Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
The Role of PKM2 in Diabetic Microangiopathy
Received 14 March 2022
Accepted for publication 28 April 2022
Published 4 May 2022 Volume 2022:15 Pages 1405—1412
DOI https://doi.org/10.2147/DMSO.S366403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ming-Hui Zou
Chao Tu, Liangzhi Wang, Lan Wei
Department of Internal Medicine, the Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, 213000, People’s Republic of China
Correspondence: Lan Wei, Department of Internal Medicine, the Third Affiliated Hospital of Soochow University, 185 Juqian Road, Changzhou, Jiangsu, 213000, People’s Republic of China, Tel +86 0519 68871132, Email [email protected]
Abstract: Diabetic microangiopathy is among the most common complications affecting patients with diabetes, and includes both diabetic retinopathy (DR) and diabetic nephropathy (DKD). Diabetic microangiopathy remains a persistent threat to the health and quality of life of affected patients. Mechanistically, the severity of DR and DKD is tied to mitochondrial and glucose metabolism abnormalities, with the activation of the glycolytic enzyme pyruvate kinase M2 (PKM2) contributing to mitochondrial and glomerular dysfunction, abnormal renal hemodynamics, and retinopathy. PKM2 can activate inflammatory bodies in macrophages to promote the release of inflammatory mediators, and serves as a key regulator of inflammatory factors, chemokines and adhesion molecules. As such, there is sufficient evidence that PKM2 can be used as a biomarker for the diagnosis of diabetes and diabetic microangiopathy. Here, we survey the mechanisms whereby PKM2 contributes to diabetes-related microvascular diseases, associated regulatory roles, post-translational modifications, and the potential utility of PKM2 as a therapeutic target. Through this literature review, we have determined that PKM2 offers promise as both a diagnostic marker and therapeutic target with direct relevance to research pertaining to diabetic microangiopathy.
Keywords: pyruvate kinase M2, inflammation, diabetic retinopathy, diabetic nephropathy, diabetic microangiopathy
Introduction
Persistent hyperglycemia, insulin resistance (IR), and the dysfunction or loss of islet β cells can contribute to the incidence of diabetes mellitus. An estimated 20–30% of diabetes patients will ultimately develop chronic kidney disease (CKD) and a subset will ultimately be affected by diabetic retinopathy (DR).1 Diabetic nephropathy (DKD) is the most common global cause of CKD, chronic renal failure, and end-stage renal disease (ESRD) in the world, with the morbidity and associated mortality rates for this condition having risen recently in recent years.2 DKD is a key driver of microvascular complications in patients with diabetes, resulting in chronic inflammation that promotes macrophage activation, the progressive disruption or dysregulation of glomerular filtration barriers contributing to glomerular hypertrophy, extracellular matrix accumulation, podocyte damage, injury to the renal tubules, and the development of tubulointerstitial fibrosis.3 Diagnoses of DKD have historically been reliant upon measurements of blood glucose and urinary microalbumin levels, but these tests are relatively non-specific and lack sensitivity, limiting their utility for early-stage DKD diagnosis. Other studies have shown that diabetic nephropathy is primarily caused by increases in toxic glucose metabolite accumulation as a result of abnormal mitochondrial activity. PKM2 plays a central role in the function of glomerular mitochondria, and dimerized PKM2 translocates to the nucleus through its non-standard protein kinase activity, whereupon it participates in the regulation of inflammatory genes, thus affecting the pathogenesis of diabetic nephropathy.4 DR is among the leading causes of blindness and visual dysfunction in individuals with diabetes. PKM2 is a key rate-limiting glycolytic enzyme that influences metabolic activity, gene expression patterns, and overall human health. Indeed, there is evidence that PKM2 can play a key role in the pathogenesis of conditions including diabetes, DKD, retinopathy, atherosclerosis, and cancer. In cancer cells, for example, interactions between PKM2 and epidermal growth factor receptor (EGFR) can regulate the metabolic activity, proliferation, and migration of cancer cells. PKM2 expression in macrophages can contribute to inflammatory mediator production. There is also evidence for the role of PKM2 in the production of brown adipose tissue.5 Interactions between PKM2 and protein tyrosine phosphatase −1B (PTP-1B) have been linked to the incidence of diabetes, and the loss of podocyte PKM2 expression can drive more rapid mitochondrial dysfunction, podocyte apoptosis, and glomerulopathy in the context of diabetes.6 The hypoxia-inducible factor 1 α (HIF-1 α)/PKM2 signaling pathway regulated the metabolic processing of glucose, driving increases in glucose uptake and the secretion of lactic acid, both of which contribute to DR development. PKM2 is a key regulator of many inflammatory molecules, including chemokines, adhesion molecules, and pro-inflammatory cytokines. This inflammatory metabolic pathway under the regulation of PKM2 is thus closely tied to the incidence of microvascular diseases including both DR and DKD, highlighting its importance as a research target.
