Back to Journals » Journal of Hepatocellular Carcinoma » Volume 10
The Safety and Efficacy of Hepatic Arterial Infusion Chemotherapy Combined with PD-(L)1 Inhibitors and Molecular Targeted Therapies for the Treatment of Intermediate and Advanced Hepatocellular Carcinoma Unsuitable for Transarterial Chemoembolization
Authors Tang HH, Zhang MQ, Zhang ZC, Fan C, Jin Y, Wang WD
Received 14 October 2023
Accepted for publication 6 December 2023
Published 12 December 2023 Volume 2023:10 Pages 2211—2221
DOI https://doi.org/10.2147/JHC.S441024
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr David Gerber
Hao-Huan Tang,1,* Ming-Qing Zhang,2,* Zi-Chen Zhang,3,* Chen Fan,1 Yong Jin,2 Wei-Dong Wang1
1Department of Interventional Radiology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, 214023, People’s Republic of China; 2Department of Interventional Radiology, The Second Affiliated Hospital of Soochow University, Suzhou, 215006, People’s Republic of China; 3Department of Interventional Vascular Medicine, Hefei Hospital Affiliated to Anhui Medical University, The Second People’s Hospital of Hefei, Hefei, 230011, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yong Jin, Department of Interventional Radiology, The Second Affiliated Hospital of Soochow University, No. 1055, Sanxiang Road, Suzhou, 215006, People’s Republic of China, Tel/Fax +86 512 67784269, Email [email protected] Wei-Dong Wang, Department of Interventional Radiology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, No. 299, Qingyang Road, Wuxi, 214023, People’s Republic of China, Tel/Fax +86 510 85350121, Email [email protected]
Objective: To investigate the efficacy and safety of hepatic arterial infusion chemotherapy (HAIC) combined with PD-(L)1 inhibitors and molecular targeted therapies (MTT) for intermediate and advanced HCC that are unsuitable for transarterial chemoembolization (TACE).
Methods: We conducted a retrospective analysis of data from patients with TACE-unsuitable HCC who were receiving triple therapy from January 2020 to December 2021 at two medical centers. The primary outcome was overall survival (OS), and the secondary outcomes were progression-free survival (PFS), objective response rates (ORR), disease control rates (DCR), and incidence of adverse events (AEs).
Results: A total of 55 patients were enrolled in the study with median treatment periods of 4 and 6 for HAIC and PD-(L)1 inhibitors, respectively. The median OS and PFS were 15.0 and 10.0 months, respectively, with a median follow-up of 11.0 months (range: 4.0– 27.5 months). According to the mRECIST criteria, the optimal ORR was 43.6% (24/55) and the DCR was 61.8% (34/55). The incidence of AEs was 58.2%, with grade 3 and above accounting for 20.0%; elevated AST (18.2%), hyperbilirubinemia (16.4%), and thrombocytopenia (16.4%) were most common. There were no treatment-related fatalities and all AEs were effectively managed. Multifactorial analysis showed that NLR > 3.82 (HR 2.380, 95% CI 1.116-2-5.079, P = 0.025), ECOG 1 (HR 2.906, 95% CI 1.373– 6.154, P = 0.005), and extrahepatic metastases (HR 8.373, 95% CI 3.492– 20.078, P < 0.001) were associated with the median OS.
Conclusion: Triple therapy with HAIC, PD-(L)1 inhibitors, and MTT was safe and effective for patients with intermediate and advanced HCC for TACE-unsuitability.
Keywords: advanced hepatocellular carcinoma, hepatic arterial infusion chemotherapy, molecular targeted therapies, PD-(L)1 inhibitors
Introduction
Hepatocellular carcinoma (HCC) is a lethal tumor that is highly prevalent worldwide, particularly in China, accounting for 45.3% liver cancer cases globally, and it predominantly has a hepatitis B virus infection background predominantly.1,2 Despite improvements in early detection, the majority of patients with HCC are diagnosed at an advanced stage, with limited options for radical treatment and dismal prognosis.3
Pursuant to the Barcelona Clinic Liver Cancer (BCLC) and China liver cancer (CNLC) staging systems, transarterial chemoembolization (TACE) is the most common treatment for unresectable HCC.2,4 However, repeated TACE may lead to the deterioration of liver function and affect prognosis. In addition, the efficacy of TACE is suboptimal in patients with BCLC stage B, the point at which there are massive, infiltrative, or intrahepatic multiple disseminated nodules.5 Hepatic arterial infusion chemotherapy (HAIC) has recently received increasing attention in Asian countries. Compared to TACE, HAIC caused fewer grade 3–4 adverse events (AEs) and fewer impaired liver function events in the short-term.6,7 Specifically, HAIC demonstrated favorable objective response rates (ORR) and overall survival (OS) in patients with infiltrating or main portal vein tumor thrombosis (PVTT).8,9 Furthermore, HAIC alone or in conjunction with sorafenib improved OS in advanced HCC patients with a high rate of downstage resection.10–12 Consequently, HAIC has emerged as a promising treatment as a complementary modality to TACE.
