Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 15
The Role of Natural Extracts in the Management of Infantile Hemangiomas and Vascular Tumors
Authors Roca IC , Cojocaru E , Rusu CD, Trandafir LM, Săveanu CI, Lupu VV , Butnariu LI, Ţarcă V, Moscalu M, Bernic J , Lupu A, Ţarcă E
Received 9 September 2023
Accepted for publication 28 December 2023
Published 6 January 2024 Volume 2024:15 Pages 1—16
DOI https://doi.org/10.2147/PHMT.S439537
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Roosy Aulakh
Iulia Cristina Roca,1,* Elena Cojocaru,2 Carmen Daniela Rusu,1 Laura Mihaela Trandafir,3 Cătălina Iulia Săveanu,4 Vasile Valeriu Lupu,3,* Lăcrămioara Ionela Butnariu,5 Viorel Ţarcă,6,* Mihaela Moscalu,6 Jana Bernic,7 Ancuța Lupu,3,* Elena Ţarcă1
1Department of Surgery II, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 2Department of Morphofunctional Sciences I – Pathology, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 3Department of Mother and Child Medicine–Pediatrics, “Grigore T. Popa” University of Medicine and Pharmacy, Iaşi, 700115, Romania; 4Surgical Department, Discipline of Preventive Dentistry, Faculty of Dental Medicine, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, 700115, Romania; 5Department of Medical Genetics, Faculty of Medicine, “Grigore T. Popa” University of Medicine and Pharmacy, Iași, 700115, Romania; 6Department of Preventive Medicine and Interdisciplinarity, “Grigore T. Popa” University of Medicine and Pharmacy, Iași, 700115, Romania; 7Discipline of Pediatric Surgery, “Nicolae Testemițanu” State University of Medicine and Pharmacy, Chisinau, Moldova
*These authors contributed equally to this work
Correspondence: Carmen Daniela Rusu; Cătălina Iulia Săveanu, Tel +40765485446, Email [email protected]; [email protected]
Abstract: Hemangiomas are vascular tumors resulting from the proliferation of endothelial-like cells; they are the most common childhood tumors, affecting approximately 5– 10% of newborns and infants. Besides hemangiomas, which are definitely benign tumors despite their overgrowth potential, there are other vascular tumors like hemangioendotheliomas, which may display intermediate characteristics between benign hemangiomas and highly malignant angiosarcomas. Standard therapy may be constricted by serious adverse effects, high cost, or traumatic influence. Diet is a major resource for health preservation, disease prevention, and treatment. The therapeutic property of edible berries, marine products, or medicinal plants have long been known and used in traditional medicine; a plant-based nutrition can prevent the development and progression of diseases associated with extensive neo-vascularization. The purpose of our review is to highlight those natural treatments that hemangioma and vascular tumor patients can receive in the future, both for their benefit and that of their families. We performed the review according to the Preferred Reporting Items for Systematic Reviews and Metanalysis Statement. We used the Web of Science, PubMed, and EMBASE engines for the study, and searched for the association of hemangioma with naturopathic treatment/plant extract/plants in published articles. We found that natural extracts from plants and fruits are cost-effective and safe treatments for hemangiomas and vascular tumors, as well as for other forms of cancer. In any case, more in vitro and in vivo studies are needed to confirm the proposed signaling pathways in tumors and validate the improvement parameters after natural products administration. The era of molecularly targeted therapy and personalized medicine is approaching and naturally occurring substances are very useful tools for tumor treatment and prevention. Plant extract substances have strong specificity and pertinence, are non- toxic and have few side effects, and may become an emerging cancer treatment.
Keywords: vascular abnormalities, plants, plant extract, naturopathic treatment
Graphical Abstract:
Introduction
Hemangiomas are benign vascular tumors resulting from the proliferation of endothelial-like cells, hypoxia playing a crucial role. Infantile hemangiomas are the most common childhood tumors, affecting approximately 5–10% of newborns and infants.1 Rarely, hemangiomas are present from birth, they usually appear in the first postnatal weeks as a small red spot, and grow rapidly in size in the first months of life, up to the age of one year. After the age of one year, the natural evolution is towards stagnation and spontaneous regression, so that after the age of 5 or 6 years they can slowly and completely involute.2 The bad news is that, when they are located on the face, genitals, hand or flexion regions, the increase in size of the hemangioma can affect the functionality of the nearby organ, with the appearance of serious complications; approximately 1–2% of all hemangiomas are life threatening.3 Also, the rapid increase in size can be complicated by ulceration, infection, repetitive bleeding, pain, functional impairment or permanent disfigurement, and if the localization is multiple (more than five) or affects the internal organs (liver or gastrointestinal tract), complications such as heart failure or intravascular coagulation can occur.2–5 If visceral hemangiomas are suspected, evaluation utilizing ultrasound, magnetic resonance imaging, angiography or computed tomographic imaging will differentiate a benign lesion from a malignant process.6 In such cases, the standard medical or surgical treatment, according to the protocols of the respective hospital, must be applied, while the remainders of the affected children live with their ugly lesion for 5–9 years until the tumor involutes. The standard therapy may be constricted by serious adverse effects, high cost, or traumatic influence, being difficult to administer by parents and difficult to tolerate by children. Besides, there are some cases in which the hemangioma, although it is located in a non-dangerous area (on the back of the thorax or on the thigh for example), creates anxiety and discomfort for the parents due to the increase in size and color. These unsightly infantile hemangiomas (IH) create anxiety for parents, who insist on applying a form of treatment. The early treatment and results are requested by the families for whom visible IHs may cause social stigma. Both for the parents’ peace of mind, and to save the patient from an unnecessary medical or surgical treatment, alternative medicine and naturopathic treatment could be a solution. There are recent studies providing information and better understanding of the pathogenesis and biological features of these vascular tumors, leading to new therapeutic approaches. Angiogenesis, vasculogenesis and tumorigenesis are the main pathogenic mechanisms of hemangioma neovascularization, linked to alterations in cellular signaling pathways. These pathways have to be targeted from a therapeutic perspective, to reverse, delay or prevent infantile hemangioma growth. The major pathways implicated in IH are the VEGF/VEGFR, Notch, β-adrenergic, Tie2/angiopoietins, PI3K/AKT/mTOR, HIF-α-mediated and PDGF/PDGF-R-β pathways.7,8 Both in vitro and in vivo studies evaluated anti-proliferation and anti-angiogenesis effects on hemangiomas cells by natural plants extracts.9 For example, 15.16-Dihydrotanshinone I (DHTS), an extracted Tanshen compound, was compared with propranolol, the first-line treatment for IH currently. Cai et al demonstrated that DHTS was much more effective than propranolol in inhibiting hemangioma cells proliferation and angiogenesis, both in vitro and in vivo.10 DHTS also downregulated HIF-1α expression by interfering in its posttranscriptional processing, and the RNA-binding protein HuR participated in this mechanism [Duan]. Tanshen is the rhizome of Salvia miltiorrhiza and is used in traditional Chinese medicine for multiple therapeutic remedies, as an antioxidant and anticoagulant agent, as well as for its antibacterial and antineoplastic activities.11 Besides hemangiomas, which are definitely benign tumors despite their overgrowth potential, there are other vascular tumors like hemangioendotheliomas (HE), which may display intermediate characteristics between benign hemangiomas and highly malignant angiosarcomas.12 Some malignant tumors, including vascular ones, have benefited over time from radiotherapy, but when it comes to children, the therapeutic doses must be drastically reduced in order not to cause more harm than good. Lower doses of radiation combined with conventional chemotherapy may be used, but chemotherapeutic drugs are also highly cytotoxic both for tumor cells but also for healthy cells in the child. Therefore, chemosensitizers, radiosensitizers, and enhancers are of great interest. In this context, the association of chemotherapy or ionizing radiation therapy with the administration of dietary active substances from natural sources can be of real benefit in the treatment of vascular cancers in children.12,13
Diet is a major tool in health preservation, disease prevention, and treatment. The therapeutic property of edible berries, marine products, or medicinal plants have long been known and used in traditional medicine;14,15 a plant-based nutrition can prevent the development and progression of disorders associated with extensive neovascularization.15,16 Regarding vascular tumors with a risk of malignancy, huge efforts are being made by numerous teams of researchers to find those natural extracts with anti-angiogenic and anti-cancer effects, and with as few adverse effects as possible.
