expressed miRNAs
The differential expression of miRNAs suggests that they may play an essential role in the related biological processes. We explored the important roles of miRNAs in the biological processes associated with allergic asthma and obtained 161 differentially expressed miRNAs by performing small RNA sequencing, of which 52 differentially expressed miRNAs were significantly upregulated and 109 differentially expressed miRNAs were downregulated (Figure 5 and Table 2). Notably, 21 novel miRNAs were differentially expressed in children with allergic asthma, including novel771_mature, novel95_mature, novel156_mature, and novel206_mature, and the secondary structures of the novel miRNAs were identified using RNAfold software, as shown in Figure 6.
 |
Table 2 Top 50 of Differentially Expressed miRNAs Between Allergic Asthma and Healthy Controls |
Prediction and Functional Analysis of Targeted mRNAs
Based on accumulating evidence, miRNAs control various biological and pathological processes by silencing target mRNAs. A total of 8929 targeted mRNAs (44,186 transcripts) associated with differentially expressed miRNAs were predicted using miRanda software, and these large amounts of data might provide insights into the mechanism of childhood allergic asthma. The most highly enriched topological relationships identified in the GO and KEGG functional analyses of targeted mRNAs are shown in Figure 7.
Gene Ontology (GO) is available for the functional annotation and categorization of targeted mRNAs associated with differentially expressed miRNAs in three broad categories: biological process (BP), cellular component (CC), and molecular function (MF). From the results of GO analysis, the mechanisms of differentially expressed miRNAs affect the initiation and maintenance of allergic asthma in children. The analysis of biological processes (BPs) revealed that target mRNAs were enriched in transcription (DNA template), transcriptional regulation (DNA template), and signal transduction. The analysis of cellular components (CCs) implied that targeted mRNAs are localized to the cell membrane, nucleus, cytoplasm, and plasma membrane to perform their biological functions. The molecular function (MF) analysis suggested that target mRNAs are involved in metal ion binding, ATP binding, DNA binding, and RNA binding (Figure 7A). On the other hand, Kyoto Encyclopedia of Genes and Genomes (KEGG) mapping has been used to analyze mRNA products and functions during metabolic processes (Table 3). According to KEGG classifications, we found that targeted mRNAs were significantly enriched in the cGMP-PKG signalling pathway, cholinergic synapse, and salivary secretion, among which the cGMP-PKG signalling pathway was the most highly enriched pathway (Figure 7B).
 |
Table 3 Statistics of KEGG Enrichment Top 20 |
The Network of miRNAs-mRNAs in the cGMP-PKG Signalling Pathway
We constructed a network of the interactions between differentially expressed miRNAs and target mRNAs in the cGMP-PKG signalling pathway to explore the role of differentially expressed miRNAs in the pathogenesis of allergic asthma using Cytoscape_v3.5.1 software and the KEGG classification results. The results showed that hsa-miR-370-3p targeted PKG and MLCP in the cGMP-PKG signalling pathway (Figure 8). Accordingly, we hypothesized that hsa-miR-370-3p may participate in the pathogenesis of allergic asthma. However, more studies are warranted for further elucidation.
qRT–PCR Validation of Differentially Expressed miRNAs
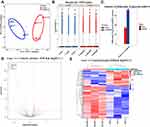
We subsequently verified the expression levels of a set of selected candidate miRNAs to minimize bias, with screening criteria including transcript per million (TPM), the significance of differential expression, and the predicted function of the targeted mRNA. Briefly, the expression levels of the five selected miRNAs were examined using qRT–PCR to validate the sequencing data, among which novel771_mature, hsa-miR-1275 and hsa-miR-370-3p were in the network of the interactions between differentially expressed miRNAs and target mRNAs in the cGMP-PKG signalling pathway. Four of the five miRNAs were differentially expressed in the validation cohort, including hsa-miR-708-5P (P=0.027), novel771_mature (P=0.002), hsa-miR-1275 (P=0.006), and hsa-miR-134-5p (P=0.024), consistent with the expression trend obtained using the sequencing data. Although hsa-miR-370-3p (P=0.054) expression was not significantly different between patients with allergic asthma and healthy controls, similar to the sequencing data, a trend towards downregulation of its expression was observed (Figure 9).