Despite encouraging results for intrahepatic malignancies, HAIC did not outperform targeted therapies for extrahepatic metastases (EHM).13 Overcoming the low rates of responses to molecularly targeted or immune single agents, clinical trials such as the IMbrave 150 trial14 and ORIENT-32 trial15 identified that PD-(L)1 inhibitors together with molecularly targeted therapies (MTT) resulted in higher OS and progression-free survival (PFS), and the combination has become the first-line treatment for advanced HCC. Triple therapy in combination with TACE has proven efficacy and safety.16,17 However, there is still a lack of evidence-based medical support for HAIC triple combination in patients who are not suitable for TACE.18–22
Therefore, we conducted this retrospective study to evaluate the safety and efficacy of HAIC along with PD-(L)1 inhibitors and MTT for the treatment of TACE-unsuitable HCC in real settings.
Patients and Methods
Patients
Consecutive HCC patients treated with HAIC at two tertiary care centers from January 2020 to December 2021 were retrospectively analyzed (Figure 1). The inclusion criteria included the following: patients with BCLC stage B or stage C for TACE-unsuitable HCC (for definitions, refer to the Asia-Pacific Expert Consensus Statement on Primary Hepatocellular Carcinoma:5 confluent multinodular type, massive or infiltrative type, simple nodular type with extranodular growth, poorly differentiated type, or intrahepatic multiple disseminated nodules); age ≥ 18 years; HCC with a pathological or clinical diagnosis; Child-Pugh class A-B; Eastern cooperative oncology group (ECOG) performance score of 0–1; and at least one measurable lesion using the modified Response Evaluation Criteria in Solid Tumours (mRECIST). The exclusion criteria included the following: white blood cells < 3.0×109 /L; platelets < 50×109 /L; creatinine > 2 mg/dL or creatinine clearance < 30 mL/min; patients with active autoimmune diseases, severe cardiovascular disorders, major infections, or allergies to relevant chemotherapeutic agents; patients who had received other local or systemic treatments; patients who had received fewer than two cycles of triple therapy; and patients with incomplete clinical data or lost follow-up.
 |
Figure 1 Study flow diagram. |
This study was approved by the Institutional Ethics Review Board of Wuxi People’s Hospital, affiliated with Nanjing Medical University (Wuxi, Jiangsu Province, China) (No. KY23101), and was conducted in accordance with the principles of the Declaration of Helsinki. Considering the retrospective nature of the study, the Institutional Ethics Review Board of Wuxi People’s Hospital, affiliated with Nanjing Medical University, waived the requirement for written informed consent. We declare that patient data are strictly confidential.
HAIC Procedure
The HAIC protocols all involved FOLFOX with catheter placement through the femoral artery, and the procedures were comparable to those previously reported.12,23 Intraoperatively, the gastroduodenal artery was embolized as necessary with a microspring coil, and then the microcatheter was inserted into the tumor’s principal blood supply artery. All chemotherapeutic agents were infused via microcatheter arterial infusion on the ward. The protocol was an 85 mg/m2 or 130 mg/m2 oxaliplatin drip for 2 hours, a 400 mg/m2 calcium folinic acid drip for 2 hours, and a 400 mg/m2 5 fluorouracil regiment followed by 2400 mg/m2 continuous infusion for 46 hours. The therapy was repeated every 3–4 weeks for a maximum of 6 cycles.