The purpose of our review is to highlight those natural treatments that hemangioma and other vascular tumor patients can receive in the future, both for their benefit and that of their families.
Materials and Methods
Search Strategy of Electronic Databases
The main outcome of our study is the systematization of current data from evidence-based medicine regarding the positive effect of natural extracts or specific nutrients in the treatment of vascular tumors. We performed this review according to the Preferred Reporting Items for Systematic Reviews and Metanalysis Statement (http://www.prisma-statement.org/ (accessed on 25 May 2023)). Five researchers independently performed data search and extraction, as well as quality assessment of the articles. We used the Web of Science, PubMed, and EMBASE engines for the study, and searched for the association of hemangioma with naturopathic treatment/plant extract/plants in published articles. The inclusion criteria were original studies written in English, literature or systematic reviews, randomized controlled trials, observational studies, series of cases, and case report studies, from the earliest time possible until May 2023. We excluded works that were not written in English, those that did not refer to the treatment of vascular tumors or that did not contain a reference to the treatment with substances derived from plants.
Results
From Web of Science, 16338 articles were found discussing about the hemangioma; anyway, only 8 results were obtained after searching for “hemangioma” AND “plant extract”/“plants” OR “hemangioma” AND “naturopathic treatment”.
A total of 27,734 results were obtained from PubMed when searched for hemangiomas, and only 262 when we added the Medical Subject Headings (MeSH) terms “plant extract”/“plants” AND “naturopathic treatment”, to select the appropriate articles for our review. From these, only 82 articles were found to be eligible for our review, based on title and abstract assessment.
A total of 72,057 articles were obtained from EMBASE for the hemangioma search; after using the association “plant extract” OR “plants” OR “naturopathic treatment”, 36, 6, and 1 articles were identified. Of these, only 30 were articles and reviews suited for our review (Publication type – filters applied).
During the second step, we reviewed the titles, abstracts, and the full texts of 243 papers from the last 30 years to extract pertinent data for our review (Figure 1). The bibliography was searched for additional studies to be reviewed. Seventy-five relevant studies with their citations were included in our reference list.
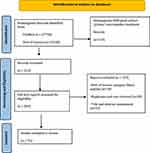 |
Figure 1 PRISMA flowchart. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34.17 Creative Commons. |
Pathogenesis
The origin and pathogenesis of infantile hemangiomas and vascular tumors is still unclear, being the result of multiple factors, such as inactivation of tumor suppressor genes and the activation of proto-oncogenes.18,19 Compared with other vascular malformations, glucose transporter-1 (GLUT-1), an erythrocyte-type glucose transporter protein, has been identified as a highly selective marker of IH at all stages, from development to involution.20 GLUT-1 is a downstream target of hypoxia-inducible factor-1-alpha (HIF-1α), together with vascular endothelial growth factor A (VEGF-A) and insulin-like growth factor 2 (IGF-2), thus sustaining the hypothesis that hypoxia may trigger the appearance of IH.21
GLUT1 serves as a marker for distinguishing between vascular tumors and vascular malformations but also as a primary key molecule in endothelial cell glycolysis, facilitating glucose transport into the cell for energy supply. Inhibition of the glycolytic activator phosphofructokinase-1 (PFK-1) suppresses hemangioma-derived endothelial cell (HemEC) proliferation and migration, induces cell arrest, and reduces glucose uptake and lactate and ATP production.22,23 Glucose metabolism in HemECs is different from normal endothelial cells, and altering the expression of glycolysis-associated molecules will influence the phenotype of HemECs, providing new therapeutic approaches for IH treatment.22 Endothelial cell glycolysis was confirmed to be closely associated with the pathogenesis of infantile hemangioma, and influencing the expression of glycolytic key enzymes such as PFKFB3 (6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3) may inhibit the occurrence and development of vascular tumors through IH angiogenesis suppression and apoptosis induction.23
The VEGF – HIF-1α Pathway in the Appearance of Hemangiomas
Infantile hemangioma appears from excessive angiogenesis/vasculogenesis or hypoxia-induced vascular endothelial cell proliferation, via HIF-1α and vascular endothelial growth factor (VEGF).24 It is demonstrated that in hemangioma tissue there is a significant increase in the protein and mRNA expression of HIF-1 compared with normal vascular tissue.25 HIF-1α stimulates the angiogenesis and proliferation of endothelial cells, especially in hypoxia conditions. Furthermore, HIF-1α induces the expression of VEGF gene (downstream of HIF-1α), which stimulates the proliferation and migration of vascular endothelial cells, leading to the formation of new vessels.26 VEGF binds to tyrosine kinase receptors (RTKs) and promotes the activity of vascular endothelial growth factor receptors (VEGFRs), mediating mitogenic signals via VEGFR1 and VEGFR2.2 The decreased level of VEGFR1 in endothelial cells facilitates VEGFR2 persistent activation in IH. The combination of VEGFR2 and VEGF triggers specific signals and stimulates endothelial cell proliferation and induces more angiogenesis. VEGFR2 signal further activates PI3K/Akt (serine/threonine protein kinase B) pathway and formation of new vessels (Figure 2). Under hypoxia conditions, the spontaneous apoptosis of hemangioma endothelial cells is inhibited via the regulation of mTOR and p70S6K.2 This whole mechanism is like an autocrine cycle that promotes the proliferation of endothelial cells and the formation of new blood vessels, leading to the rapid increase in the size of hemangiomas.2
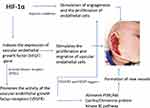 |
Figure 2 The VEGF – HIF-1α pathway in the appearance of hemangiomas. |
New theories on the pathogenesis of infantile hemangioma include placental origin, somatic endothelial mutation, or other intrinsic defect, and external factors that create an environment conducive to growth.18
The Role of Transcription Factor Fli-1 in the Appearance of Vascular Tumors
The occurrences of malignant and potentially malignant sarcomas originating from blood vessels are rarely seen and include hemangiosarcomas, Kaposi’s sarcomas, and hemangioendothelimas, usually with no known cause. The transcription factor Friend leukemia virus integration 1 (Fli-1) belongs to the E26 Transformation-Specific (ETS) transcription factor family and is expressed in normal hematopoietic stem cells and vascular endothelial cells. But Fli-1 is also abnormally expressed in numerous malignant tumors (Ewing sarcoma, erythroleukemia, lymphoma, breast cancer, etc.) and benign or malignant hemangioma.19 Fli-1 promotes p110 and GATA-1 expression in leukemia cells and inhibits the transcriptional activity of Rb and SHIP-1 by regulating the PI3K/Akt signaling pathway (also implicated in the appearance of vascular tumors), thus inducing cell proliferation. Fli-1 favors the transcriptional activity of MDM2 and Bcl-2 and inhibits the activity of phosphorylated Bax and Bad, thus inhibiting cell apoptosis; Fli-1 also interacts with the TGF-β/Smad pathway and inhibits the activity of DAPK, TIMP-1, and Beclin1, promoting the occurrence and development of tumors.19 In some studies, the knockout of Fli-1 gene was conducted to decrease growth and death of mouse erythroid leukemia cells; therefore, maybe Fli-1 could be used as a target for specific tumor therapy.27,28
Treatment
Standard Therapy
There are very few countries where a standardized approach is applied to hemangiomas; usually, the management is “wait and see” for the IH not implying the face, genitals, or hand. For these special localizations, a variety of therapies may be applied: systemic or topical drugs (propranolol, timolol, corticosteroids, Vincristine, immunosuppressant, and itraconazole), pulsed dye laser treatment, or surgical excision.2,3,29 In the last decade, propranolol became the first-line treatment for IH, at a dose between 2 and 3 mg/kg/day, with a positive response of 80–100%.29 Because of the rare, but cited side effects of systemic drugs like neurocognitive impairment, bronchospasm, hypotension, bradycardia, hyperkalemia, or hypoglycemia, some physicians and parents do not agree with this treatment.30 Corticosteroids, at a dose of 2–3 mg/kg/day of prednisone, may be effective in more than 80% of cases, but the adverse reactions are numerous: mood disorders, gastric irritation, Cushing’s phase, weight gain, immunosuppression, spastic diplegia, developmental delays, or adrenal suppression.31,32 Topical timolol may have reduced efficiency if utilized early in the development of the IH, or on small thin lesions, but adverse side effects such as local xerosis from medication, ulceration, and infection of the IH were reported.33
Regarding laser therapy, also effective, traumatic impairments and high cost are major disadvantages. Sclerotherapy and surgical excision of IH in children are associated with the adverse effects of general anesthesia, bleeding, wound infection, or unsightly scars.