 |
Figure 9 Expression analysis of selected candidate miRNAs assessed by qRT-PCR between allergic asthma (n = 15) and healthy controls (n = 15). Abbreviation: NS, no significant changes. Notes: (A): has-miR-708-5P; (B): novel771_mature; (C): hsa-miR-370-3p; (D): hsa-miR-1275; (E) hsa-miR-134-5p. The relative expression of miRNAs was calculated by applying the comparative cycle threshold method (2−∆∆Ct), where 5S was an internal control. All data in Figure 5 were statistically analyzed by Student’s t-test or non-parametric Mann–Whitney test and P < 0.05 was considered statistically significant (P*<0.05, P**<0.01 between allergic asthma and healthy controls). |
Discussion
We identified the miRNA profile of PBMCs in children with allergic asthma and identified 161 differentially expressed miRNAs. Mechanistically, 8929 target mRNAs associated with differentially expressed miRNAs were predicted and significantly enriched in the cGMP-PKG signalling pathway, cholinergic synapse, and salivary secretion. We also found that miRNA-370-3p targeted PKG and MLCP molecules in the cGMP-PKG signalling pathway, which provides a novel insight into understanding the pathogenesis of allergic asthma and investigating new targets for the treatment of allergic asthma in children.
Accumulating evidence has suggested that noncoding RNAs (ncRNAs) play important roles in a variety of inflammatory diseases, particularly allergic asthma.12 Importantly, ncRNAs, including long noncoding RNAs (lncRNAs) with sizes larger than 200 nucleotides, microRNAs (miRNAs), and circular RNAs (circRNAs), among others, are being recognized as important molecules regulating coding genes at the transcriptional or posttranscriptional level in individuals with allergic asthma.13 Multiple studies have emphasized that the effects of miRNAs on the initiation and maintenance of asthma are mainly achieved by regulating the expression of mRNAs at the posttranscriptional level.14 In contrast, lncRNAs and circRNAs employ a similar mechanism by functioning as an miRNA sponge as a competing endogenous RNA (ceRNA) in individuals with asthma. Numerous researchers have established that ceRNA is a new transcriptional regulatory model whereby lncRNAs and circRNAs share the same miRNA response element (MRE) to indirectly inhibit the expression of miRNAs and weaken the interactions of miRNAs and mRNAs.15 Importantly, these findings highlight the indispensable position and value for basic and translational research of miRNAs in allergic asthma.
Undeniably, the exploration of miRNAs associated with the frequent occurrence of allergic asthma in children is still in its infancy. Although the great potential of miRNAs to serve as biomarkers of airway-related has been revealed, the underlying mechanisms leading to airway hyperreactivity and the value of miRNAs as biomarkers of clinical outcomes and diagnostic biomarkers of allergic asthma, particularly in children, are enigmatic. Considerable evidence has confirmed the differential expression of miRNAs in the serum, sputum, bronchial biopsies, and bronchial epithelium of patients with asthma,16–18 but most of these studies focused on the general definition of asthma. Similar to our findings, the expression of miRNAs was differentially altered in asthmatic mice and seasonal asthmatic mice. For example, Weidner et al19 found that miRNAs, including miR-146a, miR-223, and miR-155, had the ability to distinguish between allergic asthma and nonallergic asthma in murine models, providing experimental evidence for miRNA involvement in allergic asthma. Additionally, the phenomenon of differential expression of miRNAs in the sera of children with seasonal allergic asthma was reported by Tiwari et al. They suggested correlations between miRNA expression and seasonal asthma symptoms: reduced let-7d-3p expression and increased asthma symptoms in the spring, and increased miR-328-3p expression and increased asthma symptoms in the autumn.20 The indispensable position of an miRNA in the development and progression of allergic asthma was established based on theoretical and experimental evidence. However, more in-depth investigations of whether differentially expressed miRNAs are drivers or outcomes of allergic asthma are needed.