PD-(L)1 Inhibitors and MTT
While laboratory testing was not contraindicated, treatments with PD-(L)1 inhibitors and MTT were initiated 1–3 days after the first HAIC. The PD-(L)1 inhibitors included atezolizumab, stintiliumab, tislelizumab, and camrelizumab. The MTTs were mainly sorafenib, lenvatinib, donafenib, apatinib, and bevacizumab. The doses and time intervals for the PD-(L)1 inhibitors and MTT administration were adjusted based on patient tolerability and relevant clinical trials and instructional recommendations.
Follow-Up
Patients with viral hepatitis received standard antiviral medication. After the initial triple therapy, abdominal CTs or MRIs with enhancement and chest CTs were reviewed every 4–8 weeks. Routine blood markers, liver and kidney function, tumor markers, and immune-related indicators were monitored before and after each therapy cycle. The final follow-up visit was scheduled for December 31, 2022, and patients were followed as outpatients or inpatients or via telephone, with detailed records of AEs and survival status. Unless disease progression or intolerable toxicities occurred, HAIC was used for 4–6 full cycles, followed by maintenance treatment with PD-(L)1 inhibitors and MTT.
Outcomes
The primary outcome was OS, and the secondary outcomes included PFS, ORR, disease control rate (DCR), and incidence of AEs. The optimality of the tumor response was assessed by two independent radiologists with 5–10 years of diagnostic abdominal imaging experience utilizing the mRECIST criteria. The ORR was defined as the sum of the CR and PR, whereas the DCR was defined as the sum of the CR, PR, and SD. OS was defined as the interval between the first HAIC therapy and mortality from any cause or the last follow-up, while PFS was defined as the period between the first HAIC treatment and the onset of PD, mortality from any cause, or the final follow-up. All AEs were recorded and evaluated using the National Cancer Institute Common Terminology Criteria for AEs (CTCAE) version 5.0.
Statistical Analyses
Continuous variables are expressed as mean ± standard deviation or median and interquartile range (IQR), and categorical variables are presented as n (%) and were compared using chi-square tests. A one-way analysis was undertaken to identify variables that might be associated with OS (P < 0.1), followed by a Cox regression analysis (forward stepwise likelihood quotient) to discover independent predictors of OS. Cumulative survival was depicted as KaplanMeier curves and compared using Log rank tests. The contribution of each parameter to the risk of the outcome was recorded as a hazard ratio (HR) with a 95% confidence interval (CI). All tests were two-sided, and significance was determined as P < 0.05. SPSS 26.0 (IBM Corporation, Somers, NY) and R 3.4.1 (http://www.R-project.org) were used to perform statistical analyses and calculate the optimal cutoff value for the neutrophil/lymphocyte ratio (NLR) (Figure 2).
 |
Figure 2 NLR cutoff value calculation. |
Results
Patient Characteristics
A total of 55 eligible patients were enrolled in this study (Table 1). All patients had more than three tumors, with 36 patients (65%) having tumors located in both lobes, and 24 patients (43.6%) having a tumor with a maximum diameter greater than 10 cm, and 10 patients (18.2%) having an infiltrative type of tumor. ALBI grade 2 was observed in 38 (69.1%) patients. A total of 33 patients (60%) had PVTT, with 15 patients (27.3%) suffering from VP type III and 18 patients (32.7%) having VP type IV. Ten patients (18.2%) had EHM. The primary sites of metastases were the lungs, adrenal glands, abdominal lymph nodes, and vertebrae. The optimal cutoff value for the NLR was 3.82.
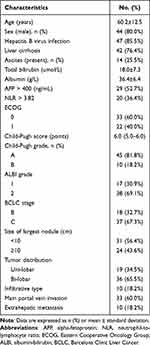 |
Table 1 Baseline Characteristics of Patients |
The median duration of follow-up was 11.0 months (range: 4.0–27.5), and the median numbers of interventions for HAIC and PD-(L)1 inhibitors were four (IQR: 2–4) and six, respectively (IQR: 4–11). As shown in Table 2, the combination regimens of PD-(L)1 inhibitors and MTT were diverse.
