Back to Journals » International Journal of General Medicine » Volume 15
The Role of Serum CD26 in the Diagnosis of Gastric Cancer
Received 17 June 2022
Accepted for publication 7 September 2022
Published 12 September 2022 Volume 2022:15 Pages 7179—7187
DOI https://doi.org/10.2147/IJGM.S378620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ju Yup Lee,1,2 Mae-Ja Park3
1Department of Internal Medicine, Keimyung University School of Medicine, Daegu, South Korea; 2Institute for Cancer Research, Keimyung University, Daegu, South Korea; 3Department of Anatomy, School of Medicine, Kyungpook National University, Daegu, South Korea
Correspondence: Mae-Ja Park, Department of Anatomy, School of Medicine, Kyungpook National University, Daegu, South Korea, Tel/Fax +82-53-420-4802, Email [email protected]
Purpose: The value of serum cluster of differentiation 26 (CD26) in gastric cancer remains unknown. We investigated serum CD26 as a non-invasive serological marker for the diagnosis of gastric cancer and its relationship with serum human epidermal growth factor receptor-2 (HER2) levels.
Patients and Methods: We enrolled 393 gastric cancer patients treated with endoscopic resection or surgery, and 90 healthy controls. HER2 positivity in tissue was evaluated by immunohistochemistry staining, and the serum CD26 and HER2 levels were measured using an enzyme-linked immunosorbent assay.
Results: Serum CD26 levels were significantly lower in gastric cancer patients than in healthy controls (582.2 ± 254.3 vs 862.7 ± 410.6 ng/mL, P< 0.001). Serum CD26 levels were significantly lower in advanced gastric cancer compared to early gastric cancer (642.2 ± 333.9 vs 503.4 ± 332.7 ng/mL, P< 0.001), and tended to decrease with gastric cancer progression. To diagnose gastric cancer, the optimal cut-off value of serum CD26 was 762.7 ng/mL with 75.6% sensitivity and 64.4% specificity. Serum CD26 levels were weakly correlated with serum HER2 levels (rs=0.363, P< 0.001). However, no difference in serum CD26 levels was observed between tissue HER2-negative and HER2-positive gastric cancer groups (586.2 ± 362.1 vs 579.6 ± 264.8 ng/mL, P=0.898).
Conclusion: CD26 is a useful non-invasive serological marker for gastric cancer diagnosis; however, its levels do not correlate with HER2 status.
Keywords: gastric cancer, biomarker, CD26, HER2
Introduction
Gastric cancer is one of the main health issues worldwide, and the most common cancer in South Korea.1 In South Korea, cancer screening is implemented as a part of the national cancer screening program; men and women over 40 years of age undergo upper endoscopy every 2 years for the screening of gastric cancer. In addition, upper endoscopy is recommended every year for those with a family history of gastric cancer, or those that are at high risk for gastric cancer such as patients with chronic atrophic gastritis or with intestinal metaplasia.2,3 Although endoscopy is the best method for screening, the presence of a serological marker that can predict cancer, such as α-fetoprotein for hepatocellular carcinoma or carcinoembryonic antigen for colorectal cancer, will be of great help in screening. Development of serological biomarkers in countries with a high prevalence of gastric cancer is important for screening and early detection; however, there are no serological markers specific for gastric cancer.
Several studies have reported that the cluster of differentiation 26 (CD26)/dipeptidyl peptidase-4 (DPP-4) gene affects the invasiveness of various cancer cells.4–8 CD26 has been revealed as a useful serological marker for the detection of colorectal cancer, as well as a prognostic factor that promotes metastasis of human colorectal cancer.4,9 However, the value of serum CD26 for the diagnosis of gastric cancer remains unknown.10
The human epidermal growth factor receptor-2 (HER2) protein, a member of the transmembrane tyrosine kinase receptor family, plays pivotal roles in cell growth, differentiation, and survival of gastric cancer.11 Several studies have revealed that serum HER2 levels are associated with HER2 overexpression in cancer cells.12 In a study analyzing the relationship between serum CD26 and gastric cancer tissue HER2, it was revealed that the serum CD26 levels were low in HER2-positive gastric cancer, suggesting a negative relationship between them.10
Regarding the prevalence of gastric cancer, non-invasive serological markers would be beneficial for screening, especially in early gastric cancer (EGC). Here we aimed to investigate the role of serum CD26 as a non-invasive serological marker for the screening of gastric cancer and its relationship with serum HER2 levels.