Here, we documented the diagnostic and therapeutic values of our selected differentially expressed miRNAs in children with allergic asthma by performing a literature review. A number of the top 50 differentially expressed miRNAs attracted our attention, including hsa-miR-487b-3p, hsa-miR-136-3p, hsa-miR-708-5p, hsa-miR-150-5p, hsa-miR-124, and hsa-miR-370-3p. According to previous reports, miR-487b-3p directly inhibits the expression of interleukin-33 (IL-33), a critical proinflammatory cytokine known to mediate allergic airway inflammation, but the finding that the expression levels of miR-487b-3p are similar between control mice and OVA-challenged wild-type mice is difficult to understand.21 Our study may provide evidence that miR-487b-3p expression is reduced in the serum of patients with allergic asthma. In addition, the interest in the importance of miR-1248 in the pathogenesis of allergic asthma has been renewed. Panganiban et al22 reported that miR-1248 expression is increased within PBMCs of patients with asthma, suggesting that miR-1248 is a positive regulator of IL-5 expression to exacerbate asthma by increasing Th2 cytokine levels. Similarly, miR-1248 has again attracted attention for its role in allergic asthma due to its significantly altered expression in our study. Notably, miR-370 was identified as a significantly upregulated miRNA in murine or cellular models of asthma, which alleviates asthma progression by inhibiting the FGF1/MAPK/STAT1 axis,23 and the same expression trend was observed in our study. Meanwhile, miRNAs such as miR-150,24 hsa-miR-381-3p,25 and hsa-miR-708-5p26 were also detected in studies of asthma or allergic asthma.
However, the value of differentially expressed miRNAs as biomarkers for diagnosing allergic asthma in children must be validated by conducting a large-sample study due to the limited number of subjects participating in the present study. Importantly, ceRNA is an important direction for studying the regulation of differentially expressed miRNAs. Undeniably, the mechanisms by which lncRNAs or circRNAs functioning as ceRNAs drive differentially expressed miRNAs to regulate chronic inflammation and airway hyperreactivity and whether DEM blockade might be of therapeutic value remain unclear, particularly in individuals with allergic asthma. Thus, more research is warranted to elucidate the precise molecular mechanisms of differentially expressed miRNAs and ceRNAs associated with allergic asthma in children. In addition, our results also suggested that miRNA-370-3p and the cGMP-PKG signalling pathway27,28 should be the priority of our subsequent studies.
Conclusively, this study confirms the specific microRNA profile and functional networks in children with allergic asthma. This study provides novel insights into understanding the pathogenesis of allergic asthma and investigating new targets for the treatment of allergic asthma in children.
Data Sharing Statement
The molecular sequences presented in this study was successfully deposited the data with BioSample accessions: SAMN28187458, SAMN28187459, SAMN28187460, SAMN28187461, SAMN28187462, SAMN28187463. The temporary Submission ID is SUB11457029 in the BioProject database. The project information will be accessible with the link (http://www.ncbi.nih.gov/bioprojrct/837058).
Ethics Approval and Consent
The ethics committee of Weifang People’s Hospital (KYLL2021120) approved the study, and all subjects signed written informed consent forms.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval for the version to be published; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation, China (82171790), and Science and Technology Development Program, Weifang, China (2020YX013).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Akar-Ghibril N, Casale T, Custovic A, et al. Allergic endotypes and phenotypes of asthma. J Allergy Clin Immunol Pract. 2020;8(2):429–440. doi:10.1016/j.jaip.2019.11.008
2. Tsang MS, Sun X, Wong CK. The role of new IL-1 family members (IL-36 and IL-38) in atopic dermatitis, allergic asthma, and allergic rhinitis. Curr Allergy Asthma Rep. 2020;20(8):40. doi:10.1007/s11882-020-00937-1
3. Ghafouri-Fard S, Shoorei H, Taheri M, et al. Emerging role of non-coding RNAs in allergic disorders. Biomed Pharmacother. 2020;130:110615. doi:10.1016/j.biopha
4. Weidner J, Bartel S, Kılıç A, et al. Spotlight on microRNAs in allergy and asthma. Allergy. 2021;76(6):1661–1678. doi:10.1111/all.14646
5. Specjalski K, Jassem E. MicroRNAs: potential biomarkers and targets of therapy in allergic diseases? Arch Immunol Ther Exp. 2019;67(4):213–223. doi:10.1007/s00005-019-00547-4
6. Griffiths-Jones S, Bateman A, Marshall M, et al. Rfam: an RNA family database. Nucleic Acids Res. 2003;31(1):439–441. doi:10.1093/nar/gkg006
7. Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi:10.1016/S0022-2836(05)80360-2
8. Griffiths-Jones S, Saini HK, van Dongen S, et al. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–158. doi:10.1093/nar/gkm952
9. Friedländer MR, Mackowiak SD, Li N, et al. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012;40(1):37–52. doi:10.1093/nar/gkr688