Visceral hemangiomas that create shunts and have the potential to cause congestive heart failure are man-aged with systemic treatments (propranolol, interferon-alpha, prednisone, or sirolimus), but refractory cases may need percutaneous embolization or surgery, depending on the size; as for adults, surgical approaches range from embolization, radiofrequency ablation, radiotherapy to hepatic resection or liver transplantation.34 Zhang et al reported a successfully treated case of hepatic hemangioma with azithromycin, a widely used macrolide antibiotic which, in addition to its antimicrobial activity, exerts anti-inflammatory and antiproliferative effects through inhibition of VEGFR2-mediated downstream signaling pathways.4,35
Ionizing radiation therapy targets rapidly proliferating tumor cells and may be very effective for the treatment of vascular tumors, but depending on its dosage, adverse side effects are of serious concern in children.36 To achieve maximum health benefits, lower doses of radiation may be used in combination with conventional chemotherapy. In any case, the cumulated adverse side effects of radiation and chemotherapy raise serious concerns for the quality of life of the surviving patient.12
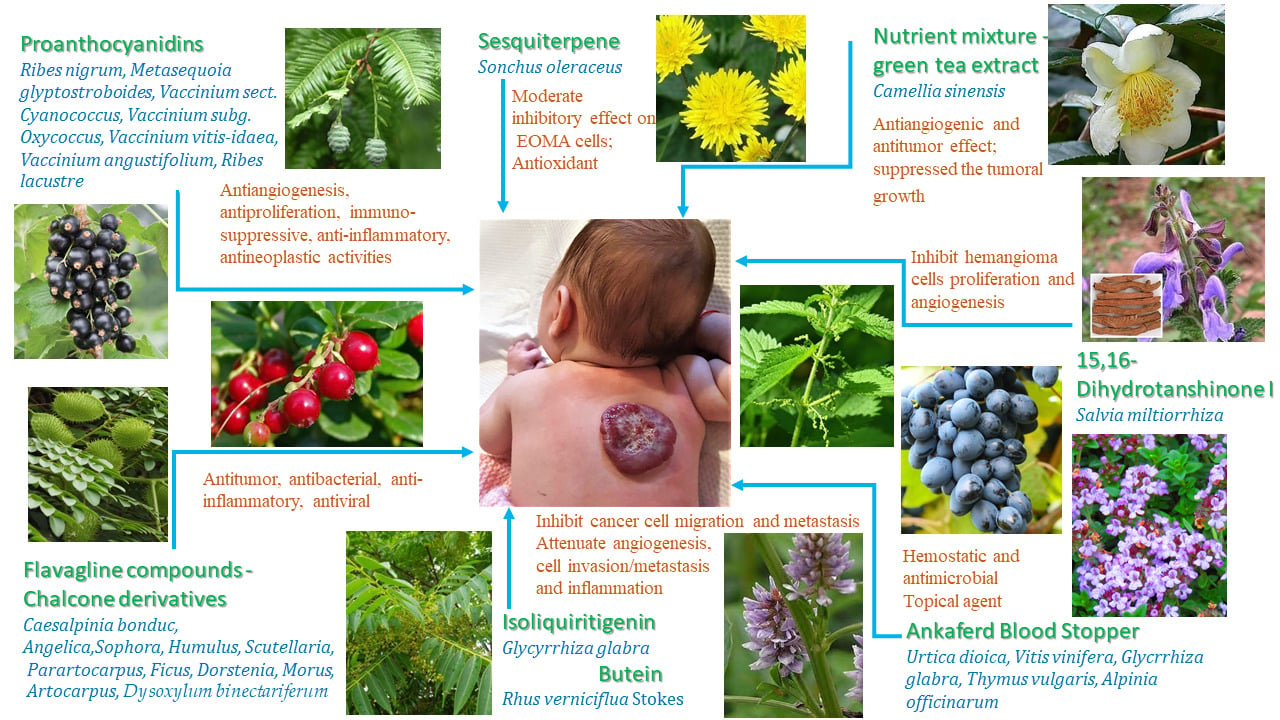
Role of Proanthocyanidins in Treating Hemangiomas and Vascular Tumor
Proanthocyanidins are natural extracts from grape seeds and red wine, black currant (Ribes nigrum, family Grossulariaceae), dawn redwood (Metasequoia glyptostroboides, subfamily Sequoioideae of the family Cupressaceae), blueberry (Vaccinium sect. Cyanococcus, family Ericaceae), cranberries (Vaccinium subg. Oxycoccus, family Ericaceae), and other fruits. Proanthocyanidins are condensed tannins from a group of biologically active polyphenolic bioflavonoids synthesized by many plants; they have demonstrated human health benefits. These phytochemicals are oligo- or polymers of monomeric flavan-3-ols, and they result as an end product of flavonoid biosynthetic pathway, in ingredients containing catechin, epicatechin, gallic acid, or epigallocatechin subunit chains doubly linked by C4-C6 and C4-C8 interferon bonds.37,38 Proanthocyanidins have natural effects on antiangiogenesis, antiproliferation, immuno-suppressive, anti-inflammatory, and antineoplastic activities, as well as antidiabetic, lipid lowering, anti-obesity, and cardio and neuroprotective effects.37,39 And because there are very few side effects, they can be administered even in infants and pregnant women.40,41
Due to their powerful effects on antiproliferation and antiangiogenesis through suppressing VEGF signaling pathway, proanthocyanidins may have applications in those angiogenesis-related diseases, such as vascular tumors, hemangiomas, and inflammatory diseases. It was already demonstrated that their beneficial effect on osteoarthritis, urinary tract infections in children, and due to their antiangiogenic and anticancer activities, proanthocyanidins could be used as an antitumor agent under various signaling pathways, participating in cell survival, apoptosis, migration, and invasion.41–43
There are studies in the literature that demonstrate the favorable effect of proanthocyanidins in the treatment of hemangiomas, although the beneficial role of these substances in conditions involving the proliferation of vascular endothelial cells and uncontrolled angiogenesis has already been demonstrated.37,38,42 Proanthocyanidins bind to VEGF and inhibit HIF-1α translation; the decrease of VEGF expression through mediating the PI3K/Akt and mTOR/p70S6K signaling pathways arrests the proliferation, invasion, and metastasis of tumor cell. This may be the process of proanthocyanidin controlling hemangioma growth.2
Evidence for Anti-Angiogenic and Anticancer Properties of Berries
Berry fruits, especially lingonberries (Vaccinium vitis-idaea, family Ericaceae), blueberries (Vaccinium angustifolium), or black currants (Ribes lacustre) are very rich in phenolic compounds with high antioxidant, anti-inflammatory, antiseptic, anti-tumoral, and anti-angiogenic capacity. Lingonberries are called “superfruits”, as they are very rich in antioxidants (vitamins C, A, and E), polyphenols, and functional compounds like fibers and minerals.44 Anthocyanin glycosides are the responsible pigments for the blue or red colors in berries and are the most frequent phenolic compounds in lingonberries. Lingonberries are hard to find in the marketplace compared to cranberries, strawberries, or other red fruits, but blueberries for example are consumed frequently in Europe, Asia, and also in the United States. There are several in vitro, in vivo and also clinical studies demonstrating the beneficial effect of these aliments as raw fruits or as nutritional supplements on treatment or prevention of carcinogenesis.45
Lingonberry extracts inhibit cancer cell multiplication and tumor progression in mice model systems; the anti-proliferative effect is due to the tannin-rich extract, composed of proanthocyanidins.46 The inhibition is also correlated with vitamin C and the synergistic effect of vitamin C and other substances contained in the fruits.47 In mice, supplementation of food with 10% of freeze-dried lingonberries substantially inhibited adenoma growth by reducing tumor size by over 60% in the distal part of the small intestine. The expression of adenosine deaminase (ADA) and 5’ecto-nucleotidase (5-NT) genes was inhibited, and Prostaglandin E2 (PGE2) receptor subtype EP4 expression was significantly decreased; in this way, the number and size of colonic adenomas were notably reduced in animal models.48