Materials and Methods
Subjects
We enrolled 393 gastric cancer patients who underwent endoscopic resection or surgery at Keimyung University Dongsan Medical Center between June 2006 and June 2016. Ninety subjects without a history of upper gastrointestinal disease were enrolled as healthy controls. Patients with previous gastrointestinal surgery, with other malignancy, and with severe systemic comorbidities were excluded. The medical records of the registered subjects were reviewed retrospectively. Data such as sex, gastric cancer stage, H. pylori status, and histological type of gastric cancer by Lauren classification were collected. Cancer stage was based on the 7th edition of the American Joint Committee on Cancer/Union for International Cancer Control tumor, node, metastasis staging system.13 H. pylori positivity was defined as histologic confirmation of H. pylori by modified Giemsa or Warthin-starry silver stain, or CLOtest (Delta West, Bentley, Australia) positive. The histological type of gastric cancer was classified into intestinal (well-differentiated type) and diffuse (poorly differentiated type) according to the Lauren classification. This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (DSMC 2016–06-034). Informed consent obtained from the study participants for the use of their medical records data prior to study commencement. The biospecimens used in this study were provided by the Keimyung Human Bio-Resource Bank (KHBB), a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols that in accordance with the Declaration of Helsinki.
Determination of Serum CD26 and HER2
The concentrations of serum CD26 and HER2 were determined by specific human CD26/DPP4 and human ErbB2 (HER2) enzyme-linked immunosorbent assay kits, respectively (Thermo Fisher Scientific, Waltham, MA, USA). Enzyme-linked immunosorbent assays were performed according to the manufacturer’s instructions.
Determination of HER2 Expression in Gastric Cancer Tissues
Gastric cancer tissue HER2 immunohistochemistry (IHC) was performed by an experienced pathologist and followed the scoring system proposed by Hofmann et al14 and Rüschoff et al15 from 0 to 3+. In case of the IHC 2+, amplification of the HER2 gene was confirmed by fluorescence in situ hybridization (FISH). IHC 3+, IHC 2+, and positive FISH cases were considered as tissue HER2-positive.
Statistical Analyses
Continuous variables are expressed as median ± interquartile range (IQR). Categorical variables are presented as numbers and percentages. The Mann–Whitney U-test was used to compare the medians of continuous variables and Chi-square test was applied to compare categorical variables. Receiver operator characteristic (ROC) curves were used to obtain appropriate cut-off values of serum CD26 for the diagnosis of gastric cancer. The relationship between serum CD26 and serum HER2 was analyzed using the Spearman rank correlation (rs). Two-sided P values of less than 0.05 were considered statistically significant. All statistical analyses were performed using Analyse-it (version 5.90, Analyse-it Software, Ltd., http://analyse-it.com/; 2021).
Results
Baseline Characteristics of Subjects
Table 1 shows the baseline characteristics of gastric cancer patients and control patients. The median age of the gastric cancer group was 64.0 ± 16.0 years and that of the control group was 58.0 ± 20.1 years. The male to female ratio was 251/142 in the gastric cancer group. The proportion of intestinal-type gastric cancer was 52.4%, and that of diffuse-type gastric cancer was 46.8%. The ratio of EGC to advanced gastric cancer (AGC) was 227/166. The H. pylori positivity was 73.0% in the gastric cancer group.
 |
Table 1 Baseline Characteristics of Study Subjects |
Serum CD26 Levels in Gastric Cancer Patients vs Controls
First, healthy control and gastric cancer patients were compared and lower serum levels of CD26 were observed in gastric cancer patients than that in control patients (862.7 ± 401.6 vs 582.2 ± 327.3 ng/mL, P<0.001) (Figure 1). In the comparison between EGC and AGC, serum levels of CD26 were lower in AGC than that in EGC (642.2 ± 333.9 vs 503.4 ± 332.7 ng/mL, P<0.001, respectively) (Figure 2). Analysis was performed according to the gastric cancer stage and a trending decrease in serum CD26 was observed as the stage of gastric cancer progressed (P<0.001; stage 1 versus stage 2, P=0.002; stage 1 versus stage 3, P<0.001) (Figure 3).
 |
Figure 1 Serum level of CD26 in gastric cancer versus controls. The serum CD26 level was significantly lower in the gastric cancer group than in the control group. |
 |
Figure 3 Serum level of CD26 according to the T stage of gastric cancer. Serum CD26 levels were significantly lower in stage 2 and stage 3 compared to stage 1. |
Power of Serum CD26 in the Diagnosis of Gastric Cancer
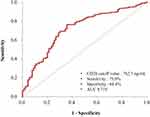
ROC curve analysis was performed to evaluate the gastric cancer diagnostic ability of serum CD26 value. The optimal cut-off value of serum CD26 was 762.7 ng/mL for the diagnosis of gastric cancer, with a sensitivity of 75.6% and a specificity of 64.4% (area under the curve (AUC): 0.719, 95% CI: 0.660–0.778) (Figure 4).
