Back to Journals » Drug Design, Development and Therapy » Volume 15
The Role of Pretreatment 18F-FDG PET/CT for Early Prediction of Neoadjuvant Chemotherapy Response in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma
Authors Yao J , Wang Y, Lin Y, Yang Y, Wan J, Gong X, Zhang F, Zhang W, Marks T, Wang S, Jin H, Shan H
Received 19 July 2021
Accepted for publication 17 September 2021
Published 1 October 2021 Volume 2021:15 Pages 4157—4166
DOI https://doi.org/10.2147/DDDT.S330154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Jijin Yao,1,2,* Ying Wang,3,* Yujing Lin,4 Yingying Yang,5 Jingjing Wan,3 Xiaohua Gong,1 Fanwei Zhang,3 Wangjian Zhang,6 Tia Marks,7 Siyang Wang,1 Hongjun Jin,2 Hong Shan2,8
1The Cancer Center of the Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China; 2Guangdong Provincial Key Laboratory of Biomedical Imaging, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China; 3Department of Nuclear Medicine, Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China; 4Department of Pathology, The Fifth Affiliated Hospital of Sun Yat-sen University, Zhuhai, Guangdong Province, 519001, People’s Republic of China; 5Department of Pharmacy, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China; 6Department of Medical Statistics, School of Public Health, Sun Yat-sen University, Guangzhou, 510080, People’s Republic of China; 7Department of Environmental Health Sciences, School of Public Health, University at Albany, State University of New York, Rensselaer, NY, 12144, USA; 8Department of Interventional Medicine, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongjun Jin
Guangdong Provincial Key Laboratory of Biomedical Imaging, Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China
Email [email protected]
Hong Shan
Department of Interventional Medicine, Guangdong Provincial Key Laboratory of Biomedical Imaging, Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province, 519000, People’s Republic of China
Email [email protected]
Introduction: To evaluate the role of maximal standardized uptake values (SUVmax) and total lesion glycolysis (TLG) from serial 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) for early prediction of neoadjuvant chemotherapy (NAC) response in locoregionally advanced nasopharyngeal carcinoma (LANPC).
Methods: A total of 121 LANPC patients who completed pretreatment 18F-FDG PET/CT between June 2017 and July 2020 were retrospectively included. The median age of all the participants was 50 years old (range: 19– 74 years), with 94 (77.7%) males and 27 (22.3%) females. The SUVmax from the primary tumor site (SUVmax-PT) and the total lesion glycolysis from the primary tumor site (TLG-PT) were recorded. Tumor response was calculated according to the Response Evaluation Criteria in Solid Tumor (RECIST) 1.1 Criteria at two-week post-secondary NAC cycle. Patients who achieved an objectively partial or full reaction after two cycles of NAC were defined as ‘responders’, and patients who obtained stability or progression were classified as ‘non-responders’.
Results: After two cycles of NAC, 96 patients were categorized as “responders” and 25 patients as “non-responders”. The optimal thresholds of the SUVmax-PT were 11.8 and 38.5 for the TLG-PT. Non-responders were significantly associated with high SUVmax-PT (HR, 3.49; 95% CI, 1.17– 10.36; p = 0.024) and TLG-PT (HR, 4.45; 95% CI, 1.44– 13.78; p = 0.010) in multivariate analysis. Recursive partitioning analysis (RPA) categorized patients into three prognostic groups based on SUVmax-PT and TLG-PT: high-response group, intermediate-response group, and low-response group, with corresponding favorable response rates of 94%, 80%, and 55%, respectively. Moreover, a nomogram was created based on metabolic parameters that precisely projected an individual’s response of NAC (C-index, 0.787; 95% CI, 0.533– 1.000).
Conclusion: Pretreatment 18F-FDG PET/CT to measure SUVmax-PT and TLG-PT could be a useful non-invasive method for early indication of NAC efficacy. The nomogram based on PET/CT parameters may potentially provide direction for treatment decisions based on NAC levels.