Natural berry extract (NBE) was awarded Food and Drug Administration—Investigational New Drug (FDA–IND; 140318) for human testing against infantile hemangioma.12 It was also demonstrated that a standardized anthocyanin-rich NBE was effective in prolonging survival of hemangioendothelioma bearing mice.13
The mouse hemangioendothelioma endothelial (EOMA) cells treated with a blend of powdered NBE highly decreased the activity of multidrug resistance protein-1 (MRP-1) compared to vehicle controls. This was conducted for nuclear accumulation of oxidized glutathione (GSSG) and apoptotic EOMA cell death, as demonstrated by immunocytochemistry. NBE-treated EOMA cells were injected into mice and led to the emergence of smaller tumors compared to vehicle-treated EOMA cells. NBE treatment significantly prolonged survival compared to vehicle-treated controls for tumor-bearing mice, as demonstrated by Kaplan–Meier survival curves.13
Recently, the same scientific team also showed that only the mitochondrial dynamics of tumor cells and not of non-tumor cells can be specifically targeted by the standardized natural product NBE and a combination therapy, including weak X-ray therapy (XRT, 0.5 Gy) and NBE significantly (threefold) extended survival of HE-affected mice.12 Gordillo et al demonstrated that NBE inhibits basal and nonmitochondrial respiration of EOMA cells and does not affect murine aortic endothelial (MAE) cells used as controls. Weak XRT had no effect on basal respiration but inhibited nonmitochondrial respiration in EOMA. The combination of NBE and weak XRT had no effect on healthy MAE cells but inhibited basal and nonmitochondrial respiration in EOMA cells. The mechanism was as follows: NBE decreased the rate of adenosine triphosphate (ATP) production and depleted ATP amount, elevated ADP, and decreased ATP/ADP ratio in EOMA cells but not in MAE cells. The synergistic effect explains the potentiation of EOMA cell death as a response to the combination of NBE treatment followed by weak XRT [12]. It was again demonstrated that anthocyanins, rich in berries, are able to influence mitochondrial dynamics.
Besides fruits, the leaves of some berries have a much higher antioxidant capacity than the fruits.49 Molecular mechanisms include inhibition of pro-inflammatory molecule production, inhibition of oxidative stress and products of this process such as DNA damage, inhibition of cancer cell proliferation, and increased apoptosis.50,51 The ingestion of fruits and plant extracts rich in polyphenolic compounds has neuroprotective effects and other positive effects on urinary, gastrointestinal tract, and cardiovascular system.49,52,53 The types of berries, active substances, properties, and food methods are summarized in Table 1.
 |
Table 1 Types of Berries, Active Substances, Properties, and Food Methods |
Methods of consumption/administration of active substances from fruits or plants foods with healing properties can be consumed in various forms. Red fruits will usually be eaten raw, but when they are astringent and difficult for children to tolerate, they can be turned into jams, compotes, jellies, and juices. Fermented berry juice is preferred by adults and shows an anti-proliferative and anti-invasive effect on highly proliferative and invasive oral tongue squamous carcinoma cells such as HSC-3 and SCC-25.54
In in vivo conditions, raw food goes through gastrointestinal digestion, suffering changes and reduction in the bioactive compound content, but despite these changes, digested berry extracts still showed bioactivity.55 Therefore, polyphenols from a diet rich in cultivated or wild berries are bioavailable, but significant inter-individual variation in all compounds’ plasma concentrations is found, depending on the differences in the intestinal microflora of each individual.56
It has been shown that wild fruits contain higher concentrations of polyphenols compared to the same cultivated variety, but the wild fruits are difficult to procure, instead their leaves are equally valuable. Wild berries are exposed to environmental stress, and they show an enhanced defense mechanism by producing increased number of polyphenolics and protecting the plants from external agents.56 In aqueous extracts from fruit or leaves, the flavanol contents ranged between 30% and 36%, and the flavonol glycosides are in the range of 7–9%.57,58 The solubility of phenolics is higher in alcohols, so the ethanol–water extract may be used with higher results than the infusion.59
Comparative analysis of several berry extracts led to the conclusion that wild blueberry and a berry mix were most effective in suppressing inducible VEGF expression and in vitro and in vivo angiogenesis.15 The blueberry and berry mix extracts had a high antioxidant function as determined by the oxygen radical absorbing capacity.15 The ORAC (oxygen radical absorbance capacity, a measure of anti-oxidant strength) values of strawberry powder and grape seed proanthocyanidin extract are higher than cranberry, elderberry, or raspberry seeds, but wild bilberry and blueberry extracts possessed the highest ORAC values.60 The consumption of forest fruits in general should be encouraged, not only those grown in farms (such as strawberries) but as varied as possible.