 |
Table 2 Regimens of the Triple Combination Therapy |
Treatment Efficacy
According to the analysis, 29 patients (52.7%) had died by the end of the investigation, with a median OS of 15.0 months (HR = 2.640, 95% CI, 9.826–20.174) (Figure 3A) and a PFS of 11.0 months (HR = 1.505, 95% CI, 8.051–13.95) (Figure 3B). Table 3 documents the best tumor response based on mRECIST criteria assessment with 8 (14.5%) CR cases (none of these patients had EHM prior to the first HAIC), 16 (29.1%) PR cases, 10 (18.2%) SD cases and 21 (38.2%) PD cases. The ORR and DCR were 43.6% (24/55) and 61.8% (34/55), respectively. By the end of the study, no patients had undergone surgical resection. This was a decision made by the patients and their families, who took the MDT opinion, the cost of surgery, the patient’s physical status, and the therapeutic efficacy of the current program into account.
 |
Table 3 Summary of Best Response |
 |
Figure 3 Long-term survival outcomes after HAIC triple therapy. (A) OS; (B) PFS. |
Prognostic Factor Analysis
Univariate analysis (Table 4) revealed that liver cirrhosis, ALBI grade, NLR > 3.82, ECOG, and EHM were associated with OS. After adjusting for confounders, NLR > 3.82 (HR 2.380, 95% CI 1.116-2-5.079, P = 0.025), ECOG 1 (HR 2.906, 95% CI 1.373–6.154, P = 0.005), and extrahepatic metastases (HR 8.373, 95% CI 3.492–20.078, P < 0.001) were independent risk factors for OS (Table 4). The Kaplan-Meier curve is illustrated in Figure 4A–C.
 |
Table 4 Univariate and Multivariate Analysis of Risk Factors for OS and PFS |
 |
Figure 4 Kaplan-Meier analysis of NLR > 3.82 (A), ECOG (B), and EHM (C) with OS. |
Comparable to the findings from the OS analysis, the risk factors for the median PFS (Table 4) were predominantly ECOG 1 (HR 3.170, 95% CI 1.607–6.256, P = 0.001) and extrahepatic metastases (HR 5.870, 95% CI 2.615–13.175, P < 0.001).
Adverse Events
Thirty-two patients (58.2%) suffered at least one treatment-related AE during the study (Table 5), and no therapeutic-related deaths occurred. The most common AEs were AST elevation (18.2%), bilirubin elevation (16.4%), thrombocytopenia (16.4%), neutropenia (12.7%), and malaise (10.9%). Among them, 29 cases (52.7%) were grade 1–2 AEs and 10 cases (18.2%) were grade 3 AEs.
 |
Table 5 Treatment Related Adverse Events |
One patient experienced a grade 4 AE manifesting as immune-associated myocarditis, which improved with hormonal therapy, and terminated PD-(L)1 indefinitely while continuing HAIC plus MTT. After endoscopic treatment for ruptured esophagogastric variceal bleeding, one patient discontinued bevacizumab and switched to a tyrosine kinase inhibitor. Three patients stopped triple therapy due to hyperbilirubinemia. Additionally, three patients had their doses of oxaliplatin reduced to 85 mg/m2 because of abdominal pain.
Discussion
In our real-world study, HAIC, in combination with PD-(L)1 inhibitors and MTT, demonstrated controlled toxicities with an ORR and DCR of 43.6% and 61.8%, and a median OS and PFS of 15.0 months and 11.0 months, respectively. The triple therapy regimen is safe and effective in treating intermediate and advanced HCC that is unsuitable for TACE; however careful patient selection is still warranted.
Local perfusion avoids the first-pass effect and increases chemotherapeutic drug concentrations in liver tumors while minimizing hepatic damage or toxic side effects.24 Triple therapy has been made possible by the synergistic antitumor effects of HAIC with MTT or PD-(L)1 inhibitors, which have also been confirmed in previous studies.10,11,25 Researches in the literature have shown a median OS of approximately 15.9 months, a median PFS of 8.8 to 11.1 months, and an ORR and DCR of 40.0%~67.6% and 77.6%~94.6%, respectively, for triple therapy.18,26–29 Our research included more patients with substantial tumor loads, infiltrative types, or concurrent PVTT. Most patients had an ECOG 1 or ALBI 2 status at baseline. The patients’ tumor or hepatic function characteristics indicated that the efficacy of TACE was limited or that TACE was not appropriate. Overall, this study revealed a favorable median OS, PFS, and ORR and an acceptable DCR with the HAIC triple combination.