An Overview of PKM2
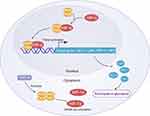
As a glycolytic rate-limiting enzyme, pyruvate kinase can increase aerobic glycolysis rates by catalyzing phosphoenolpyruvate (PEP) conversion into pyruvate and activating the pentose phosphate pathway (PPP).7 Competitive pyruvate kinase is one of four different subtypes of this enzyme (M1, M2, L, and R) encoded by two different genes (PKM and PKLR),8 with the PKM2 subtype being composed of four domains (A, B, C, N). A high degree of sequence homology is evident between human and murine PKM2, which incorporates exon 10 but not exon 9 of the parental gene. Exon 10 consists of 167 bases encoding 56 amino acids,9 and is inserted into the PKM2 transcript by the serine/arginine-rich splicing factor 3 (SRSF3) rich in serine/arginine. Expression of PKM2 is evident beginning during embryonic development and persists throughout life in all tissues. The PKM2 enzyme can be present within cells in both the inactive tetramerized T-state or in the active monomeric or dimeric R-state.10 Many of the residues of PKM2 can undergo various post-translational modifications such as phosphorylation, oxidation, hydroxylation, acetylation, ubiquitylation, succinylation, or glycosylation, all of which can impact the structure and function of the encoded protein.11 A number of different metabolites influence the multimerization of PKM2, including fructose 1-diphosphate (FBP), with post-translational acetylation and phosphorylation additionally shaping such activity. Wang et al12 found that in the absence of FBP, PKM2 primarily exists in monomeric or dimeric forms, while in the presence of FBP it primarily exists in a tetramerized state. When phosphorylated at Tyr-105, PKM2 is stabilized in the dimeric configuration, impairing its glycolytic activity. Decreased glycolytic activity in the context of PKM2 Cys-358 oxidation results in the entry of glucose into the pentose phosphate pathway. Oxidative factors including nitric oxide (NO), endothelial nitric oxide synthase (ENOS), and hydrogen peroxide (H2O2) can also impact the localization and activation of PKM2 within cells.13 FBP serves as an allosteric regulator of PKM2, promoting its tetramerization and activation, leading to increased glycolytic activity and glucose utilization. Tetrameric PKM2 can, together with lactate dehydrogenase A1 (LDHA1), contribute to extensive lactic acid production.14 The activation of PKM2 can also occur in response to carbamides and serine, while phenylalanine functions as an allosteric inhibitor of PKM2, reducing its affinity for PEP.15,16
Dimerized PKM2 functions independently of competitive activity, accumulating with the nucleus wherein it functions as a protein kinase to directly phosphorylate STAT3 (Tyr705), thus leading to increased MAP kinase 5 (MEK5/ERK5) expression.14,17 PKM2 also can also promote the phosphorylation of histone H3 (Thr11) in the context of EGFR activation, leading to the dissociation of histone deacetylase 3 (HDAC3) from the promoter regions associated with the CyclinD1 and Myc genes, contributing to subsequent histone H3 Lys9 acetylation. As such, PKM2-mediated histone H3 modifications play a central role in regulating the EGF-induced expression of both c-Myc and cyclin D1.18 In the context of mitosis, PKM2 can bind to the spindle checkpoint protein Bud3, promoting its phosphorylation at Tyr207 and thereby promoting the association of the Bud3-Bud1 complex with the kinetochore while also interacting with Blinkin. This is critical, given that this Bud3-Bud1 complex is necessary for appropriate kinetochore-microtubule attachment, mitosis-spindle assembly checkpoints, chromosomal segregation, thus supporting the survival and proliferation of cells in addition to regulating EGFR-mediated tumorigenesis.19 Mitochondrial PKM2 can also interact with and phosphorylate Thr69 of Bcl2, interfering with the ability of the E3 ubiquitin ligase to bind and induce Bcl2 degradation, with HSP90α1 facilitating the interactions between PKM2 and Bcl2.20 HSP90α1 can also complex with GSK-3β and PKM2, stabilizing PKM2 by phosphorylating it at Thr328.