Fli-1 as a Target for Antitumor Drug Development
The high expression of proto-oncogene Fli-1 in vascular tumors is much related to good prognosis and enhanced immunity, because of its action as a tumor suppressor gene. A new trend in the treatment of cancer is molecular targeted therapy, which specifically targets oncogenic sites and causes specific death of tumor cells. Recently, a group of researchers studied the traditional Chinese medicine and ethnic medicine chemical resource databases, aiming to select anti-tumor drugs from it.19 They found that the flavagline compounds A1544 and A1545, the diterpenoids A661 and A665, the chalcone derivatives C10 and ZH-254, the Cardenolides, the calcium ionophores, lumefantrine, and some others have significant inhibitor efficacy as regulators targeting Fli-1.19
Chalcone derivatives are polyphenolic flavonoid substances (phytochemicals) which contain isoflavones, with antitumor, antibacterial, anti-inflammatory, antiviral, and other pharmacological activities.61 Chalcones are simple chemical scaffolds belonging to the flavonoid family, found in natural plant products such as spices, vegetables, fruits, and teas. Chalcone family substances are the subject of many studies due to their biological activities like antitumoral, anti-inflammatory, antidiabetic, antioxidant, and antimicrobial activities.62,63 Due to their special chemical scaffolding, chalcones are useful templates for the development of novel anticancer agents. Chalcones were also documented to modulate the following steps in angiogenesis: vascular endothelial factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β) signaling pathway, or hypoxia-inducible factor-1 (HIF-1); in this way, they can be used for the treatment of hemangiomas.64,65 Chalcones isolated from young Caesalpinia bonduc (Family Caesalpiniaceae) twigs and leaves show Fli-1 agonism and regulate the expression of VEGF-1, TGF-β2, p53, intercellular cell adhesion molecule-1 (ICAM-1), and MMP-1 genes, which are associated with tumor apoptosis, migration, and invasion in cancer cells.61,66
Chalcones represent the core of many natural biological compounds, being found in the roots, heartwoods, buds, leaves, flowers, rhizomes, and seeds of species of genera Angelica, Sopho ra, Humulus, Scutellaria, Glycyrrhiza, Parartocarpus, Ficus, Dorstenia, Morus, and Artocarpus.67 Isoliquiritigenin (ISL) is a bioactive compound with a chalcone structure isolated from licorice roots (Glycyrrhiza glabra, family Fabaceae). ISL has therapeutic potential against various cancers, inhibiting cancer cell migration and metastasis by suppressing cell proliferation, induces apoptosis and autophagy, arrests the cell cycle, inhibits angiogenesis, obstructs metastasis, and enhances chemosensitivity.68,69 Butein, is a biologically active flavonoid extracted from the bark of Rhus verniciflua Stokes; it exhibits significant anticancer activity and can attenuate angiogenesis, cell invasion/metastasis, and inflammation.70
The flavagline compounds from Dysoxylum binectariferum (Family Meliaceae), a Chinese medicinal plant, effectively inhibit the activity of Fli-1 and consequently negatively regulate the expression of the downstream target genes C-Kit, GATA-1, and Bcl-2.27 These natural compounds can represent a new strategy in the treatment of leukemia, lymphoma, and other tumors with Fli-1 overexpression.
Other Plants That Can Be Used for the Treatment of Vascular Tumors
EOMA cell proliferation could be suppressed by a sesquiterpene isolated from Sonchus oleraceus (family Asteraceae), a medicinal plant frequently found and used by many people as antioxidant and antidiabetic, and as a natural treatment for many other diseases. The sesquiterpenes were isolated by the methods of column chromatography, and Western Blot assay indicated that they induced apoptosis through the Bax/caspase-3 pathway. The plant extracts elicited a moderate inhibitory effect on EOMA cells with IC50 value of 26.5 μM.71
Grapes and grape seed extract are well-known sources of antioxidants, used for centuries in rejuvenation treatments, wound healing, inflammation, for cardiovascular disease treatment or in the fight against cancer, being known for their antiangiogenic effects. Grape seed proanthocyanidin extract (GSPE) was studied by comparison with six berries extract, and it was found that its antioxidant activity was higher than cranberry, elderberry, or raspberry seed but significantly lower than the other samples studied.60 GSPE containing 5000 ppm resveratrol facilitates oxidant-induced VEGF expression in keratinocytes and have beneficial therapeutic effects in related skin disorders, promoting dermal wound healing or vascular tumor regression.72
Nutrient mixture (NM) containing green tea (Camellia sinensis - Theaceae family) extract, lysine, proline, and ascorbic acid have shown significant anti-angiogenic and anti-tumor effects against a number of cancer cell lines. Using in vitro tests and a mouse hemangioendothelioma model, Roomi et al demonstrated that NM inhibited the growth of tumors by 50%. In vitro, NM induced dose-dependent apoptosis of EOMA cells.73 This study concluded that NM has therapeutic potential in treating infantile HE and possibly some other cutaneous vascular tumors. Another study demonstrated that nutrient supplementation with 0.5% NM strongly suppressed tumoral growth with no adverse effects in nude mice, suggesting the nutrient combination has potential as an anticancer agent. The finding was supported by histological studies showing inhibition of MMP-9 and VEGF secretion and mitotic index (critical parameters for cancer prevention and control).74
“Ankaferd Blood Stopper” (ABS) is a hemostatic and antimicrobial topical agent produced as a mixture of five different plant extracts (Urtica dioica - family Urticaceae, Vitis vinifera - family Vitaceae, Glycyrrhiza glabra - family Fabaceae, Thymus vulgaris - family Lamiaceae, and Alpinia officinarum - family Zingiberaceae). ABS is a natural hemostatic agent used in traditional Turkish medicine, well known for its rapid inhibiting effect on dental or cutaneous bleeding as well as postoperative external bleedings, and also for its antimicrobial effect.75,76 Annagur et al presented a seven-year-old boy with recurrent bleeding and infected hemangioma on the lower lip, successfully treated with topical ABS in terms of control of bleeding and infection.77 ABS provides hemostasis independently from coagulation factors and the standard coagulation cascade, by forming a structural net through interaction of fibrinogen in blood and, hence, providing vital aggregation of erythrocytes.78
Tanshen is the rhizome of Salvia miltiorrhiza Bunge (also known as red sage or Danshen) and is used in traditional Chinese medicine to treat hematological abnormalities, as an antioxidant and anticoagulant agent, as well as for its antibacterial and antineoplastic activities.11 Its compound DHTS was much more effective than propranolol in inhibiting hemangiomas proliferation and angiogenesis and may have potential therapeutic applications for future treatment of IH.9,11 Water-soluble phenolic acids such as salvianolic acid B are bioactive constituents from S. miltiorrhiza, possessing good bioactivities like antioxidant, anti-inflammatory, anti-cancer, and other health-promoting activities.79
The main findings of our research are centralized in Table 2.
 |
Table 2 Natural Agents that Were Tested for Hemangiomas or Another Vascular Tumor Treatment |
Discussion
Vascular anomalies are a heterogeneous group of blood vessel disorders subcategorized for the first time as separate entities by Mulliken and Glowacki in 1982 (based on unique characteristics) into vascular tumors and malformations.80 IH are the most common benign vascular tumors consisting of rapidly dividing endothelial cells. Medical and surgical options are available for the management of “problematic” hemangiomas, both options having various drawbacks. IH involving the head and neck will put patients at risk for ocular axis occlusion, amblyopia, astigmatism, tear-duct occlusion, and overall risk for ulceration and disfigurement. Therefore, when heart failure, ear disturbances, airway obstruction, nutritional disturbances, and certain cosmetic problems like disfigurement and scarring threaten to appear, a patient-specific treatment is recommended.3 Among alternatives there are propranolol, systemic or topical steroids, interferon-alpha, chemotherapeutic agents, laser therapy, and surgical excision.81 When the adverse effects are not tolerable or when the patient does not have a clear indication of standard treatment, alternative therapy based on active substances from the diet or plant extracts can be recommended for hemangiomas. Therefore, there is a great need to explore novel treatments for IH, with less side effects. Regarding vascular tumors with a risk of malignancy, huge efforts are being made by numerous teams of researchers to find those natural extracts with anti-angiogenic and anti-cancer effects, and with as few adverse effects as possible.
The use of proanthocyanidins, flavonoids, and chalcones from plant extracts is a potential therapeutic strategy to reduce inflammation and tumor angiogenesis. Chalcones play a central role in the flavonoid synthesis pathway and are frequently found in various natural products. These natural substances may also be used as therapeutics or as chemosensitizers for clinical drug resistance because they improve the pharmacokinetics of poorly absorbed chemotherapeutic cancer drugs.61 Despite medicinal applications of chalcones, their wide bioactivity spectrum represents a challenge for clinical development because used empirically or in inappropriate situations they can have adverse effects such as hepatotoxicity.82,83 However, more research on chalcones in marine natural products is needed and many in vivo, in vitro and clinical studies are necessary to be able to recommend the consumption of these substances as part of the treatment of some diseases, especially when it comes to children.