Correlation Between Serum HER2 and Tissue HER2 Status
The correlation between serum HER2 and tissue HER2 was analyzed and serum HER2 levels were significantly correlated with tissue HER2 status (P=0.003) (Figure 5A). ROC curve analysis was performed and the AUC for serum HER2 was 0.638 (95% CI: 0.543–0.732) and the optimal cut-off value of serum HER2 was 11.3 ng/mL for diagnosing HER2-positive gastric cancer, with 63.6% sensitivity and 66.8% specificity (Figure 5B). When the cut-off value was reset to 13.8 ng/mL, the sensitivity and specificity for HER2-positive gastric cancer diagnosis were 18.2% and 91.4%, respectively.
Relationship Between Serum CD26 and Serum HER2 Levels
Finally, correlation analysis was performed to analyze the correlation between serum CD26 and serum HER2 levels. Serum CD26 levels had a weak positive correlation with serum HER2 levels (rs=0.363, P<0.001) (Figure 6A). However, no statistically significant difference in serum CD26 levels was observed between tissue HER2-negative and -positive gastric cancer groups (586.2 ± 362.1 vs 579.6 ± 264.8 ng/mL, P=0.898) (Figure 6B).
Discussion
In this study, we found that serum CD26 levels were significantly lower in gastric cancer patients than that in control patients. In particular, we observed that the serum CD26 levels significantly reduced from EGC to AGC and from stage I to III. In addition, it was shown that the serum HER2 levels reflects the tissue HER2 levels. The serum HER2 levels had a weak positive correlation with the serum CD26 levels; however, no significant difference was observed in serum CD26 levels between HER2-negative and HER2-positive gastric cancers.
CD26/DPP-4 is a multifunction glycoprotein with intrinsic DPP-4 activity, which is expressed on epithelial cells and lymphocytes.16,17 This protein functions as a proline-specific dipeptidyl peptidase that is involved in T-cell activation and can cleave several chemokines.18 The major substrates of CD26 are gastric inhibitory polypeptide and glucagon-like peptide-1; therefore, CD26 inhibitors have been used in the treatment of type II diabetes.19 In addition, CD26 is involved in tumorigenesis and may act as a tumor suppressor or activator, depending on the tumor microenvironment.20,21 Recently, over-expression of CD26 was founded in several cancers, including malignant mesothelioma,22 thyroid cancer,23 urothelial cancer,24 and pancreatic cancer.25 In contrast, under-expression of CD26 has also been reported in colorectal cancer (CRC). Cordero et al reported decreased serum CD26 levels in patients with CRC compared to healthy controls in early CRC.26 De Chiara et al measured serum CD26 levels in 299 patients who underwent colonoscopy and reported that CRC patients had lower serum CD26 levels than those without CRC.27
Although data related to CD26/DPP-4 expression in gastric cancer are currently limited, relevance has been reported as a marker for gastric cancer stem cells (CSCs).28 In this study, the authors used the CD44 and CD26 markers to identify gastric CSCs with distinct tumorigenicity. In vivo, CD26+/CD44+ cells demonstrated the highest tumorigenicity, while CD26-/CD44- CSCs demonstrated very low or no tumorigenicity. Another study demonstrated CD26 as a marker of the invasive gastric CSCs phenotype, and that CD26-/CXCR4- gastric CSCs transformed into a more invasive phenotype.29 In a clinical study by Boccardi et al,10 which measured serum CD26 levels in gastric cancer patients, the gastric cancer group had lower serum CD26 levels than the control group. The authors suggested that serum CD26 could be an early detection marker for gastric cancer and may serve as a prognostic marker.10
Another noteworthy result of the study by Boccardi et al was that HER2-positive patients with gastric cancer had lower CD26 levels compared to HER2-negative gastric cancer patients.10 HER2 is an oncogene that promotes cell proliferation and inhibits cell death, and is overexpressed in 6–35% of gastric cancer cases.30 HER2 overexpression is used as a predictive index for gastric cancer and is associated with a low survival rate.31 In addition, HER2-positive patients were treated with trastuzumab (Herceptin), a targeted treatment for gastric cancer. In another study, the Herceptin combination therapy group showed a superior survival rate.32 Therefore, for all patients diagnosed with gastric cancer, it is very important to perform HER2 testing at the time of initial diagnosis for prognosis evaluation and to guide treatment decisions. In this study we observed that the non-invasive serum HER2 level reflects the tissue HER2 status well, and it can be used as a tool to replace biopsy in the future. However, unlike the study by Boccardi et al, in this study, although there was a weak positive correlation between serum HER2 and serum CD26 (rs=0.363), there was no significant difference in serum CD26 levels between the HER2-positive and HER2-negative gastric cancer groups. Although the results are considered more reliable with a large sample of 393 gastric cancer patients, this is a retrospective study; hence, the possibility of various biases cannot be excluded. Therefore, the relationship between CD26 and HER2, ie, whether the status of HER2 can be predicted by measuring serum CD26 levels, needs to be investigated in future studies.