Within the nucleus, PKM2 can control the expression of a range of genes, with its regulatory activity being attributed to its ability to interact with a range of transcription factors and other proteins, thereby forming functionally diverse complexes.21 The death-associated protein kinase (DAPK) serine/threonine kinase is a nucleoprotein that plays key roles in the regulation of inflammatory responses and is in turn regulated by calcium/calmodulin kinase. There is evidence that DAPK can promote PKM2 activation, with PKM2 subsequently undergoing oxidation of Cys358 in vitro, resulting in the dissociation of the tetrameric form of PKM2 into its dimeric form.
The cytoplasmic protein β-catenin is under the control of the Wnt signaling pathway, and it can undergo Tyr333 phosphorylation mediated by sSrc. When phosphorylated, β-catenin is able to bind to PKM2 (Lys433), thereby promoting downstream gene expression.22,23 When The Tyr333 residue in β-catenin is mutated, this interferes with PKM2 phosphorylation and results in the translocation of PKM2 to the nucleus, resulting in consequent PI3K/Akt and Wnt/β-catenin pathway activation, thereby potentiating an inflammatory response.22 PKM2 can also serve as a regulatory factor for other transcription factors including HIF-1α, with dimeric PKM2 serving as a transcriptional activator that directly interacts with HIF-1α in the nucleus, driving the upregulation of a range of glycolysis-related genes including Hexokinase 1 (HK1), Phagocytosis and killing function −1 (PKF-1), Lactate dehydrogenase (LDH), and Glucose transporter 1 (GLUT-1).24 As such, PKM2 can influence glycolysis and the renal tubular epithelial-mesenchymal transition process (Figure 1).
The Role of PKM2 in Diabetes
Diabetes is associated with the dysregulation of normal glucose metabolism, abnormal mitochondrial functionality, and the generation of high levels of reactive oxygen species (ROS).25 Rising levels of glucose within cells drive the accumulation of toxic metabolites including diacylglycerol (DAG), sorbitol, and methylglyoxal (MG). PKM2 activation can enhance mitochondrial metabolic activity, and mitochondrial dysfunction can be alleviated or reversed by decreasing levels of oxidants induced in response to high levels of glucose including metalloproteinases (MMPs) and peroxisome proliferation-activated γ -coactivator 1α (PGC-1α).26 Allen et al15,27 identified a key role played by PKM2 within pancreatic β-cells, demonstrating its ability to activate the Wnt/CTNNB1 pathway, thereby promoting β-cell proliferation and insulin secretion, thus lowering blood glucose levels. In response to high concentrations of glucose, PKM2 was found to undergo acetylation, constraining its enzymatic activity and the nuclear translocation of dimeric PKM2. Tetrameric PKM2 localizes to the cytoplasm wherein it can regulate glycolysis and exhibits robust catalytic activity. When activated, PKM2 forms stable tetramers that exhibit high levels of pyruvate kinase activity, enhancing glycolytic flux and lowering blood glucose levels.28
The pathogenesis of diabetes is related to inflammation, and increases in PKM2 levels reflect chronic inflammatory activity. Some studies have shown that the expression of plasma PKM2 is related to C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). As such, PKM2 levels may contribute to diabetes diagnosis and disease surveillance. Li et al21 conducted qualitative and quantitative analyses of macrophage proteins, revealing that hyperglycemic conditions suppressed pyrin-like receptor family containing pyrin domain 3 (NLRP3) inflammatory body/stress granule signaling mediated by tetrameric PKM2. The NLRP3-mediated secretion of inflammatory factors including Interleukin-18 (IL-18) and IL-1β can contribute to atherosclerosis and atherosclerotic plaque formation, resulting in a worse prognosis for individuals affected by diabetes. Dimeric PKM2 also serves as an important regulator of lipopolysaccharide (LPS)-induced IL-1β expression through its ability to bind to the IL-1β promoter, whereas tetrameric PKM2 can suppress IL-1β in response to LPS. PKM2 may thus be an important therapeutic target in the context of diabetes.28,29