Unsatisfactory outcomes for EHM treatment have constrained the use of HAIC.13 Lyu et al30 discovered that the median PFS was merely 2.9 months for HCC patients with EHM who were treated with a single regimen of HAIC, and date from subgroup analyses of patients with EHM undergoing targeted combination immunotherapy are still limited. Patients who received triple therapy had an improved PFS, but this still barely reached 6.6 months.27 Patients with EHM constitute a subgroup that is challenging to manage and demands timely intervention. As we mentioned, these patients had high progression rates, low tumor response rates, and limited survival, whereas the worse prognosis may be associated with HAIC as a local treatment only and the hyper-immunosuppressive character of the tumor microenvironment in HCC.31 Nevertheless, frequent liver function markers and intrahepatic lesion progression determined advanced HCC patients’ survival, which might be improved with effective management and active control of extrahepatic lesions.32
Neutrophils constitute a distinct component of the inflammatory response and are linked to immunosuppression.33 In contrast, lymphocyte depletion has been associated with impaired antitumor immune responses.34 NLR, which indicates the balance between the pro-tumor inflammatory state and the anti-tumor immune response, is increasingly being used to predict HCC survival.35 High NLR (≥4) was associated with diminished survival and inadequate treatment response in HAIC-treated patients.36 NLR ≤ 3.82 is a protective factor for OS, as was determined by our study, which also led to a similar result. This suggests that these two clinically accessible serum markers, which have robust predictive properties, have value for future intensive analysis in the triple therapy population.
Multifactorial analysis indicated that ECOG 1 predicts poor outcomes, suggesting that triple therapy may be more physically demanding. The parameters determining OS and PFS in the research were mostly consistent, and PFS was relatively close to OS, which showed subsequent progression under triple treatment and that receiving alternative regimens provided little benefit and patient survival declined dramatically. How postline therapy can improve survival may be one of the research hotspots in the field of intermediate and advanced HCC. There are some details that need to be concerned. In addition to monitoring dynamic changes in liver function indicators and adverse reactions to local or systemic therapy, the nutritional status of the patient, hepatitis activity, and the degree of esophagogastric varices should also be considered. These factors by themselves, or their consequences, are likely to influence the course of tumor therapy. The work we have carried out so far provides some references for future researchers, and an in-depth exploration of interventions after the progression of triple therapy is an urgent issue.
Our study involved fewer AEs, but grade 3–4 AEs were similar to prior findings (17.7–72.2%).18,19,27 Certain AEs, such as adverse skin events, hand-foot skin reactions, and hypertension, associated with improved survival after treatment with sorafenib or lenvatinib, could predict the prognosis of HCC.37,38 Furthermore, immune-related AEs were also linked to the efficacy of immune checkpoint inhibitors, whether administered alone or in combination with MTT.39 However, AEs were temporal exposure factors with diverse intensity and organ involvement, making risk regression models unsuitable for analysis. As our analysis showed, targeted and immune medications vary in the real world, and combination schemes have to consider patients, economic burden and health insurance policies. Moreover, the HAIC treatment protocols, dosages, and techniques differed between investigations.40 These might complicate the experimental design and the interpretation of results, which requires extra consideration.
The present research has some limitations. First, retrospective studies with small samples have selection bias. Second, this real-world study included an extensive range of patients, the baseline characteristics were not homogeneous, and a small percentage of patients had progressed before HAIC treatment, which potentially reduced the benefits of treatments. Third, the PD-(L)1 inhibitor and the inconsistency of the MTT scenarios probably affected the results. Fourth, although subgroup analyses were performed, interpretation and generalization of the optimal cutoff values for NLR and other high-risk factors should be undertaken extraordinarily cautious due to sample size limitations, and they need to be validated through multicenter large-sample retrospective or randomized controlled trials in the future.
Conclusion
Our real-world study demonstrated that a triple combination of HAIC, PD-(L)1 inhibitor, and MTT initially showed acceptable safety and efficacy; thus, it represents a potential treatment strategy for patients with intermediate- and advanced-stage HCC who are not suitable for TACE.