The advantage of this study is that it presented the role of serum CD26 as a gastric cancer screening tool more effectively than previous studies, by collecting and analyzing serum from a relatively large number of gastric cancer patients. In addition, through analysis according to the tumor stage, it was possible to observe the change in the serum CD26 levels according to tumor progression. However, as this was a retrospective study that analyzed data from patients who underwent endoscopic resection or surgery, there was almost no serum from stage IV gastric cancer patients. The fact that no analysis was performed for stage IV patients is a limitation of this study.
Conclusion
In conclusion, serum CD26 levels are considered useful non-invasive serologic markers for the diagnosis of gastric cancer. This new biomarker can be used as an auxiliary tool for endoscopic gastric cancer screening to enable early diagnosis, which can be expected to reduce mortality and medical costs. Serum CD26 levels do not appear to be correlated with HER2 status in gastric cancer; however, more research is needed in the future to further evaluate the relationship between CD26 and HER2.
Institutional Review Board Statement
This study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center, Korea (DSMC 2016-06-034).
Data Sharing Statement
The data that support the results of this study are available from Dr. Ju Yup Lee ([email protected]) upon reasonable request.
Ethics Approval and Informed Consent
All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols that in accordance with the Declaration of Helsinki.
Acknowledgments
The biospecimens used in this study were provided by the Keimyung Human Bio-Resource Bank (KHBB), a member of the National Biobank of Korea, which is supported by the Ministry of Health and Welfare. All samples derived from the National Biobank of Korea were obtained with informed consent under institutional review board-approved protocols.
Funding
This study was supported by the research promoting grant from the Keimyung University Dongsan Medical Center in 2016.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Jung KW, Won YJ, Kong HJ, et al. Community of population-based regional cancer registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi:10.4143/crt.2018.143
2. Shin WG, Kim HU, Song HJ, et al. Korean college of helicobacter and upper gastrointestinal research. Surveillance strategy of atrophic gastritis and intestinal metaplasia in a country with a high prevalence of gastric cancer. Dig Dis Sci. 2012;57:746–752. doi:10.1007/s10620-011-1919-0
3. Yoon H, Kim N, Lee HS, et al. Effect of endoscopic screening at 1-year intervals on the clinicopathologic characteristics and treatment of gastric cancer in South Korea. J Gastroenterol Hepatol. 2012;27:928–934. doi:10.1111/j.1440-1746.2011.07038.x
4. Pang R, Law WL, Chu AC, et al. A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell. 2010;6:603–615. doi:10.1016/j.stem.2010.04.001
5. Aliyari Serej Z, Ebrahimi Kalan A, Mehdipour A, et al. Regulation and roles of CD26/DPPIV in hematopoiesis and diseases. Biomed Pharmacother. 2017;91:88–94. doi:10.1016/j.biopha.2017.04.074
6. Busek P, Duke-Cohan JS, Sedo A. Does DPP-IV inhibition offer new avenues for therapeutic intervention in malignant disease? Cancers. 2022;14:2072. doi:10.3390/cancers14092072
7. Lu Z, Qi L, Bo XJ, et al. Expression of CD26 and CXCR4 in prostate carcinoma and its relationship with clinical parameters. J Res Med Sci. 2013;18:647–652.
8. Zheng X, Liu J, Li X, et al. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021;497:190–201. doi:10.1016/j.canlet.2020.10.009
9. De Chiara L, Rodríguez-Piñeiro AM, Cordero OJ, et al. Postoperative serum levels of sCD26 for surveillance in colorectal cancer patients. PLoS One. 2014;9:e107470. doi:10.1371/journal.pone.0107470
10. Boccardi V, Marano L, Rossetti RR, et al. Serum CD26 levels in patients with gastric cancer: a novel poten-tial diagnostic marker. BMC Cancer. 2015;15:703. doi:10.1186/s12885-015-1757-0
11. Roskoski R Jr. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34–74. doi:10.1016/j.phrs.2013.11.002
12. Saito M, Yamashita K, Arimura Y, et al. Serum HER2 as an adjunct to assess HER2 status for advanced gastric cancer: a prospective multicenter trial (Sherlock). Acta Oncol. 2016;55:309–317. doi:10.3109/0284186X.2015.1107189
13. Kwon SJ. Evaluation of the 7th UICC TNM staging system of gastric cancer. J Gastric Cancer. 2011;11:78–85. doi:10.5230/jgc.2011.11.2.78
14. Hofmann M, Stoss O, Shi D, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi:10.1111/j.1365-2559.2008.03028.x
15. Rüschoff J, Dietel M, Baretton G, et al. HER2 diagnostics in gastric cancer-guideline validation and development of standardized immunohistochemical testing. Virchows Arch. 2010;457:299–307. doi:10.1007/s00428-010-0952-2