Reductions in L-cysteine concentrations can readily restore glucose-induced ATP production and insulin secretion to appropriate levels. This occurs in part owing to the ability of L-cysteine to promote the decomposition of active tetrameric PKM2 into the inactive dimeric and monomeric forms both in vitro and in insulin-secreting cells, resulting in reduced pyruvate production.1,30,31 This inactivation of PKM2 can contribute to impaired glucose-induced ATP production and insulin secretion. PKM2 activity, however, can be restored by either treatment with a PKM2 activator or by removing L-cysteine, thereby restoring appropriate levels of ATP production and insulin secretion, ultimately lowering blood glucose levels. The activation of PKM2 may thus represent an effective means of remediating high L-cysteine concentrations and normalizing insulin secretion activity as an approach to treating diabetes.32
By serving as a HIF-1a co-activator, PKM2 can regulate insulin-induced glucose metabolism and lower blood glucose levels. The cellular growth and survival-related serine/threonine kinase mammalian target of rapamycin (mTOR) has been shown to be inhibited by miR-99a overexpression, thus leading to the suppression of HIF-1a activity and altered glucose consumption following insulin therapy.33,34 This suggests that PKM2 plays a role in regulating insulin-induced glucose metabolism through the co-activation with HIF-1a, thereby decreasing blood glucose levels.
The Role of PKM2 in Diabetic Nephropathy
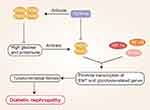
Diabetic nephropathy (DKD) is a condition in which chronic inflammation mediated by macrophages leads to the progressive compromise of glomerular filtration barriers, with associated glomerular hypertrophy, extracellular matrix accumulation, and the activation of the transforming growth factor (TGF-β) signaling pathways in renal tubular epithelial cells.35 TGF-β stimulates renal tubular epithelial cell transformation. Through the resultant epithelial-mesenchymal transition (EMT) induction and abnormal glycolytic activity, these cells ultimately undergo transformation into pathogenic cells that secrete high levels of extracellular matrix proteins, contributing to renal tubulointerstitial fibrosis and DKD incidence. The transcription factors nuclear factor kappa B (NF-kB), STAT3, and HIF-1a are all integral to the onset and progression of DKD.36 PKM2 can also regulate the expression of a range of adhesion molecules, chemokines, and inflammatory cytokines. PKM2 can undergo phosphorylation within glomerular endothelial cells in the context of DKD, resulting in its isomerization and translocation into the nucleus wherein it regulates inflammatory activity.37 Such regulatory activity can promote STAT3 and NF-kB phosphorylation and the expression of the adhesion protein intercellular adhesion molecule-1 (ICAM-1), contributing to the initiation of inflammatory cell infiltration and thereby contributing to the progression of DKD.38 Therefore, reducing inflammation can reduce oxidative stress and cell damage in patients with diabetic nephropathy.