Natural berry extract is awarded Food and Drug Administration—Investigational New Drug for human testing against infantile hemangioma and it is proved to be very effective for treating HE in mice.12,13 Standardized NBE has been demonstrated to be effective in managing endothelial cell tumor in vivo through inhibition of MRP-1 and also in reducing cancer cell chemoresistance. An important potential role of phytochemicals as an adjunctive treatment to improve the effectiveness of chemotherapy was discovered. NBE inhibition of MRP-1 is worth consideration and more investigations are needed, as proanthocyanidins may be a notable therapeutic intervention with application for HE and other cancers with elevated MRP-1 activity. While NBE alone is effective in causing tumoral cell death, Gordillo et al demonstrated on mice with HE that the effect is significantly enhanced by cotreatment with weak XRT. Thus, a combination therapy, including weak X-ray therapy and NBE extended threefold the survival of HE-affected mice.12 Therefore, safe natural products such as berry extracts target tumor cell mitochondria and sharply lower radiation dosage required for tumor management, warranting more clinical testing.
During their course, up to 40% of IHs may lead to certain complications, depending on localization, dimensions, and the growth rate of the lesion. The ulceration because of the mechanical trauma, infection, and bleeding or scar appearance during healing can easily be prevented or treated with plant extracts like topical ABS, a product that may be easily applied by family members. ABS is a cheap and efficient hemostatic, antimicrobial, and antineoplastic agent with no adverse effects, which stops the bleeding in a very short time in cases of external bleeding such as that seen in children with IHs.84
Many clinical studies often confirm that the targeted therapy of cancer had an unexpectedly curative effect compared with conventional chemotherapeutic drugs, resulting in great progress in cancer management. The natural potential is extremely high, and numerous medicinal plants have already proven their effectiveness in improving and even curing numerous ailments. Every effort must be made to fully exploit this potential, for the benefit of our children and their families.
Limitation of the study: although in our review we tried to analyze as many sources of information as possible, we found very few original studies conducted on animals and even fewer conducted on human patients (clinical experience) regarding the naturopathic treatment of vascular tumors. But there are certainly other unexplored databases and case reports not included in this study, as well as reports at various conferences or preliminary results of clinical trials not yet reported, which could bring more information in the future. We need certainties, through studies based on clinical trials and randomized studies; this review only summarizes the current knowledge in the field of the beneficial actions of medicinal plants on hemangiomas and other vascular tumors.
Conclusion
Infantile hemangiomas are the most common benign vascular tumor consisting of rapidly dividing endothelial cells and may sometimes cause remarkable complications. Medical and surgical options are available for the management of “problematic” hemangiomas, both options having various drawbacks like the cost and side effects, which stop patients from adopting them. Natural extracts from plants and fruits are cost-effective and safe treatments for hemangiomas and vascular tumors, as well as for other forms of cancer, as demonstrated in our systematic review. In any case, more in vitro and in vivo experiments are needed to validate the proposed signaling pathways in tumors and confirm the improvement parameters after natural products administration. The era of personalized medicine and molecularly targeted therapy is approaching, and both naturally occurring and synthetic substances are very useful tools for tumor treatment and prevention. Plant extract substances have strong specificity and pertinence, are non- toxic and have few side effects, and may become an emerging cancer treatment. The results of this review are encouraging, demonstrating that there is enough evidence to support the promotion of a diet rich in natural active ingredients for the management of vascular tumors; anyway, more studies based on clinical trials and randomized studies are needed to demonstrate the benefits of a medical diet.
Funding
This research received no external funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390(10089):85–94. doi:10.1016/S0140-6736(16)00645-0
2. Tang R, Xian D, Xu J, Peng H, Pan S, Zhong J. Proanthocyanidins as a potential novel way for the treatment of hemangioma. Biomed Res Int. 2021;2021:5695378. doi:10.1155/2021/5695378
3. Ţarcă E, Cojocaru E, Roşu ST, Butnariu LI, Plămădeală P, Moisă ŞM. Differential diagnosis difficulties related to infantile hemangioma - case report and literature review. Rom J Morphol Embryol. 2019;60(4):1375–1379.
4. Zhang J, Ye Z, Tan L, Luo J. Giant hepatic hemangioma regressed significantly without surgical management: a case report and literature review. Front Med Lausanne. 2021;8:712324. doi:10.3389/fmed.2021.712324
5. Darrow DH, Greene AK, Mancini AJ, Nopper AJ; Section on dermatology, section on otolaryngology–head and neck surgery, and section on plastic surgery. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136(4):e1060–e1104. doi:10.1542/peds.2015-2485
6. Smith CJF, Friedlander SF, Guma M, Kavanaugh A, Chambers CD. Infantile hemangiomas: an updated review on risk factors, pathogenesis, and treatment. Birth Defects Res. 2017;109(11):809–815. doi:10.1002/bdr2.1023
7. Ji Y, Chen S, Li K, Li L, Xu C, Xiang B. Signaling pathways in the development of infantile hemangioma. J Hematol Oncol. 2014;7(1):13. doi:10.1186/1756-8722-7-13
8. Yang K, Zhang X, Chen L, Chen S, Ji Y. Microarray expression profile of mRNAs and long noncoding RNAs and the potential role of PFK-1 in infantile hemangioma. Cell Div. 2021;16(1):1. doi:10.1186/s13008-020-00069-y
9. Duan P, Huang Y, Chen K, Cheng C, Wu Z, Wu Y. 15,16-dihydrotanshinone I inhibits EOMA cells proliferation by interfering in posttranscriptional processing of hypoxia-inducible factor 1. Int J Med Sci. 2021;18(14):3214–3223. doi:10.7150/ijms.60774
10. Cai Y, Lv F, Kaldybayeva N, Zhamilya A, Wu Z, Wu Y. 15, 16-dihydrotanshinone I inhibits hemangiomas through inducing pro-apoptotic and anti-angiogenic mechanisms in vitro and in vivo. Front Pharmacol. 2018;9:25. doi:10.3389/fphar.2018.00025
11. Wang X, Morris-Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of tanshen. Med Res Rev. 2007;27(1):133–148. doi:10.1002/med.20077
12. Gordillo GM, Biswas A, Singh K, et al. Mitochondria as target for tumor management of hemangioendothelioma. Antioxid Redox Signal. 2021;34(2):137–153. doi:10.1089/ars.2020.8059
13. Biswas A, Clark EC, Sen CK, Gordillo GM. Phytochemical inhibition of multidrug resistance protein-1 as a therapeutic strategy for hemangioendothelioma. Antioxid Redox Signal. 2017;26(17):1009–1019. doi:10.1089/ars.2016.6881