Keywords: nasopharyngeal carcinoma, neoadjuvant chemotherapy, tumor response, PET/CT, nomogram
Introduction
Nasopharyngeal carcinoma (NPC) is an extremely common head and neck cancer affecting 10 to 25 cases per 100,000 in Southern China.1 Unfortunately, more than 70% of patients with NPC are categorized with locoregionally advanced disease at initial diagnosis.2,3 Despite the use of intensity-modulated radiotherapy and chemotherapy, outcomes for patients with advanced disease still remain unsatisfactory, with 30–50% of patients suffering from disease relapse post treatment.3 It is challenging for clinicians to manage locoregionally advanced NPC (LANPC). The standard treatment for LANPC is concurrent chemoradiotherapy (CCRT).4 Neoadjuvant chemotherapy (NAC), given before CCRT, has been used increasingly to control LANPC. Several randomized trials5,6 showed the superiority of additional NAC over CCRT alone in terms of overall survival (OS) and disease-free survival (DFS). However, current meta-analyses by the MAC-NPC Collaborative Group failed to identify the advantages of NAC followed by CCRT compared to CCRT alone.7 In contrast, patients treated with NAC plus CCRT had more grade 3–4 adverse events than those treated with CCRT alone.5 Hence, early assessment of NAC response is of utmost importance for NPC due to its contribution in minimizing ineffectual and toxic chemotherapies.
To assess NAC response, conventional imaging methods (ultrasound, SPECT/CT [single-photon emission computed tomography-computed tomography] and MRI [magnetic resonance imaging]) have been utilized.8–10 Yet, these approaches have offered limited accuracy. Du et al9 proposed that prediction of chemotherapy responses in NPC patients can be elicited from the uptake ratio of 99mTc-MIBI in early phases. However, calculating 99mTc-MIBI by utilizing planar images can be subject to personal error given lower resolution, contributing to inconsistency in results across various studies.11 Segara et al12 offered a scoring metric to evaluate the pathologic response to NAC, demonstrating that breast MRI provides increased accuracy over other imaging modalities. Nevertheless, MRI has been reported with varied accuracy and diminished specificity for the breast.13 A promising field of radiomics has emerged recently, and several studies suggest that radiomics may be a beneficial instrument in individualized NAC treatment of LANPC.14,15 However, the clinical applicability of radiomics analysis, which demands specialized workstations and trained professionals, has been largely limited by the complexity of its application at present. Hence, it is of utmost importance to identify novel factors to guide NAC treatment.
18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is frequently utilized for clinical diagnosis and determination of tumor staging for NPC. Prior to this, the utilization of PET/CT was not limited to the diagnosis and detection of metastatic lesions in NPC.16 The maximum standardized uptake value (SUVmax) of PET/CT is an index that reflects tumor metabolism. Several studies17,18 have suggested that higher SUVmax from the primary tumor site (SUVmax-PT) was associated with poorer prognosis in NPC patients. Apart from SUVmax-PT, total lesion glycolysis (TLG) from the primary tumor site (TLG-PT) may be a more accurate prognosis marker, which quantifies the metabolic activity and volumetric burden of tumors.19 A recent meta-analysis of 18F-FDG PET/CT reported by Li et al20 showed that both SUVmax-PT and TLG-PT were significantly associated with NPC patients’ treatment and outcomes. However, there are few studies to evaluate baseline 18F-FDG PET/CT and its utilization in predicting the NAC response among patients with NPC. Notably, studies have confirmed 18F-FDG PET/CT to be useful in early prediction of NAC response to breast and bladder cancer.21,22 We therefore hypothesize that 18F-FDG PET/CT may be useful in predicting NAC response in NPC.
Given this background, we conducted this study to determine the accuracy of baseline 18F-FDG PET/CT in assessing the response to NAC among patients with LANPC. Moreover, a combination of metabolic parameters and clinical variables was included to create a nomogram model to more accurately predict NAC response.
Materials and Methods
Participant Inclusion
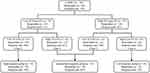
Between June 2017 and July 2020, newly diagnosed NPC patients who received treatment at our institution were retrospectively analyzed. The eligibility criteria were as follows: (1) non-metastatic, histologically demonstrated NPC; (2) stage III or IVA NPC (8th AJCC staging system); (3) received no less than two cycles of NAC; (4) whole-body 18F-FDG PET/CT performed by our institution prior to NAC; (5) completed MRI prior to NAC and after the second cycle of NAC; (6) available medical records, including age, gender, TNM stage, NAC regimens, serum Epstein‑Barr virus (EBV) DNA load, and lactate dehydrogenase (LDH). Patients with additional active cancers, pregnant or lactating, or with improperly medicated cardiac disease were excluded. Figure 1 flowchart represents patient selection. In total, 121 patients who met the eligibility criteria were retrospectively analyzed in the current study. The median age of all the participants was 50 years old (range: 19–74 years), with 94 (77.7%) males and 27 (22.3%) females. Detailed information on the treatment is available in Supplement 1. The Ethics Committee of The Fifth Affiliated Hospital, Sun Yat-sen University has approved this retrospective study, and it was run in accordance with the 1964 Declaration of Helsinki and all its amendments.