Qi et al26 determined that PKM2 activation is associated with reductions in toxic glucose metabolite levels and with the maintenance of renal function, and as such, inhibiting PKM2 phosphorylation may suppress renal inflammation, protecting against DKD incidence. Tetrameric PKM2 can decrease the levels of phosphorylated PKM2, inhibit abnormal glycolytic activity and the expression of ICAM-1, type I collagen a3, and TGF-β1 in the context of DKD, in addition to preventing renal fibrosis associated with high levels of albuminuria in individuals with diabetes.39,40 In its tetrameric form, PKM2 can also suppress the adhesion of macrophages and NF-kB and STAT3 pathway activation, thereby further protecting against renal fibrosis.10,41,42 The TEPP-46 and DASA-58 small molecules can promote tetrameric PKM2 formation through the binding of PKM2 monomers and inhibiting their phosphorylation, impairing the expression of glycolysis-related genes, Epithelial-mesenchymal transformation (EMT) induction, and reducing rates of renal fibrosis.28,29,43 Yokoyama et al44 highlighted the promise of PKM2 as a therapeutic target in DN. Given that PKM2 serves as a protective protein that can mitigate renal failure in diabetic individuals, pharmacological interventions that induce PKM2 upregulation may aid in preventing DKD progression. If tetrametric PKM2 content decreases in DKD, this will result in reduced glucose metabolic flux and the increased accumulation of toxic glucose metabolites within podocytes, further supporting the identity of PKM2 as a protective mediator with the potential to prevent or alleviate advanced diabetic microangiopathy.39,45,46 Previous studies have shown that TEPP-46 and other compounds induce PKM2 tetramerization by targeting PKM2, thereby inhibiting the transcription of pro-inflammatory genes and reducing both immune cell proliferation and inflammatory factor production, thus reducing the inflammatory response of DKD (Figure 2).
The Role of PKM2 in Diabetic Retinopathy
Diabetic retinopathy (DR) is a clinically important form of microangiopathy that can arise over time in individuals suffering from diabetes, and it is one of the most common diabetes-related causes of visual impairment and blindness, contributing to alterations in both contrast sensitivity and color discrimination. DR incidence rates are steadily rising, and efforts to treat affected patients primarily center on the prevention of overall vision loss. Retinal rod and cone cells both exhibit high levels of metabolic activity, and PKM2 can influence visual function via the regulation of phosphodiesterase 6β (Pde6β), which is an enzyme that is central to photoreceptor functionality.47 Reductions in PKM2 protein expression can impair transcriptional co-activator activity, leading to reduced Pde6β expression, whereas a loss of Pde6β can impair the function of rod photoreceptors and compromise normal retinal anabolic activity.47–49 Rajala et al50 employed a mouse model of type 2 diabetic mouse model to explore the importance of PKM2 in the context of DR, and found that retinal PKM2 protein levels declined in db/db mice whereas PKM1 expression was unaffected, with corresponding reductions in retinal Pde6β expression and the impairment of rod cell function. Retinal pyruvate kinase activity increased in these db/db mice, while the redox/reduction ratio declined. Other research suggests that PKM2 downregulation can alleviate PKM1 inhibition, potentially contributing to the overall enhancement of pyruvate kinase activity. As such, impaired visual acuity in diabetic individuals may be at least partially attributable to reduced PKM2 levels in these patients.
DR develops under conditions of sustained hyperglycemia in which retinal vascular endothelial cells produce high levels of inflammatory mediators and ROS leading to advanced glycation end product accumulation and nervous system abnormalities that ultimately contribute to damage to the retinal barrier and retinal microangiopathy and retinal barrier damage.51 Heng et al52 demonstrated that STEAP4 (prostate six transmembrane epithelial antigen 4, also known as STAMP2 or TIARP) is an essential membrane protein that is closely related to hyperglycemia-induced inflammation and injury. STEAP4 expression can suppress retinal vascular endothelial cell (HRCEC) inflammation and apoptosis, decrease atherosclerosis, and alleviate associated dysfunction.53 Overexpressing STEAP4 can suppress HIF-1α/PKM2 signaling, which is noteworthy given the close relationship between such signaling and glucose metabolism, leading to increased glucose uptake and lactic acid secretion and thus contributing to the pathogenesis of diabetes. By inhibiting HIF-1/PKM2 signal transduction it may be possible to modulate glucose metabolism and thereby prevent hyperglycemia-related retinal cell apoptosis and associated injury. This HIF-1a/PKM2 axis has been suggested as a promising therapeutic target in the treatment of DR. PKM2 is closely related to the process of inflammation, and this HIF-1a/PKM2 axis has been considered as a promising target for the treatment of DR. For example, Liu et al54 found that HIF-1α and PKM2 expression levels were significantly increased in HRCEC cells exposed to hyperglycemic conditions, while HIF-1 α downregulation inhibited glucose levels induced by PKM2 and insulin. Moreover, through animal model studies, Wang et al55 found that inhibiting PKM2 was sufficient to reduce HIF-1α and VEGF expression, thereby reducing damage to HRCECs and associated apoptosis, preventing the incidence of retinal ischemia in rats and underscoring the relevance of the HIF-1α/PKM2 signaling axis in retinal disease (Figure 3). As such, PKM2 holds great promise as a target for the treatment of DR.