10. Denman RB. Using RNAFOLD to predict the activity of small catalytic RNAs. Biotechniques. 1993;15(6):1090–1095.
11. Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi:10.1101/gr.1239303
12. Soni DK, Biswas R. Role of non-coding RNAs in post-transcriptional regulation of lung diseases. Front Genet. 2021;12:767348. doi:10.3389/fgene.2021.767348
13. Gysens F, Mestdagh P, De Bony de Lavergne E, et al. Unlocking the secrets of long non-coding RNAs in asthma. Thorax. 2022;77(5):514–522. doi:10.1136/thoraxjnl-2021-218359
14. Cañas JA, Rodrigo-Muñoz JM, Sastre B, et al. MicroRNAs as potential regulators of immune response networks in asthma and chronic obstructive pulmonary disease. Front Immunol. 2021;11:608666. doi:10.3389/fimmu.2020.608666
15. Wang X, Chen H, Liu J, et al. Emerging advances of non-coding RNAs and competitive endogenous RNA regulatory networks in asthma. Bioengineered. 2021;12(1):7820–7836. doi:10.1080/21655979.2021.1981796
16. Roffel MP, Boudewijn IM, van Nijnatten JLL, et al. Identification of asthma-associated microRNAs in bronchial biopsies. Eur Respir J. 2022;59(3):2101294. doi:10.1183/13993003.01294-2021
17. Singh P, Sharma A, Jha R, et al. Transcriptomic analysis delineates potential signature genes and miRNAs associated with the pathogenesis of asthma. Sci Rep. 2020;10(1):13354. doi:10.1038/s41598-020-70368-5
18. Maes T, Cobos FA, Schleich F, et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J Allergy Clin Immunol. 2016;137(5):1433–1446. doi:10.1016/j.jaci.2016.02.018
19. Weidner J, Ekerljung L, Malmhäll C, et al. Circulating microRNAs correlate to clinical parameters in individuals with allergic and non-allergic asthma. Respir Res. 2020;21(1):107. doi:10.1186/s12931-020-01351-x
20. Tiwari A, Wang AL, Li J, et al. Seasonal variation in miR-328-3p and let-7d-3p are associated with seasonal allergies and asthma symptoms in children. Allergy Asthma Immunol Res. 2021;13(4):576–588. doi:10.4168/aair.2021.13.4.576
21. Yamazumi Y, Sasaki O, Imamura M, et al. The RNA binding protein Mex-3B is required for IL-33 induction in the development of allergic airway inflammation. Cell Rep. 2016;16(9):2456–2471. doi:10.1016/j.celrep.2016.07.062
22. Panganiban RP, Pinkerton MH, Maru SY, et al. Differential microRNA expression in asthma and the role of miR-1248 in regulation of IL-5. Am J Clin Exp Immunol. 2012;1(2):154–165.
23. Li C, Deng C, Zhou T, et al. MicroRNA-370 carried by M2 macrophage-derived exosomes alleviates asthma progression through inhibiting the FGF1/MAPK/STAT1 axis. Int J Biol Sci. 2021;17(7):1795–1807. doi:10.7150/ijbs.59715
24. Lin L, Li Q, Hao W, et al. Upregulation of LncRNA Malat1 induced proliferation and migration of airway smooth muscle cells via miR-150-eIF4E/Akt signaling. Front Physiol. 2019;10:1337. doi:10.3389/fphys.2019.01337
25. Lin CC, Law BF, Hettick JM. Acute 4,4’-methylene diphenyl diisocyanate exposure-mediated downregulation of miR-206-3p and miR-381-3p activates inducible nitric oxide synthase transcription by targeting calcineurin/NFAT signaling in macrophages. Toxicol Sci. 2020;173(1):100–113. doi:10.1093/toxsci/kfz215
26. Dileepan M, Sarver AE, Rao SP, et al. MicroRNA mediated chemokine responses in human airway smooth muscle cells. PLoS One. 2016;11(3):e0150842. doi:10.1371/journal.pone.0150842
27. Chu S, Zhang X, Sun Y, et al. Atrial natriuretic peptide inhibits epithelial-mesenchymal transition (EMT) of bronchial epithelial cells through cGMP/PKG signaling by targeting Smad3 in a murine model of allergic asthma. Exp Lung Res. 2019;45(8):245–254. doi:10.1080/01902148.2019.1660734
28. Bayarri MA, Milara J, Estornut C, et al. Nitric oxide system and bronchial epithelium: more than a barrier. Front Physiol. 2021;12:687381. doi:10.3389/fphys.2021.687381
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.