18F-FDG PET/CT Data Acquisition
Prior to the examination, all patients were mandated to fast for a minimum of 6 hours prior to examination. 18F-FDG was manufactured in line with standards set by our hospital, utilizing a 112-ring digital light guide PET/CT scanner (uMI780, United Imaging, China). The radiochemical purity was maintained at 98%, with the final products being diluted with saline and sterilized via a 0.22-μm Millipore filter through a sterile syringe. Doses of intravenously injected 18F-FDG were calculated utilizing each patient’s weight (3.7 MBq [0.1 mCi/kg]). The bounds for the images were set to CT scan from the head to upper thighs. PET scans were performed promptly post CT scan with 1.5 minutes of scanning time per image, utilizing 3-D mode for all images.
Two experienced nuclear medicine physicians (YW and JJW) examined all PET/CT images. For quantitative analysis, the SUVmax-PT was normalized to body weight and automatically calculated for the primary tumor. TLG-PT was calculated using SUVmean × MTV (metabolic tumor volume, whcih recorded at the absolute SUV threshold of 2.5 and the relative SUVmax-PT threshold of 70%). In the current study, we did not include SUVmax and TLG for the neck lymph nodes in the analysis, given that neck lymph node inflammation and the complex biology of lymph nodes may inadvertently influence the results of the research.23
Magnetic Resonance Imaging (MRI) Scan
The MRI study was done using a 3.0-T system (Verio; Siemens Healthcare, Erlangen, Germany). A combined head-and-neck coil was applied to inspect the area from the sternal end of the clavicle to the suprasellar cistern. Complete data on MRI protocol is described in the Supplement 2. All patients completed two MRI studies. The MRI scans were performed prior to treatment and repeated two weeks after the second NAC administration.
Tumor Response Evaluation
Tumor response was evaluated by two radiologists (JBG and GJW) specializing in NPC MRI based on Response Evaluation Criteria for Solid Tumor (RECIST) 1.1 Criteria,24 with disagreements resolved by consensus. Specifically, the NAC response was determined by combining the results of the evaluation of nasopharynx lesion and of metastatic lymph nodes in this study. The variation of the maximum diameter of nasopharynx lesion and the short axis of neck lymph nodes was calculated. The nasopharynx lesion with the longest diameter was recorded and only neck lymph nodes with the short axis of at least 15 mm were considered, and no more than five target lesions were evaluated. In addition, all the diameters and short axis measurements were done on the transverse plane. The overall biological responses after NAC were grouped as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Participants who experienced CR/PR were categorized as “responders”; while participants who suffered from SD/PD were categorized as “non-responders” in this study.
Statistical Analysis
Receiver-operating characteristic (ROC) curves were utilized on metabolic parameters to assess an optimal cut-off value for NAC response. The AUC ([area under the ROC]) was computed to understand the sensitivity and specificity of all metabolic parameters in calculating NAC response. Assessed covariates include SUVmax-PT (low vs high), TLG-PT (low vs high), gender, age (<50 vs ≥50 years), T stage (T1–2 vs T3–4), N stage (N0–1 vs N2–3), serum EBV DNA (<4000 vs ≥4000 copy/mL), LDH (<245 vs ≥245 U/L), and NAC regimen (GP [cisplatin 80 mg/m2 D1 and gemcitabine 1 mg/m2 D1, D8] vs TP [cisplatin 75 mg/m2 D1 and docetaxel 75 mg/m2 D1 or paclitaxel 150–180 mg/m2 D1]/PF [cisplatin 80–100 mg/m2 D1 and 5–fluorouracil 800–1,000mg/m2, by 120-h continuous intravenous infusion] vs TPF [cisplatin 60 mg/m2 D1, docetaxel 60 mg/m2 D1 or paclitaxel 135 mg/m2 D1, and 5-fluorouracil 500–800 mg/m2, by 120-h continuous intravenous infusion]). The cutoff value was set at 4000 copies/mL and was selected to categorize patients into high- and low pre-EBV DNA groups as described in prior NPC studies.25 Normal LDH ranged from 109 to 245 U/L; thus, a cutoff value of 245 U/L was selected to categorize patients into high- and low LDH.