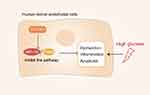 |
Figure 3 Mechanistic overview of the role of PKM2 in diabetic retinopathy: STEAP4 reduces HG-induced HRCEC dysfunction, inflammation, and apoptosis by inhibiting the HIF-1α/PKM2 signaling pathway. |
Conclusions
The studies discussed in this review emphasize the fact that PKM2 functions not only as a key rate-limiting glycolytic enzyme, but also an important regulator of gene expression, metabolic activity, and inflammatory diseases. Tetrameric PKM2 can inhibit LPS-induced IL-1β production, remediated high L-cysteine concentrations, influence insulin-induced glucose metabolism, and promote enhanced insulin secretion via inducing HIF-1a co-activation. Through its interactions with TGF-β and the HIF-1α/PKM2 pathway, PKM2 can thus influence the pathogenesis of diabetes, DR, DKD, and a range of other diseases. In addition, the combination of PKM2 and TEPP-46 can reduce the phosphorylation of STAT3 and NF-kB, suppress the production of inflammatory factors such as IL-1 β and HIF-1α, and thus decrease the magnitude of inflammatory responses associated with diabetes and diabetic microangiopathy. These prior studies thus provide a foundation for future work exploring the potential relevance of PKM2 as a therapeutic target in the treatment of diabetes and associated diabetic microangiopathy.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This review is not supported by funds.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu M, Fang J, Wang H, Zhou S. Proteome and phosphoproteome analyses reveal the kinase regulatory network involved in glycogen synthesis kinase 3beta. Front Genet. 2021;12:657140.
2. Heerspink HJL, Parving HH, Andress DL, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (sonar): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393(10184):1937–1947.
3. Burns JS, Manda G. Metabolic pathways of the warburg effect in health and disease: perspectives of choice, chain or chance. Int J Mol Sci. 2017;18(12):2755.
4. Bertelsen LB, Hansen ESS, Sadowski T, Ruf S, Laustsen C. Hyperpolarized pyruvate to measure the influence of pkm2 activation on glucose metabolism in the healthy kidney. NMR Biomed. 2021;34(11):e4583.
5. Kong Q, Li N, Cheng H, et al. Hspa12a is a novel player in nonalcoholic steatohepatitis via promoting nuclear pkm2-mediated m1 macrophage polarization. Diabetes. 2019;68(2):361–376.
6. Yang P, Li Z, Li H, Lu Y, Wu H, Li Z. Pyruvate kinase m2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell Signal. 2015;27(7):1525–1532.
7. Luo W, Semenza GL. Emerging roles of pkm2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23(11):560–566.
8. Israelsen WJ, Vander Heiden MG. Pyruvate kinase: function, regulation and role in cancer. Semin Cell Dev Biol. 2015;43:43–51.
9. Lang N, Wang C, Zhao J, Shi F, Wu T, Cao H. Long noncoding rna bcyrn1 promotes glycolysis and tumor progression by regulating the mir149/pkm2 axis in nonsmallcell lung cancer. Mol Med Rep. 2020;21(3):1509–1516.
10. Zhang Z, Deng X, Liu Y, Liu Y, Sun L, Chen F. Pkm2, function and expression and regulation. Cell Biosci. 2019;9:52.
11. Dong G, Mao Q, Xia W, et al. Pkm2 and cancer: the function of pkm2 beyond glycolysis. Oncol Lett. 2016;11(3):1980–1986.
12. Wang P, Sun C, Zhu T, Xu Y. Structural insight into mechanisms for dynamic regulation of pkm2. Protein Cell. 2015;6(4):275–287.
13. Alquraishi M, Puckett DL, Alani DS, et al. Pyruvate kinase m2: a simple molecule with complex functions. Free Radic Biol Med. 2019;143:176–192.
14. Zhu S, Guo Y, Zhang X, et al. Pyruvate kinase m2 (pkm2) in cancer and cancer therapeutics. Cancer Lett. 2021;503:240–248.