14. Saqib S, Ullah F, Naeem M, et al. Mentha: nutritional and health attributes to treat various ailments including cardiovascular diseases. Molecules. 2022;27(19):6728. doi:10.3390/molecules27196728
15. Atalay M, Gordillo G, Roy S, et al. Anti-angiogenic property of edible berry in a model of hemangioma. FEBS Lett. 2003;544(1–3):252–257. doi:10.1016/s0014-5793(03)00509-x
16. Luca AC, Miron IC, Mîndru DE, et al. Optimal nutrition parameters for neonates and infants with congenital heart disease. Nutrients. 2022;14(8):1671. doi:10.3390/nu14081671
17. Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34
18. Lo K, Mihm M, Fay A. Current theories on the pathogenesis of infantile hemangioma. Semin Ophthalmol. 2009;24(3):172–177. doi:10.1080/08820530902805438
19. Li L, Yu J, Cheng S, Peng Z, Luo H. Transcription factor Fli-1 as a new target for antitumor drug development. Int J Biol Macromol. 2022;209(Pt A):1155–1168. doi:10.1016/j.ijbiomac.2022.04.076
20. Leon–Villapalos J, Wolfe K, Kangesu L. GLUT-1: an extra diagnostic tool to differentiate between haemangiomas and vascular malformations. Br J Plast Surg. 2005;58:348–352. doi:10.1016/j.bjps.2004.05.029
21. de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308(4):219–227. doi:10.1007/s00403-016-1635-x
22. Chen J, Wu D, Dong Z, Chen A, Liu S. The expression and role of glycolysis-associated molecules in infantile hemangioma. Life Sci. 2020;259:118215. doi:10.1016/j.lfs.2020.118215
23. Yang K, Qiu T, Zhou J, et al. Blockage of glycolysis by targeting PFKFB3 suppresses the development of infantile hemangioma. J Transl Med. 2023;21(1):85. doi:10.1186/s12967-023-03932-y
24. Babiak-Choroszczak L, Giżewska-Kacprzak K, Gawrych E, et al. Serum concentrations of VEGF and bFGF in the course of propranolol therapy of infantile hemangioma in children: are we closer to understand the mechanism of action of propranolol on hemangiomas? Adv Clin Exp Med. 2018;27(5):703–710. doi:10.17219/acem/84800
25. Chen YZ, Bai N, Bi JH, et al. Propranolol inhibits the proliferation, migration and tube formation of hemangioma cells through HIF-1α dependent mechanisms. Braz J Med Biol Res. 2017;50(12): e6138. doi:10.1590/1414-431X20176138
26. Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis. 2011;2(5):e164–e164. doi:10.1038/cddis.2011.48
27. Song J, Yuan C, Yang J, et al. Novel flavagline-like compounds with potent fli-1 inhibitory activity suppress diverse types of leukemia. FEBS J. 2018;285(24):4631–4645. doi:10.1111/febs.14690
28. Mazzotta C, Marden G, Farina A, Bujor A, Trojanowski MA, Trojanowska M. FLI1 and ERG protein degradation is regulated via cathepsin B lysosomal pathway in human dermal microvascular endothelial cells. Microcirculation. 2021;28(1):e12660. doi:10.1111/micc.12660
29. Luca AC, Miron IC, Trandafir LM, et al. Morphological, genetic and clinical correlations in infantile hemangiomas and their mimics. Rom J Morphol Embryol. 2020;61(3):687–695. doi:10.47162/RJME.61.3.07
30. Leaute-Labreze C, Hoeger P, Mazereeuw-Hautier J, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med. 2015;372(8):735–746. doi:10.1056/NEJMoa1404710
31. Lomenick JP, Reifschneider KL, Lucky AW, et al. Prevalence of adrenal insufficiency following systemic glucocorticoid therapy in infants with hemangiomas. Arch Dermatol. 2009;145(3):262–266. doi:10.1001/archdermatol.2008.572
32. George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Arch Dermatol. 2004;140(8):963–969. doi:10.1001/archderm.140.8.963
33. Muñoz-Garza FZ, Ríos M, Roé-Crespo E, et al. Efficacy and safety of topical timolol for the treatment of infantile hemangioma in the early proliferative stage: a randomized clinical trial. JAMA Dermatol. 2021;157(5):583–587. doi:10.1001/jamadermatol.2021.0596
34. Prodromidou A, Machairas N, Garoufalia Z, et al. Liver transplantation for giant hepatic hemangioma: a systematic review. Transplant Proc. 2019;51(2):440–442. doi:10.1016/j.transproceed.2019.01.018
35. Li F, Huang J, Ji D, et al. Azithromycin effectively inhibits tumor angiogenesis by suppressing vascular endothelial growth factor receptor 2-mediated signaling pathways in lung cancer. Oncol Lett. 2017;14(1):89–96. doi:10.3892/ol.2017.6103
36. Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician. 2010;82(4):381–388, 394.
37. Huang S, Yang N, Liu Y, et al. Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr Res. 2012;32(7):530–536. doi:10.1016/j.nutres.2012.05.012
38. Yang L, Xian D, Xiong X, Lai R, Song J, Zhong J. Proanthocyanidins against o xidative stress: from molecular mechanisms to clinical applications. Biomed Res. Int. 2018;11. doi:10.1155/2018/8584136.8584136
39. Sano A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Ood Chem Toxicol. 2017;108(Pt B):519–523. doi:10.1016/j.fct.2016.11.021
40. Yang L-J, Zhu D-N, Dang Y-L, Zhao X. Treatment of condyloma acuminata in pregnant women with cryotherapy combined with proanthocyanidins: outcome and safety. Exp Ther Med. 2016;11(6):2391–2394. doi:10.3892/etm.2016.3207
41. Afshar K, Stothers L, Scott H, MacNeily AE. Cranberry juice for the prevention of pediatric urinary tract infection: a randomized controlled trial. J Urol. 2012;188(4):1584–1587. doi:10.1016/j.juro.2012.02.031
42. Chen GY, Liu XY, Yan XE, et al. Total flavonoids of rhizoma drynariae treat osteoarthritis by inhibiting arachidonic acid metabolites through AMPK/NFκB pathway. J Inflamm Res. 2023;16:4123–4140. doi:10.2147/JIR.S418345
43. Yang J, Wang Q, Zhao R, et al. Identification of oligomer proanthocyanidins (F2) isolated from grape seeds as a formyl peptide receptor 1 partial agonist. Int Immunopharmacol. 2013;15(4):756–763. doi:10.1016/j.intimp.2013.03.007
44. Mane C, Loonis M, Juhel C, Dufour C, Malien-Aubert C. Food grade lingonberry extract: polyphenolic composition and in vivo protective effect against oxidative stress. J Agric Food Chem. 2011;59(7):3330–3339. doi:10.1021/jf103965b
45. Johnson SA, Arjmandi BH. Evidence for anti-cancer properties of blueberries: a mini-review. Anticancer Agents Med Chem. 2013;13(8):1142–1148. doi:10.2174/18715206113139990137
46. McDougall GJ, Ross HA, Ikeji M, Stewart D. Berry extracts exert different antiproliferative effects against cervical and colon cancer cells grown in vitro. J Agric Food Chem. 2008;56(9):3016–3023. doi:10.1021/jf073469n
47. Olsson ME, Gustavsson KE, Andersson S, Nilsson A, Duan RD. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J Agric Food Chem. 2004;52(24):7264–7271. doi:10.1021/jf030479p
48. Misikangas M, Pajari A-M, Päivärinta E, et al. Three Nordic berries inhibit intestinal tumorigenesis in multiple intestinal neoplasia/+ mice by modulating β-catenin signaling in the tumor and transcription in the mucosa. J Nutr. 2007;137(10):2285–2290. doi:10.1093/jn/137.10.2285
49. Kelly E, Vyas P, Weber JT. Biochemical properties and neuroprotective effects of compounds in various species of berries. Molecules. 2017;23(1):26. doi:10.3390/molecules23010026