In this study, we first conducted a univariate analysis for each independent variable with the NAC response, then selected the independent variables with a P value <0.1 and included them into the final multivariate analysis. To determine which patients are most likely to benefit from NAC, recursive partitioning analysis (RPA) based on metabolic parameters was applied. A random number generator was used to sort patients into a training cohort containing 81 patients and an internal validation cohort of 40 patients. Nomograms were created using the rms package within R, and gender was included into the nomogram given the importance of gender in clinical decision-making.3 The concordance index (C-index) was utilized to estimate bias-correction or overfitting within the model. P < 0.05 were categorized as significant, and all statistical assessments were two-sided. All statistical analyses were performed in R version 3.3.2 (http://www.r-project.org/).
Results
Patient Characteristics
The clinical characteristics of all 121 enrolled participants are categorized in Table 1. Of the 121 patients, 54 (44.6%) received GP regimen of NAC, 48 (39.7%) received TP/PF regimen, and 19 (15.7%) received TPF regimen. Disease in stage III accounted for 88 (72.7%) cases, and stage IVA was identified for 33 (27.3%) cases. The SUVmax-PT ranged from 2.7 to 25.7, with a median value of 10.6. The TLG-PT ranged from 0.6 to 205.4, with a median of 54.5. After two cycles of NAC, 96 participants were categorized as “responders” (7 having CR and 89 having PR) and 25 participants as “non-responders” (22 having SD and 3 having PD) by the RECIST 1.1 Criteria.
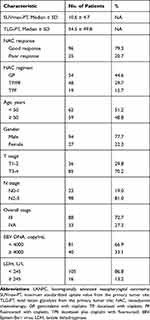 |
Table 1 Clinical Characteristics of 121 Patients with LANPC |
Correlation Between Tumor Response and Metabolic Parameters
An ROC curve was plotted to determine an absolute cut-off value for SUVmax-PT and TLG-PT in tumor response to NAC. Optimal cut-offs for SUVmax-PT and TLG-PT were 11.8 (AUC value, 0.744; specificity 69.8%, sensitivity 72.0%; p < 0.001) and 38.5 (AUC value, 0.720; specificity 63.5%, sensitivity 76.0%, p < 0.001), respectively. Among the entire group, 47 patients (38.8%) showed high SUVmax-PT, and 54 (44.6%) showed high TLG-PT. Univariate analysis showed that SUVmax-PT (HR, 5.94; 95% CI, 2.24–15.76; p < 0.001) and TLG-PT (HR, 5.52; 95% CI, 2.02–15.12; p < 0.001) were significantly associated with NAC response (Table 2). In multivariate analysis, non-responders were significantly associated with high SUVmax-PT (HR, 3.49; 95% CI, 1.17–10.36; p = 0.024), TLG-PT (HR, 4.45; 95% CI, 1.44–13.78; p = 0.010) and LDH (HR, 1.83; 95% CI, 1.15–5.34; p = 0.048), but not with chemotherapy (HR, 1.30; 95% CI, 0.53–3.21; p= 0.830) (Table 3).
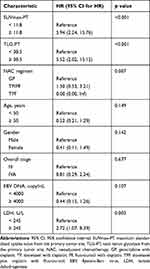 |
Table 2 Univariate Analysis of Prognostic Factors for NAC Response |
 |
Table 3 Multivariate Analysis of Prognostic Factors for NAC Response |
Benefit Stratification for NAC According to Metabolic Parameters
Since both SUVmax-PT and TLG-PT were shown to be independently related to NAC response in univariate and multivariate analyses, these above two PET parameters were integrated into an RPA model for the prediction of chemotherapy response. The RPA algorithm separated the 121 patients with NPC into 4 classes (Figure 2). Because the NAC responses of classes 2 and 3 were not significantly different (class 2 vs 3, p = 0.767), these two groups were combined. Thus, the final RPA model categorized patients into the following three prognostic groups: high response (low SUVmax-PT with low TLG-PT; n = 53), intermediate response (low SUVmax-PT with high TLG-PT, and high SUVmax-PT with low TLG-PT; n = 35), and low response (high SUVmax with high TLG-PT; n = 33), with corresponding NAC response rates of 94%, 80%, and 55%, respectively.
Establishment of Metabolic and Clinical Nomogram
For the prediction of NAC responses, a nomogram was developed utilizing results from multivariate analysis (Figure 3A). Since SUVmax-PT and TLG-PT were predictors of NAC response after multivariate analysis, this elicited inclusion in the nomogram. The established nomogram yielded a high C-index of 0.787 (95% CI, 0.533–1.000; Figure 3B) in the training cohort and 0.825 (95% CI, 0.570–1.000; Figure 3C) in the validation cohort. Individual PET parameters were utilized to assess the validity of the nomogram by comparing ROC curves. The AUC values of SUVmax-PT, TLG-PT, and the nomogram were 0.744, 0.720, and 0.787, respectively (p < 0.001; Supplement 3). The results revealed that the nomogram predicted NAC response, use of PET parameters in conjunction with clinical risk variables, was superior to an individual PET parameter.