15. Allen AE, Locasale JW. Glucose metabolism in cancer: the saga of pyruvate kinase continues. Cancer Cell. 2018;33(3):337–339.
16. Morgan HP, O’Reilly FJ, Wear MA, et al. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci U S A. 2013;110(15):5881–5886.
17. Xu J, Jiang C, Wang X, et al. Upregulated pkm2 in macrophages exacerbates experimental arthritis via stat1 signaling. J Immunol. 2020;205(1):181–192.
18. He CL, Bian YY, Xue Y, et al. Pyruvate kinase m2 activates mtorc1 by phosphorylating akt1s1. Sci Rep. 2016;6:21524.
19. Jiang Y, Li X, Yang W, et al. Pkm2 regulates chromosome segregation and mitosis progression of tumor cells. Mol Cell. 2014;53(1):75–87.
20. Chiu CF, Weng JR, Lee SL, et al. Osu-a9 induced-reactive oxygen species cause cytotoxicity in duodenal and gastric cancer cells by decreasing phosphorylated nuclear pyruvate kinase m2 protein levels. Biochem Pharmacol. 2020;174:113811.
21. Li Q, Leng K, Liu Y, et al. The impact of hyperglycaemia on pkm2-mediated nlrp3 inflammasome/stress granule signalling in macrophages and its correlation with plaque vulnerability: an in vivo and in vitro study. Metabolism. 2020;107:154231.
22. Liang J, Cao R, Wang X, et al. Mitochondrial pkm2 regulates oxidative stress-induced apoptosis by stabilizing bcl2. Cell Res. 2017;27(3):329–351.
23. Nakamura A, Terauchi Y. Present status of clinical deployment of glucokinase activators. J Diabetes Investig. 2015;6(2):124–132.
24. Sizemore ST, Zhang M, Cho JH, et al. Pyruvate kinase m2 regulates homologous recombination-mediated DNA double-strand break repair. Cell Res. 2018;28(11):1090–1102.
25. Qi W, Li Q, Gordin D, King GL. Preservation of renal function in chronic diabetes by enhancing glomerular glucose metabolism. J Mol Med (Berl). 2018;96(5):373–381.
26. Qi W, Keenan HA, Li Q, et al. Pyruvate kinase m2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med. 2017;23(6):753–762.
27. Wang S, Yang Z, Gao Y, et al. Pyruvate kinase, muscle isoform 2 promotes proliferation and insulin secretion of pancreatic beta-cells via activating wnt/ctnnb1 signaling. Int J Clin Exp Pathol. 2015;8(11):14441–14448.
28. Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase m2 regulates hif-1alpha activity and il-1beta induction and is a critical determinant of the warburg effect in lps-activated macrophages. Cell Metab. 2015;21(2):347.
29. Palsson-McDermott EM, Curtis AM, Goel G, et al. Pyruvate kinase m2 regulates hif-1alpha activity and il-1beta induction and is a critical determinant of the warburg effect in lps-activated macrophages. Cell Metab. 2015;21(1):65–80.
30. Nakatsu D, Horiuchi Y, Kano F, et al. L-cysteine reversibly inhibits glucose-induced biphasic insulin secretion and atp production by inactivating pkm2. Proc Natl Acad Sci U S A. 2015;112(10):E1067–1076.
31. Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64(3):663–672.
32. Damasceno LEA, Prado DS, Veras FP, et al. Pkm2 promotes th17 cell differentiation and autoimmune inflammation by fine-tuning stat3 activation. J Exp Med. 2020;217(10):e20190613.
33. Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102(12):4343–4410.
34. Esen I, Jiemy WF, van Sleen Y, et al. Plasma pyruvate kinase m2 as a marker of vascular inflammation in giant cell arteritis. Rheumatology. 2021;1:keab814.
35. Takagaki Y, Shi S, Katoh M, Kitada M, Kanasaki K, Koya D. Dipeptidyl peptidase-4 plays a pathogenic role in bsa-induced kidney injury in diabetic mice. Sci Rep. 2019;9(1):7519.