Data Sharing Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author (Wei-Dong Wang).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 Edition). Liver Cancer. 2020;9(6):682–720. doi:10.1159/000509424
3. Villanueva A, Longo DL. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi:10.1056/NEJMra1713263
4. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681–693. doi:10.1016/j.jhep.2021.11.018
5. Kudo M, Han KH, Ye SL, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–260. doi:10.1159/000507370
6. Yue R, Liu X. Impact of transarterial chemoembolization or hepatic artery infusion chemotherapy on liver function after hepatocellular carcinoma resection: an observational study. Digestion. 2023;1–8. doi:10.1159/000528750
7. Chen S, Yuan B, Yu W, et al. Comparison of arterial infusion chemotherapy and chemoembolization for locally advanced hepatocellular carcinoma: a multicenter retrospective study. J Gastrointest Surg. 2022;26(11):2292–2300. doi:10.1007/s11605-022-05421-x
8. An C, Zuo M, Li W, et al. Infiltrative hepatocellular carcinoma: transcatheter arterial chemoembolization versus hepatic arterial infusion chemotherapy. Front Oncol. 2021;11:747496. doi:10.3389/fonc.2021.747496
9. Hu J, Bao Q, Cao G, et al. Hepatic arterial infusion chemotherapy using oxaliplatin plus 5-fluorouracil versus transarterial chemoembolization/embolization for the treatment of advanced hepatocellular carcinoma with major portal vein tumor thrombosis. Cardiovasc Intervent Radiol. 2020;43(7):996–1005. doi:10.1007/s00270-019-02406-3
10. Zheng K, Zhu X, Fu S, et al. Sorafenib plus hepatic arterial infusion chemotherapy versus sorafenib for hepatocellular carcinoma with major portal vein tumor thrombosis: a randomized trial. Radiology. 2022;303(2):455–464. doi:10.1148/radiol.211545
11. He M, Li Q, Zou R, et al. Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol. 2019;5(7):953–960. doi:10.1001/jamaoncol.2019.0250
12. Lyu N, Wang X, Li JB, et al. Arterial chemotherapy of oxaliplatin plus fluorouracil versus sorafenib in advanced hepatocellular carcinoma: a biomolecular exploratory, randomized, Phase III trial (FOHAIC-1). J Clin Oncol. 2022;40(5):468–480. doi:10.1200/JCO.21.01963
13. Ueshima K, Ogasawara S, Ikeda M, et al. Hepatic arterial infusion chemotherapy versus sorafenib in patients with advanced hepatocellular carcinoma. Liver Cancer. 2020;9(5):583–595. doi:10.1159/000508724
14. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
15. Ren Z, Xu J, Bai Y, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, Phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
16. Huang J, Zhong B, Jiang N, et al. Transarterial chemoembolization combined with immune checkpoint inhibitors plus tyrosine kinase inhibitors versus immune checkpoint inhibitors plus tyrosine kinase inhibitors for advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2022;9:1217–1228. doi:10.2147/JHC.S386672
17. Zhu HD, Li HL, Huang MS, et al. Transarterial chemoembolization with PD-(L)1 inhibitors plus molecular targeted therapies for hepatocellular carcinoma (CHANCE001). Signal Transduct Target Ther. 2023;8(1):58. doi:10.1038/s41392-022-01235-0
18. J LB, Gao S, Zhu X, et al. Real-world study of hepatic artery infusion chemotherapy combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors for advanced hepatocellular carcinoma. Immunotherapy. 2021;13(17):1395–1405. doi:10.2217/imt-2021-0192
19. Lai Z, He M, Bu X, et al. Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: a biomolecular exploratory, Phase II trial. Eur J Cancer. 2022;174:68–77. doi:10.1016/j.ejca.2022.07.005
20. Li S, Lyu N, Han X, et al. Hepatic artery infusion chemotherapy using fluorouracil, leucovorin, and oxaliplatin versus transarterial chemoembolization as initial treatment for locally advanced hepatocellular carcinoma: a propensity score-matching analysis. J Vasc Interv Radiol. 2021;32(9):1267–1276. doi:10.1016/j.jvir.2021.06.008
21. Chen CB, Chen CM, Tzeng RH, et al. Combining HAIC and sorafenib as a salvage treatment for patients with treatment-failed or advanced hepatocellular carcinoma: a single-center experience. J Clin Med. 2023;12(5). doi:10.3390/jcm12051887