Discussion
18F-FDG PET/CT was identified as a prognostic instrument for NPC based on the high sensitivity and noninvasive assessment methods for identifying tumor metabolism.26 However, there were a limited number of reports supporting the use of 18F-FDG PET/CT for the identification of NPC tumor response to NAC. This case series using 18F-FDG PET/CT to predict NAC response in LANPC is reported to add to the literature on non-invasive and highly sensitive assessment methods. Preliminary outcomes found SUVmax-PT and TLG-PT to be highly effective in identifying the subpopulation sensitive to NAC. Furthermore, we also built a nomogram based on 18F-FDG PET/CT bounds in conjunction with other variables to precisely predict individual response to NAC, which would be useful to guide clinical decisions.
Tumour response to NAC is a significant prognostic factor in NPC.27–29 Generally, favourable NAC response was significantly associated with improved disease control, locoregional control, and overall survival for NPC. In contrast, unfavorable NAC response was correlated with poor clinical outcomes. Hence, evaluating NAC response may assist in prognostication and refining of treatments for high-risk NPC patients. Clinical studies on the efficacy of 18F-FDG PET/CT in predicting NAC response have been presented for lung cancer, breast cancer, and head and neck cancer.30–32 For example, Vallius et al assessed the benefit of 18F-FDG PET/CT to identify NAC in non-responsive patients after three or four cycles;33 and a decrease in FDG uptake after one cycle of NAC was associated with improved tumor response to ovarian cancer. However, patients with chemo-resistant NPC have been shown to have a delayed need for radiotherapy, reduced toxic exposure as well as reduced cost with the use of NAC. Thus, early screening to determine chemo-resistant NPC would allow clinicians to personalize treatment protocols.
For this study, all patients completed baseline 18F-FDG PET/CT and received two cycles of standard NAC regimen. Our results indicated that patients who responded favorably to NAC had significantly lower SUVmax-PT and TLG-PT than non-responders. The potential mechanisms underlying this observation may be explained as follows. It is well recognized that intratumoral heterogeneity contributes to drug resistance and treatment failure.34,35 Prior studies have confirmed that the intratumoral heterogeneity could be identified based on variations in glucose metabolism,36,37 with relevant molecular and cellular characteristics including, fibrosis, necrosis, and hypoxia. Consistent with our study,38 Wang et al indicated an uptake ratio of 99mTc-MIBI at the primary tumor site, which may predict a reaction to NAC in NPC. Still, the calculation of 99mTc-MIBI using planar imaging is vulnerable to human error due to limited resolution, contributing to discrepancies in the findings among studies.12 Compared to 99mTc-MIBI, PET/CT is highly advantageous for its production of images with intrinsically greater contrast allowing for increased specificity and sensitivity in lesion detection.26 Given that NPC is often enclosed by normal tissues that will take up a substantial amount of 99mTc-MIBI, adoption of 18F-FDG PET/CT improves measurement accuracy of tumor uptake ratios and minimizes the impact of standard uptake, thus maintaining stability within the results.26
To understand which patients may benefit from NAC, researchers often use multivariate analysis to elucidate relevant prognostic factors.31–33 However, these prognostic factors are often combined with independent factors, creating confusion for clinicians when several prognostic factors coexist. To avoid the interaction of prognostic factors with risk of tumor response, we analyzed the role of metabolic parameters by hierarchical analysis, which is unique compared to traditional statistical analysis methods used to date.30 RPA is one of the most successful models for stratifying patients into subgroups with homogeneous performance and has been implemented to create prognostic algorithms for numerous malignancies.39,40 We found that these objective risk factors (SUVmax-PT and TLG-PT) were confirmed to influence NAC response significantly in certain subgroups in the RPA model. As a result, the RPA model then categorized patients into different response-risk groups according to metabolic parameters. In further stratified analysis, we found that the NAC response is more apparent in intermediate- and high-response groups. By contrast, nearly 50% of patients in the low-response group responded poorly to NAC. As a result, clinicians can screen and identify patients with LANPC who will benefit most from NAC while providing them with sound advice on NAC. Moreover, some low-response patients can avoid adverse effects from NAC and experience reduced overall treatment costs.