16. Ohnuma K, Hosono O, Dang NH, et al. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem. 2011;53:51–84.
17. Ohnuma K, Takahashi N, Yamochi T, et al. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci. 2008;13:2299–2310. doi:10.2741/2844
18. Klemann C, Wagner L, Stephan M, et al. Cut to the Chase: a Review of CD26/Dipeptidyl Peptidase-4ʹs (DPP4) entanglement in the immune system. Clin Exp Immunol. 2016;185:1–21. doi:10.1111/cei.12781
19. Tseng CM, Liao WC, Chang CY, et al. Incretin-based pharmacotherapy and risk of adverse pancreatic events in the ethnic Chinese with diabetes mellitus: a population-based study in Taiwan. Pancreatology. 2017;17:76–82. doi:10.1016/j.pan.2016.10.003
20. Ohnuma K, Hatano R, Komiya E, et al. A novel role for CD26/dipeptidyl peptidase IV as a therapeutic target. Front Biosci. 2018;23:1754–1779. doi:10.2741/4671
21. Doonan BP, Ohnuma K, Dang LH, et al. Current and emerging therapy for malignant pleural mesothelioma: focus on CD26/Dipeptidyl Peptidase IV as a therapeutic target. Curr Med Chem. 2017;13:76–88.
22. Aoe K, Amatya VJ, Fujimoto N, et al. CD26 overexpression is associated with prolonged survival and enhanced chemosensitivity in malignant pleural mesothelioma. Clin Cancer Res. 2012;18:1447–1456. doi:10.1158/1078-0432.CCR-11-1990
23. Lee JJ, Wang TY, Liu CL, et al. Dipeptidyl Peptidase IV as a prognostic marker and therapeutic target in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2017;102:2930–2940. doi:10.1210/jc.2017-00346
24. Liang PI, Yeh BW, Li M, et al. DPP4/CD26 overexpression in urothelial carcinoma confers an independent prognostic impact and correlates with intrinsic biological aggressiveness. Oncotarget. 2017;8:2995–3008. doi:10.18632/oncotarget.13820
25. Yan L, Tian X, Ye C, et al. CD26 as a promising biomarker for predicting prognosis in patients with pancreatic tumors. Onco Targets Ther. 2020;13:12615–12623. doi:10.2147/OTT.S278736
26. Cordero OJ, Ayude D, Nogueira M, et al. Preoperative serum CD26 levels: diagnostic efficiency and predictive value for colorectal cancer. Br J Cancer. 2000;83:1139–1146. doi:10.1054/bjoc.2000.1410
27. De Chiara L, Rodríguez-Piñeiro AM, Rodríguez-Berrocal FJ, et al. Serum CD26 is related to histopathological polyp traits and behaves as a marker for colorectal cancer and advanced adenomas. BMC Cancer. 2010;10:333. doi:10.1186/1471-2407-10-333
28. Nishikawa S, Konno M, Hamabe A, et al. Surgically resected human tumors reveal the biological significance of the gastric cancer stem cell markers CD44 and CD26. Oncol Lett. 2015;9:2361–2367. doi:10.3892/ol.2015.3063
29. Jiang YX, Yang SW, Li PA, et al. The promotion of the transformation of quiescent gastric cancer stem cells by IL-17 and the underlying mechanisms. Oncogene. 2017;36:1256–1264. doi:10.1038/onc.2016.291
30. Bang YJ. Advances in the Management of HER2-positive advanced gastric and gastroesophageal junction cancer. J Clin Gastroenterol. 2012;46:637–648. doi:10.1097/MCG.0b013e3182557307
31. Cho JH, Lim JY, Cho JY. Survival analysis based on human epidermal growth factor 2 status in stage II–III gastric cancer. World J Gastroenterol. 2017;23:7407–7414. doi:10.3748/wjg.v23.i41.7407
32. Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a Phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi:10.1016/S0140-6736(10)61121-X
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.