50. Sergazy S, Shulgau Z, Kamyshanskiy Y, et al. Blueberry and cranberry extracts mitigate CCL4-induced liver damage, suppressing liver fibrosis, inflammation and oxidative stress. Heliyon. 2023;9(4):e15370. doi:10.1016/j.heliyon.2023.e15370
51. Bunea A, Rugină D, Sconţa Z, et al. Anthocyanin determination in blueberry extracts from various cultivars and their antiproliferative and apoptotic properties in B16-F10 metastatic murine melanoma cells. Phytochemistry. 2013;95:436–444. doi:10.1016/j.phytochem.2013.06.018
52. Hossain MZ, Shea E, Daneshtalab M, Weber JT. Chemical analysis of extracts from newfoundland berries and potential neuroprotective effects. Antioxidants. 2016;5(4):36. doi:10.3390/antiox5040036
53. Trandafir LM, Frăsinariu OE, Țarcă E, et al. Can bioactive food substances contribute to cystic fibrosis-related cardiovascular disease prevention? Nutrients. 2023;15(2):314. doi:10.3390/nu15020314
54. Hoornstra D, Vesterlin J, Pärnänen P, et al. Fermented lingonberry juice inhibits oral tongue squamous cell carcinoma invasion in vitro similarly to curcumin. In Vivo. 2018;32(5):1089–1095. doi:10.21873/invivo.11350
55. Brown EM, Nitecki S, Pereira-Caro G, et al. Comparison of in vivo and in vitro digestion on polyphenol composition in lingonberries: potential impact on colonic health. Bio Fact. 2014;40:611–623. doi:10.1002/biof.1173
56. Koli R, Erlund I, Jula A, Marniemi J, Mattila P, Alfthan G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J Agric Food Chem. 2010;58(7):3927–3932. doi:10.1021/jf9024823
57. Zorzi M, Gai F, Medana C, Aigotti R, Peiretti PG. Identification of polyphenolic compounds in edible wild fruits grown in the north-west of Italy by means of HPLC-DAD-ESI HRMS. Plant Foods Hum Nutr. 2020;75(3):420–426. doi:10.1007/s11130-020-00830-2
58. Kowalska K. Lingonberry (Vaccinium vitis-idaea L.) fruit as a source of bioactive compounds with health-promoting Effects-A review. Int J Mol Sci. 2021;22(10):5126. doi:10.3390/ijms22105126
59. Dróżdż P, Šėžienė V, Pyrzynska K. Phytochemical properties and antioxidant activities of extracts from wild blueberries and lingonberries. Plant Foods Hum Nutr. 2017;72(4):360–364. doi:10.1007/s11130-017-0640-3
60. Roy S, Khanna S, Alessio HM, et al. Anti-angiogenic property of edible berries. Free Radic Res. 2002;36(9):1023–1031. doi:10.1080/1071576021000006662
61. Ouyang Y, Li J, Chen X, Fu X, Sun S, Wu Q. Chalcone derivatives: role in anticancer therapy. Biomolecules. 2021;11(6):894. doi:10.3390/biom11060894
62. Gao F, Huang G, Xiao J. Chalcone hybrids as potential anticancer agents: current development, mechanism of action, and structure-activity relationship. Med Res Rev. 2020;40:2049–2084. doi:10.1002/med.21698
63. Lin Y, Zhang M, Lu Q, Xie J, Wu J, Chen C. A novel chalcone derivative exerts anti-inflammatory and anti-oxidant effects after acute lung injury. Aging. 2019;11:7805–7816. doi:10.18632/aging.102288
64. Mirossay L, Varinska L, Mojzis J. Antiangiogenic effect of flavonoids and chalcones: an update. Int J Mol Sci. 2017;19:27. doi:10.3390/ijms19010027
65. Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F. Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vasc Pharmacol. 2017;15:503–519. doi:10.2174/1570161115666170713094319
66. Iheagwam FN, Ogunlana OO, Ogunlana OE, Isewon I, Oyelade J. Potential anti-cancer flavonoids isolated from Caesalpinia bonduc young twigs and leaves: molecular docking and in silico studies. Bioinform Biol Insights. 2019;13:1177932218821371. doi:10.1177/1177932218821371
67. Rozmer Z, Perjési P. Naturally occurring chalcones and their biological activities. Phytochem Rev. 2014;15:87–120. doi:10.1007/s11101-014-9387-8
68. Wang C, Chen Y, Wang Y, et al. Inhibition of COX-2, mPGES-1 and CYP4A by isoliquiritigenin blocks the angiogenic akt signaling in glioma through ceRNA effect of miR-194-5p and lncRNA NEAT1. J Exp Clin Cancer Res CR. 2019;38:371. doi:10.1186/s13046-019-1361-2
69. Lin PH, Chiang YF, Shieh TM, et al. Dietary compound isoliquiritigenin, an antioxidant from licorice, suppresses triple-negative breast tumor growth via apoptotic death program activation in cell and xenograft animal models. Antioxidants (Basel). 2020;9(3):228. doi:10.3390/antiox9030228
70. Moon D-O, Choi YH, Moon S-K, Kim W-J, Kim G-Y. Butein suppresses the expression of nuclear factor-kappa B-mediated matrix metalloproteinase-9 and vascular endothelial growth factor in prostate cancer cells. Toxicol. In Vitro. 2010;24:1927–1934. doi:10.1016/j.tiv.2010.08.002
71. Lei M, Wang Q, Liu B, Che Y. Two new sesquiterpenes from Sonchus oleraceus and inhibitory mechanism on murine haemangioendothelioma (EOMA) cell lines. Nat Prod Res. 2022;36(11):2814–2820. doi:10.1080/14786419.2021.1931186
72. Khanna S. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free Radic Biol Med. 2001;31(1):38–42. doi:10.1016/s0891-5849(01)00544-5
73. Roomi MW, Kalinovsky T, Niedzwiecki A, Rath M. Antiangiogenic properties of a nutrient mixture in a model of hemangioma. Exp Oncol. 2009;31(4):214–219.
74. Roomi MW, Ivanov V, Kalinovsky T, Niedzwiecki A, Rath M. In vivo antitumor effect of ascorbic acid, lysine, proline and green tea extract on human colon cancer cell HCT 116 xenografts in nude mice: evaluation of tumor growth and immunohistochemistry. Oncol Rep. 2005;13(3):421–425.
75. Meric Teker A, Korkut AY, Kahya V, Gedikli O. Prospective, randomized, controlled clinical trial of ankaferd blood stopper in patients with acute anterior epistaxis. Eur Arch Otorhinolaryngol. 2010;267:1377–1381. doi:10.1007/s00405-010-1208-0
76. Tasdelen Fisgin N, Tanriverdi Cayci Y, Coban AY, et al. Antimicrobial activity of plant extract ankaferd blood stopper. Fitoterapia. 2009;80:48–50. doi:10.1016/j.fitote.2008.09.006
77. Annagür A, Altunhan H, Konak M, Ors R. Successful use of topical ”ankaferd blood stopper” for repetitive bleedings in an infant with infantile hemangioma. Int J Clin Exp Med. 2012;5(4):342–345.
78. Goker H, Haznedaroglu IC, Ercetin S, et al. Haemostatic actions of the folkloric medicinal plant extract ankaferd blood stopper. J Int Med Res. 2008;36:163–170. doi:10.1177/147323000803600121
79. Shi M, Huang F, Deng C, Wang Y, Kai G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in salvia miltiorrhiza. Crit Rev Food Sci Nutr. 2019;59(6):953–964. doi:10.1080/10408398.2018.1474170
80. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982;69:412–422. doi:10.1097/00006534-198203000-00002
81. Xu W, Zhao H. Management of infantile hemangiomas: recent advances. Front Oncol. 2022;12:1064048. doi:10.3389/fonc.2022.1064048
82. Tugcu G, Kirmizibekmez H, Aydin A. The integrated use of in silico methods for the hepatotoxicity potential of piper methysticum. Food Chem Toxicol. 2020;145:111663. doi:10.1016/j.fct.2020.111663
83. Țarcă V, Țarcă E, Luca FA. The impact of the main negative socio-economic factors on female fertility. Healthcare. 2022;10(4):734. doi:10.3390/healthcare10040734
84. Malkan UY, Haznedaroglu IC. Antineoplastic effects of ankaferd hemostat. Biomed Res Int. 2022;2022:2665903. doi:10.1155/2022/2665903
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