Our nomogram utilizing 18F-FDG PET/CT boundaries provided an interesting result. While methods traditionally use prognostic variables such as TNM stage, sex, and blood parameters, our nomogram provides biological heterogeneity for tumors. Thus, this prognostic nomogram may offer a simple and precise method of identification for improved patient recovery from NAC in LANPC. One recent study established an MRI-based radiomics nomogram containing 120 patients with NPC, for which it provided a high AUC value of 0.822, to predict responses to NAC. However, the establishment of MRI radiomics models to predict NPC should consider several important points. Primarily, MRI-based radiomic studies face technological challenges. Both image acquisition, which distorts tissue properties, and scanner limitations, which decrease the reproducibility of tumor features, affect the quality of MRI-based studies.41 Secondly, MRI varies in scanner properties, affecting the reproducibility of images and, in turn, the tumor features identified within them.42 Extreme variability in scans and systems limits generalizability and merit for these systems to be used in clinical decisions. In contrast to radiomic features from MRI, metabolic parameters indicated by 18F-FDG PET/CT are easier to obtain, and our nomogram may be more clinically practical.
Another biological mechanism associated with NPC is plasma EBV DNA.43 Prior studies indicate that plasma EBV DNA may be utilized for clinical and molecular monitoring in NPC patients.43 However, the prognostic benefits of plasma EBV DNA in predicting tumor reaction to NAC have not been sufficiently studied. Recently, Liu et al29 assessed the prognostic benefit of EBV DNA values on tumor reaction in NPC and identified detectable EBV DNA following NAC to be associated with poor tumor response. However, baseline EBV DNA levels failed to predict tumor response to NAC in the current study. One reason underlying this observation may be differences in the time points of EBV DNA examination between the Liu et al study and this study. Currently, NAC response is calculated most often by the change in tumor size, yet the tumor size in non-responders shrank insignificantly or increased.24 Usually, patients with a poor response to NAC are more likely to have a larger residual tumor lesions, and EBV DNA load was confirmed to be a reliable indicator of tumor burden in patients with NPC. Therefore, it is reasonable to conclude that a detectable EBV DNA after NAC treatment is associated with poor tumor response.
The current study has some limitations. Primarily, all data was obtained exclusively from a single center with few patients. Consequently, future multi-center studies with a higher number of patients may be warranted. Second, confined to the follow-up duration, the prognostic value of glucose metabolic parameters on survival outcomes was lacking for the current study. Although prior analysis of NPC indicated that the reaction to primary chemotherapy is related to outcome,28,29 prolonged follow-up times are essential to assess long-term outcomes. Finally, the efficacy of a variety of NAC regimens may have confounded the main findings of this research. Yet, to date, no evidence has been found to suggest an ideal NAC regimen, and all regimens used for this study are in line with the National Comprehensive Cancer Network Guidelines.
In summary, our findings suggest that metabolic parameters (SUVmax-PT and TLG-PT) measured using 18F-FDG PET/CT were significantly associated with NAC response in LANPC. Moreover, the nomogram based on metabolic parameters with other variables had good prognostic accuracy in predicting NAC response. We therefore cautiously suggest that 18F-FDG PET/CT would be a useful non-invasive method for early indication of NAC efficacy in guiding clinical decisions. Future studies with larger sample sizes are still needed to validate these findings.
Data Sharing Statement
Datasets can be retrieved from the corresponding author (Hong Shan) upon formal request from interested readers. Datasets cannot be directly shared with public repositories due to the National Personal Data Protection Act.
Acknowledgments
We would like to acknowledge and thank the patients who participated in the study. Moreover, the authors appreciate Dr. Jiebing Gao and Dr. Guojie Wang from the Department of Radiology who specialized in head and neck MRI, which evaluated the treatment response in MRI.
Author Contributions
All authors contributed to data analysis, drafting or revising of the article, gave final approval for the version to be published, agreed to the submitted journal, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by grants from the National Key R&D Program of China (No. 2018YFC0910600), the National Natural Science Foundation of China (No. 81901699, 81871382),and the Nursing Research Project of Guangdong Nursing Association (No. gdhlxueh2019zx072).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492.
2. Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer. 2016;122:546–558. doi:10.1002/cncr.29795
3. Yao JJ, Lin L, Gao TS, et al. Development and validation of web-based nomograms to precisely predict survival outcomes of non-metastatic nasopharyngeal carcinoma in an endemic area. Cancer Res Treat. 2021;53(3):657–670. doi:10.4143/crt.2020.899
4. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
5. Sun Y, Li WF, Chen Ny, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a Phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17:1509–1520. doi:10.1016/S1470-2045(16)30410-7
6. Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol. 2017;29:731–736. doi:10.1093/annonc/mdx770
7. Ribassin-Majed L, Marguet S, Lee AW, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. J Clin Oncol. 2017;35:498–505. doi:10.1200/JCO.2016.67.4119
8. Chagpar AB, Middleton LP, Sahin AA, et al. Accuracy of physical examination, ultrasonography, and mammography in predicting residual pathologic tumor size in patients treated with neoadjuvant chemotherapy. Ann Surg. 2006;243(2):257–264. doi:10.1097/01.sla.0000197714
9. Du C, Ying H, Zhou J, et al. Prediction of the response to docetaxel-based chemotherapy for locoregionally advanced nasopharyngeal carcinoma: the role of double-phase (99m)Tc-MIBI SPECT/CT. Med Oncol. 2014;31(2):833. doi:10.1007/s12032-013-0833-z
10. Zheng D, Chen Y, Chen Y, et al. Early assessment of induction chemotherapy response of nasopharyngeal carcinoma by pretreatment diffusion-weighted magnetic resonance imaging. J Comput Assist Tomogr. 2013;37(5):673–680. doi:10.1097/RCT.0b013e31829a2599
11. Budinger TF. Physical attributes of single-photon tomography. J Nucl Med. 1980;21(6):579–592.
12. Segara D, Krop IE, Garber JE, et al. Does MRI predict pathologic tumor response in women with breast cancer undergoing preoperative chemotherapy? J Surg Oncol. 2007;96(6):474–480. doi:10.1002/jso.20856
13. Bahri S, Chen JH, Mehta RS, et al. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Ann Surg Oncol. 2009;16(6):1619–1628. doi:10.1245/s10434-009-0441-5
14. Dong D, Zhang F, Zhong LZ, et al. Development and validation of a novel MR imaging predictor of response to induction chemotherapy in locoregionally advanced nasopharyngeal cancer: a randomized controlled trial substudy (NCT01245959). BMC Med. 2019;17(1):190. doi:10.1186/s12916-019-1422-6
15. Zhao L, Gong J, Xi Y, et al. MRI-based radiomics nomogram may predict the response to induction chemotherapy and survival in locally advanced nasopharyngeal carcinoma. Eur Radiol. 2020;30(1):537–546. doi:10.1007/s00330-019-06211-x
16. Xiao W, Xu A, Han F, et al. Positron emission tomography-computed tomography before treatment is highly prognostic of distant metastasis in nasopharyngeal carcinoma patients after intensity-modulated radiotherapy treatment: a prospective study with long-term follow-up. Oral Oncol. 2015;51(4):363–369. doi:10.1016/j.oraloncology.2015.01.009
17. Chan WK, Kwong DL, Yeung DW, Huang B, Khong PL. Prognostic impact of standardized uptake value of F-18 FDG PET/CT in nasopharyngeal carcinoma. Clin Nucl Med. 2011;36(11):1007–1011. doi:10.1097/RLU.0b013e31821a29a4
18. Yang Z, Shi Q, Zhang Y, et al. Pretreatment (18) F-FDGuptake heterogeneity can predict survival in patients with locally advanced nasopharyngeal carcinoma–a retrospective study. Radiat Oncol. 2015;10:4. doi:10.1186/s13014-014-0268-5
19. Chan SC, Chang JT, Lin CY, et al. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: predictive role for different survival endpoints and impact on prognostic stratification. Nucl Med Commun. 2011;32(11):989–996. doi:10.1097/MNM.0b013e3283495662
20. Li Q, Zhang J, Cheng W, et al. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Medicine (Baltimore). 2017;96(37):e8084. doi:10.1097/MD.0000000000008084
21. Soubra A, Gencturk M, Froelich J, et al. FDG- PET/CT for assessing the response to neoadjuvant chemotherapy in bladder cancer patients. Clin Genitourin Cancer. 2018;16(5):360–364. doi:10.1016/j.clgc.2018.05.008
22. Tian F, Shen G, Deng Y, Diao W, Jia Z. The accuracy of 18F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol. 2017;27(11):4786–4796. doi:10.1007/s00330-017-4831-y
23. Schwartz DL, Rajendran J, Yueh B, et al. FDG- PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–1367. doi:10.1001/archotol.130.12.1361