22. Huang Y, Zhang L, He M, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus sorafenib for hepatocellular carcinoma refractory to transarterial chemoembolization: retrospective subgroup analysis of 2 prospective trials. Technol Cancer Res Treat. 2022;21:2081108019. doi:10.1177/15330338221117389
23. Li Q, He M, Chen H, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin versus transarterial chemoembolization for large hepatocellular carcinoma: a randomized Phase III trial. J Clin Oncol. 2022;40(2):150–160. doi:10.1200/JCO.21.00608
24. Obi S, Sato S, Kawai T. Current status of hepatic arterial infusion chemotherapy. Liver Cancer. 2015;4(3):188–199. doi:10.1159/000367746
25. Mei J, H LS, J LQ, et al. Anti-PD-1 immunotherapy improves the efficacy of hepatic artery infusion chemotherapy in advanced hepatocellular carcinoma. J Hepatocell Carcinoma. 2021;8:167–176. doi:10.2147/JHC.S298538
26. Zhang W, Zhang K, Liu C, et al. Hepatic arterial infusion chemotherapy combined with anti-PD-1/PD-L1 immunotherapy and molecularly targeted agents for advanced hepatocellular carcinoma: a real world study. Front Immunol. 2023;14:1127349. doi:10.3389/fimmu.2023.1127349
27. Luo L, Xiao Y, Zhu G, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: a tertiary medical center experience. Front Oncol. 2022;12:1004652. doi:10.3389/fonc.2022.1004652
28. He MK, Liang RB, Zhao Y, et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol. 2021;13:17552544. doi:10.1177/17588359211002720
29. Mei J, Tang YH, Wei W, et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol. 2021;11:618206. doi:10.3389/fonc.2021.618206
30. Lyu N, Kong Y, Pan T, et al. Hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin in hepatocellular cancer with extrahepatic spread. J Vasc Interv Radiol. 2019;30(3):349–357. doi:10.1016/j.jvir.2018.09.004
31. Santagata S, Rea G, Castaldo D, et al. Hepatocellular carcinoma (HCC) tumor microenvironment is more suppressive than colorectal cancer liver metastasis (CRLM) tumor microenvironment. Hepatol Int. 2023. doi:10.1007/s12072-023-10537-6
32. Long HY, Huang T-Y, Xie X-Y, et al. Treatment strategies for hepatocellular carcinoma with extrahepatic metastasis. World J Clin Cases. 2021;9(21):5754–5768. doi:10.12998/wjcc.v9.i21.5754
33. Chen H, Zhou XH, Li JR, et al. Neutrophils: driving inflammation during the development of hepatocellular carcinoma. Cancer Lett. 2021;522:22–31. doi:10.1016/j.canlet.2021.09.011
34. Zhu H, Feng J, Xiang Y, et al. Combination of alpha-fetoprotein and neutrophil-to-lymphocyte ratio to predict treatment response and survival outcomes of patients with unresectable hepatocellular carcinoma treated with immune checkpoint inhibitors. BMC Cancer. 2023;23(1):547. doi:10.1186/s12885-023-11003-0
35. Zhang L, Feng J, Kuang T, et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint Inhibitors: a pooled analysis of 44 retrospective sudies. Int Immunopharmacol. 2023;118:110019. doi:10.1016/j.intimp.2023.110019
36. Tajiri K, Kawai K, Minemura M, et al. Neutrophil/lymphocyte ratio as a prognostic indicator of hepatic arterial infusion chemotherapy with arterial cisplatin plus continuous 5-fluorouracil. Hepatol Res. 2015;45(7):755–763. doi:10.1111/hepr.12417
37. Reig M, Torres F, Rodriguez-Lope C, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61(2):318–324. doi:10.1016/j.jhep.2014.03.030
38. Shimose S, Iwamoto H, Niizeki T, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers. 2020;12(7):1867. doi:10.3390/cancers12071867
39. Ng K, Tan J, Tan JJE, et al. Impact of immune-related adverse events on efficacy of immune checkpoint inhibitors in patients with advanced hepatocellular carcinoma. Liver Cancer. 2022;11(1):9–21. doi:10.1159/000518619
40. Chen CT, Liu TH, Shao YY, Liu KL, Liang PC, Lin ZZ. Revisiting hepatic artery infusion chemotherapy in the treatment of advanced hepatocellular carcinoma. Int J Mol Sci. 2021;22(23). doi:10.3390/ijms222312880
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