36. Ouyang X, Han SN, Zhang JY, et al. Digoxin suppresses pyruvate kinase m2-promoted hif-1alpha transactivation in steatohepatitis. Cell Metab. 2018;27(5):1156.
37. Kocak MZ, Aktas G, Atak BM, et al. Is neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50(3):e13206.
38. Amdur RL, Feldman HI, Gupta J, et al. Inflammation and progression of ckd: the cric study. Clin J Am Soc Nephrol. 2016;11(9):1546–1556.
39. Li L, Tang L, Yang X, et al. Gene regulatory effect of pyruvate kinase m2 is involved in renal inflammation in type 2 diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2020;128(9):599–606.
40. Gordin D, Shah H, Shinjo T, et al. Characterization of glycolytic enzymes and pyruvate kinase m2 in type 1 and 2 diabetic nephropathy. Diabetes Care. 2019;42(7):1263–1273.
41. Liu H, Takagaki Y, Kumagai A, Kanasaki K, Koya D. The pkm2 activator tepp-46 suppresses kidney fibrosis via inhibition of the emt program and aberrant glycolysis associated with suppression of hif-1alpha accumulation. J Diabetes Investig. 2021;12(5):697–709.
42. Zhang Z, Deng X, Liu Y, Liu Y, Sun L, Chen F. Correction to: pkm2, function and expression and regulation. Cell Biosci. 2019;9:59.
43. Niewczas MA, Gohda T, Skupien J, et al. Circulating tnf receptors 1 and 2 predict esrd in type 2 diabetes. J Am Soc Nephrol. 2012;23(3):507–515.
44. Yokoyama M, Tanuma N, Shibuya R, et al. Pyruvate kinase type m2 contributes to the development of pancreatic ductal adenocarcinoma by regulating the production of metabolites and reactive oxygen species. Int J Oncol. 2018;52(3):881–891.
45. Angiari S, Runtsch MC, Sutton CE, et al. Pharmacological activation of pyruvate kinase m2 inhibits cd4(+) t cell pathogenicity and suppresses autoimmunity. Cell Metab. 2020;31(2):391–405 e398.
46. Sweetwyne MT, Gruenwald A, Niranjan T, Nishinakamura R, Strobl LJ, Susztak K. Notch1 and notch2 in podocytes play differential roles during diabetic nephropathy development. Diabetes. 2015;64(12):4099–4111.
47. Aung MH, Kim MK, Olson DE, Thule PM, Pardue MT. Early visual deficits in streptozotocin-induced diabetic long evans rats. Invest Ophthalmol Vis Sci. 2013;54(2):1370–1377.
48. Rajala A, Wang Y, Brush RS, et al. Pyruvate kinase m2 regulates photoreceptor structure, function, and viability. Cell Death Dis. 2018;9(2):240.
49. Lambert V, Hansen S, Schoumacher M, et al. Pyruvate dehydrogenase kinase/lactate axis: a therapeutic target for neovascular age-related macular degeneration identified by metabolomics. J Mol Med (Berl). 2020;98(12):1737–1751.
50. Rajala A, Soni K, Rajala RVS. Metabolic and non-metabolic roles of pyruvate kinase m2 isoform in diabetic retinopathy. Sci Rep. 2020;10(1):7456.
51. Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O’Neal DN, Januszewski AS. Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015;12(1–2):159–195.
52. Heng LZ, Comyn O, Peto T, et al. Diabetic retinopathy: pathogenesis, clinical grading, management and future developments. Diabet Med. 2013;30(6):640–650.
53. Scarl RT, Lawrence CM, Gordon HM, Nunemaker CS. Steap4: its emerging role in metabolism and homeostasis of cellular iron and copper. J Endocrinol. 2017;234(3):R123–R134.
54. Liu L, Xu H, Zhao H, Jiang C. Steap4 inhibits hif-1alpha/pkm2 signaling and reduces high glucose-induced apoptosis of retinal vascular endothelial cells. Diabetes Metab Syndr Obes. 2020;13:2573–2582.
55. Wang HJ, Hsieh YJ, Cheng WC, et al. Jmjd5 regulates pkm2 nuclear translocation and reprograms hif-1alpha-mediated glucose metabolism. Proc Natl Acad Sci U S A. 2014;111(1):279–284.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.