24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
25. Jin YN, Yao JJ, You YF, et al. Optimal cumulative cisplatin dose during concurrent chemoradiotherapy among children and adolescents with locoregionally advanced nasopharyngeal carcinoma: a real-world data study. Radiother Oncol. 2021;161:83–91. doi:10.1016/j.radonc.2021.06.003
26. Wood KA, Hoskin PJ, Saunders MI. Positron emission tomography in oncology: a review. Clin Oncol. 2007;19:237–255. doi:10.1016/j.clon.2007.02.001
27. Chen YH, Chang KP, Chu SC, et al. Value of early evaluation of treatment response using 18F-FDG PET/CT parameters and the Epstein-Barr virus DNA load for prediction of outcome in patients with primary nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging. 2019;46(3):650–660. doi:10.1007/s00259-018-4172-3
28. Peng H, Chen L, Zhang Y, et al. The tumour response to induction chemotherapy has prognostic value for long-term survival outcomes after intensity-modulated radiation therapy in Nasopharyngeal carcinoma. Sci Rep. 2016;6:24835. doi:10.1038/srep24835
29. Liu LT, Tang LQ, Chen QY, et al. The prognostic value of plasma Epstein-Barr viral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93(4):862–869. doi:10.1016/j.ijrobp.2015.08.003
30. Lee SM, Bae SK, Kim TH, et al. Value of 18F-FDG PET/CT for early prediction of pathologic response (by residual cancer burden criteria) of locally advanced breast cancer to neoadjuvant chemotherapy. Clin Nucl Med. 2014;39(10):882–886. doi:10.1097/RLU.0000000000000531
31. Burger IA, Casanova R, Steiger S, et al. 18F-FDG PET/CT of non-small cell lung carcinoma under neoadjuvant chemotherapy: background-based adaptive-volume metrics outperform TLG and MTV in predicting histopathologic response. J Nucl Med. 2016;57(6):849–854. doi:10.2967/jnumed.115.167684
32. Powell C, Schmidt M, Borri M, et al. Changes in functional imaging parameters following induction chemotherapy have important implications for individualised patient-based treatment regimens for advanced head and neck cancer. Radiother Oncol. 2013;106(1):112–117. doi:10.1016/j.radonc.2012.09.009
33. Vallius T, Peter A, Auranen A, et al. 18F-FDG-PET/CT can identify histopathological non-responders to platinum-based neoadjuvant chemotherapy in advanced epithelial ovarian cancer. Gynecol Oncol. 2016;140:29–35. doi:10.1016/j.ygyno.2015.10.018
34. Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi:10.1038/nrclinonc.2017.166
35. Yang F, Wang Y, Li Q, et al. Intratumor heterogeneity predicts metastasis of triple-negative breast cancer. Carcinogenesis. 2017;38(9):900–909. doi:10.1093/carcin/bgx071
36. Lee JW, Park JY, Lee HJ, et al. Preoperative [18F]FDG PET/CT tumour heterogeneity index in patients with uterine leiomyosarcoma: a multicentre retrospective study. Eur J Nucl Med Mol Imaging. 2018;45(8):1309–1316. doi:10.1007/s00259-018-3975-6
37. Castelli J, Depeursinge A, Devillers A, et al. PET-based prognostic survival model after radiotherapy for head and neck cancer. Eur J Nucl Med Mol Imaging. 2019;46(3):638–649. doi:10.1007/s00259-018-4134-9
38. Wang XS, Zhang YJ, Liu XL, Zhou ZR, Hu CS, Eisbruch A. The role of technetium-99m methoxyisobutyl isonitrile scintigraphy in predicting the therapeutic effect of chemotherapy against nasopharyngeal carcinoma. Cancer. 2011;117(11):2435–2441. doi:10.1002/cncr.25802
39. Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol. 2015;33(8):836–845. doi:10.1200/JCO.2014.58.6412
40. Chen Y, Zheng ZQ, Chen FP, et al. Role of postoperative radiotherapy in nonmetastatic head and neck adenoid cystic carcinoma. J Natl Compr Canc Netw. 2020;18(11):1476–1484. doi:10.6004/jnccn.2020.7593
41. Zhao B, Tan Y, Tsai WY, et al. Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep. 2016;6:23428. doi:10.1038/srep23428
42. Fruehwald-Pallamar J, Hesselink JR, Mafee MF, Holzer-Fruehwald L, Czerny C, Mayerhoefer ME. Texture-based analysis of 100 MR examinations of head and neck tumors - is it possible to discriminate between benign and malignant masses in a multicenter trial? Rofo. 2016;188(2):195–202. doi:10.1055/s-0041-106066
43. Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer. 2019;125(1):79–89. doi:10.1002/cncr.31741
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.